Published online Aug 15, 2017. doi: 10.4291/wjgp.v8.i3.117
Peer-review started: February 8, 2017
First decision: April 17, 2017
Revised: July 4, 2017
Accepted: July 14, 2017
Article in press: July 17, 2017
Published online: August 15, 2017
Processing time: 199 Days and 21 Hours
To determine if 30-d of oral spore-based probiotic supplementation could reduce dietary endotoxemia.
Apparently healthy men and women (n = 75) were screened for post-prandial dietary endotoxemia. Subjects whose serum endotoxin concentration increased by at least 5-fold from pre-meal levels at 5-h post-prandial were considered “responders” and were randomized to receive either placebo (rice flour) or a commercial spore-based probiotic supplement [Bacillus indicus (HU36), Bacillus subtilis (HU58), Bacillus coagulans, and Bacillus licheniformis, and Bacillus clausii] for 30-d. The dietary endotoxemia test was repeated at the conclusion of the supplementation period. Dietary endotoxin (LAL) and triglycerides (enzymatic) were measured using an automated chemistry analyzer. Serum disease risk biomarkers were measured using bead-based multiplex assays (Luminex and Milliplex) as secondary, exploratory measures.
Data were statistically analyzed using repeated measures ANOVA and a P < 0.05. We found that spore-based probiotic supplementation was associated with a 42% reduction in endotoxin (12.9 ± 3.5 vs 6.1 ± 2.6, P = 0.011) and 24% reduction in triglyceride (212 ± 28 vs 138 ± 12, P = 0.004) in the post-prandial period Placebo subjects presented with a 36% increase in endotoxin (10.3 ± 3.4 vs 15.4 ± 4.1, P = 0.011) and 5% decrease in triglycerides (191 ± 24 vs 186 ± 28, P = 0.004) over the same post-prandial period. We also found that spore-based probiotic supplementation was associated with significant post-prandial reductions in IL-12p70 (24.3 ± 2.2 vs 21.5 ± 1.7, P = 0.017) and IL-1β (1.9 ± 0.2 vs 1.6 ± 0.1, P = 0.020). Compared to placebo post supplementation, probiotic subject had less ghrelin (6.8 ± 0.4 vs 8.3 ± 1.1, P = 0.017) compared to placebo subjects.
The key findings of the present study is that oral spore-based probiotic supplementation reduced symptoms indicative of “leaky gut syndrome”.
Core tip: Dietary or metabolic endotoxemia is a condition that affects approximately 1/3 of individuals living in Western society. It is characterized by increased serum endotoxin concentration during the first five hours of the post-prandial period following consumption of a meal with a high-fat, high-calorie content. The key findings of the present study, were that 30-d of oral spore-based probiotic supplementation reduced the incidence of dietary endotoxemia, which may be indicative of reduced gut permeability.
- Citation: McFarlin BK, Henning AL, Bowman EM, Gary MA, Carbajal KM. Oral spore-based probiotic supplementation was associated with reduced incidence of post-prandial dietary endotoxin, triglycerides, and disease risk biomarkers. World J Gastrointest Pathophysiol 2017; 8(3): 117-126
- URL: https://www.wjgnet.com/2150-5330/full/v8/i3/117.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v8.i3.117
Incidence of gastrointestinal (GI) distress and permeability has increased in prominence in modern society due in large part to the excessive consumption of highly processed, calorie dense, commercially available foods[1]. These same dietary choices coupled with low physical activity are believed to be the primary causes underlying the current obesity epidemic[2]. Recent efforts have focused on the use of over-the-counter probiotics (typically Lactobacillus and Bifidobacterium) to address symptoms associated with GI abnormalities[3-5]. The lay literature has generally identified a goal of improved “GI health”, but unfortunately this is so broadly defined that it is nearly impossible to identify a single research focus[6]. Further complicating matters is that probiotic supplementation does not yield consistent results[7,8]. We have speculated that if an individual doesn’t have a pre-existing GI abnormality then they would not be a “responder” to probiotic supplementation. Complicating oral probiotic supplementation efforts is the fact that few traditional probiotic supplements (i.e., Lactobacillus and Bifidobacterium) delivery fully viable bacteria to the small intestine[9,10]. Recently it has been speculated gram positive, spore-forming probiotic strains may be a good alternative because the endospores that encapsulate the strains are highly resistant to stomach acid, potentially resulting in the delivery of more viable probiotics to the small intestine[11,12]. Thus, it appears that two major limitations of the existing probiotic literature lie with an inability to identify “responder” subjects prior to enrollment and issues associated with viable probiotic delivery to the small intestine.
Dietary or metabolic endotoxemia occurs when one’s dietary consumption causes disruption in either GI permeability, the microbiota profile, or both[1,2,4,13-15]. Dietary endotoxemia transiently increases systemic inflammation, which chronically may increase one’s risk of a variety of diseases[2]. Our laboratory and others have demonstrated that consumption of a single, high-fat, high-calorie meal was associated with an increase in serum endotoxin, triglycerides, metabolic biomarkers, inflammatory cytokines, endothelial microparticles, and monocyte adhesion molecules[16-22]. The post-prandial time course varies for each biomarker, but generally the transient changes occur during the first five hours of the post-prandial period. Given the direct link between nutrition, microbiota, GI permeability, and disease risk, our laboratory and others have speculated that these changes represent an appropriate treatment target for a probiotic intervention[23,24]. To address known issues with sufficient probiotic delivery, we utilized a “spore-based” probiotic in the present study. According to the literature the biggest advantages of a “spore-based” probiotic is that it is compose of endosomes which are highly resistant to acidic pH, are stable at room temperature, and deliver a much greater quantity of high viability bacteria to the small intestine that traditional probiotic supplements[11,12]. To our knowledge, the present study is the first attempt to clinically leverage the benefits of spore-based probiotics to improve health outcomes. The primary purpose of the present study was to determine if 30-d of spore-based probiotic supplementation reduced post-prandial endotoxemia and triglycerides. The study enrollment was unique in that we developed an additional level of screening to only enroll subjects who had dietary endotoxemia (i.e., responders). Our secondary purpose was to determine if other metabolic biomarkers and cytokines, known to change after consuming a high-fat meal, would also be modified by 30-d of spore-based probiotic supplementation.
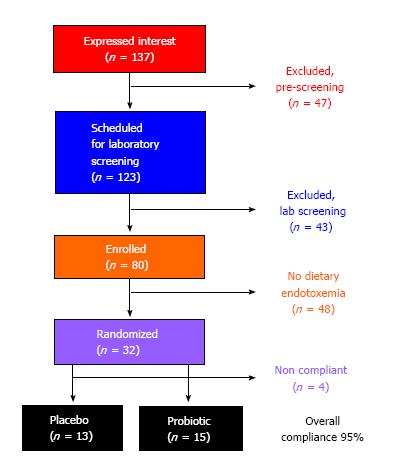
All the procedures described in the present study were reviewed and approved by the University of North Texas Institutional Review Board (IRB) for Human Subject’s Research. Subjects provided their written and verbal consent to participate before being enrolled in the study. The present study was completed following a completion of a preliminary proof of concept study in the laboratory (data not shown). From this data, we identified that only 2 of 6 subjects (“responders”) had a measurable dietary endotoxemia response (i.e., at least a 5-fold increase from pre-meal values at 5-h post-prandial). “Responder” subjects experienced a 30% reduction in serum endotoxin (effect size = 0.40) at 5-h post-prandial following a 30-d probiotic intervention (same probiotic used in the present study). Based on these criteria, we identified that we needed to enroll a minimum of n = 10 “responders” in placebo and spore-based probiotic groups (n = 20 total) in order to achieve at least 80% statistical power. Eighty subjects were screened for a dietary endotoxin response, and 25 “responders” were enrolled (Table 1) and matriculated through the study treatments (Figure 1).
| Characteristic | Placebo(n = 13) | Probiotic(n = 15) |
| Age (yr) | 21.8 ± 0.7 | 21.2 ± 0.5 |
| Height (cm) | 167.9 ± 3.2 | 170.8 ± 2.7 |
| Body mass (kg) | 74.2 ± 6.6 | 71.2 ± 3.1 |
| Body mass index (kg/m2) | 25.9 ± 1.5 | 24.3 ± 0.9 |
| Body fat (%) | 27.8 ± 4.1 | 25.2 ± 3.0 |
| Fat mass (kg) | 21.0 ± 4.3 | 17.3 ± 2.4 |
| Lean mass (kg) | 50.1 ± 3.8 | 50.0 ± 3.7 |
| Bone mineral mass (kg) | 2.9 ± 0.2 | 2.9 ± 0.1 |
| Resting energy expenditure (kcal/d) | 2243 ± 304 | 2071 ± 108 |
Prior to testing for the post-prandial endotoxemia response, subjects also completed a series of other tests to exclude for other pre-existing conditions. Screening included measurement of body composition (whole body DEXA scan; GE Lunar Prodigy, United States), medical history assessment, and resting metabolic rate (RMR; MGC Diagnostics Ultima; St. Paul, MN, United States). Subjects who were currently taking or had taken in the previous 6-mo medications for the treatment of metabolic disease, antibiotics, probiotic supplements, anti-inflammatory medications, and/or daily consumed at least 3 serving of yogurt were excluded from further participation. Within the medical history, we also excluded subjects who were currently being treated for metabolic disease (i.e., diabetes mellitus), currently being treated for cardiovascular disease, and/or were obese (by BMI and/or percent body fat from DEXA). Individuals who met the initial screening criteria were scheduled to consume the experimental meal challenge on a separate day. The experimental meal challenge was used to identify subjects with a dietary endotoxin response that we considered “responders”. Individuals classified as “responders” were enrolled in the supplementation phase of the study.
Experimental meal challenge: Subjects reported to the laboratory between 0600 and 1000 following an overnight fast (> 8-h) and abstention from exercise (> 24-h). Following collection of a pre-meal blood sample, subjects were provided a high-fat meal (85% of the daily fat RDA and 65% of the daily calorie needs based on RMR). Thin crust cheese pizza from a local vendor was used as the high-fat meal source (Table 2). Blood samples were measured for endotoxin concentration after the meal and only those subjects whose endotoxin level increased by > 5-fold at 5-h post-prandial were classified as “responders” and enrolled in the supplementation phase of the study. This same experimental meal challenge was completed at the end of the supplementation period to assess the effectiveness of spore-based probiotic supplementation at modifying the serum endotoxin response.
| Component | Placebo (n = 13) | Probiotic (n = 15) |
| Total calories (kcal) | 1630.4 ± 134.4 | 1644.7 ± 94.5 |
| Total caloric needs (% of RMR) | 72% | 79% |
| Servings (#) | 6.3 ± 0.5 | 6.4 ± 0.4 |
| Fat (g) | 88.8 ± 7.3 | 89.6 ± 5.1 |
| Fat (kcal) | 799.3 ± 6.6 | 806.4 ± 46.3 |
| Saturated fat (g) | 31.7 ± 2.6 | 32.0 ± 1.8 |
| Trans fat (g) | 0 | 0 |
| Protein (g) | 69.8 ± 5.8 | 70.4 ± 4.0 |
| Carbohydrate (g) | 145.9 ± 12.0 | 147.2 ± 8.5 |
| Carbohydrate (kcal) | 583.6 ± 48.1 | 588.8 ± 33.8 |
| Cholesterol (mg) | 152.3 ± 12.5 | 153.6 ± 8.8 |
| Sodium (mg) | 2911.9 ± 240.0 | 2937.4 ± 168.8 |
Supplementation conditions: “Responder” subjects were randomized to either a placebo (rice flour) or spore-based probiotic (Megasporebiotic; Physicians Exclusive, LLC; Glenview, IL, United States) condition. The spore-based probiotic included 4 billion spores from gram-positive, spore-forming strains [Bacillus indicus (HU36), Bacillus subtilis (HU58), Bacillus coagulans, and Bacillus licheniformis, Bacillus clausii]. Subjects were instructed to consume 2 capsules each day for a total of 30-d. Subjects were asked to promptly report any missed doses. Based on subject reporting, efficacy of intake was > 95% for the study period. All group assignments were completed using double-blind procedures. Subjects were instructed to maintain their habitual dietary and lifestyle habits during the study.
Blood sample collection: Venous blood samples were collected prior to the high-fat meal (PRE), 3-h, and 5-h post meal from a peripheral arm vein into an evacuated serum tube. Serum tubes were held at room temperature for 30-min to allow for clotting. Serum was separated by centrifugation and frozen at -80 °C until additional analysis.
Serum was analyzed for endotoxin concentration using a commercially available kinetic limulus amebocyte lysate (LAL) assay (Lonza; Allendale, NJ, United States). Briefly, serum samples were diluted 1:100 in endotoxin-free water and heated at 70 °C for 15-min to remove contaminating proteases. Treated samples were then analyzed in triplicate using an automated chemistry analyzer (Chem Well T; Palm City, FL, United States) to determine endotoxin concentration against an E. coli endotoxin standard.
Serum was analyzed in triplicate for triglyceride concentration using an endpoint enzymatic assay (Pointe Scientific; Canton, MI, United States) on an automated chemistry analyzer (ChemWell T).
Exploratory disease risk biomarkers: Previously frozen serum samples were analyzed as previously described[25-27]. Briefly, ghrelin, insulin, leptin, MCP-1, GM-CSF, interleukin (IL)-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12(p70), IL-13, and tumor necrosis factor alpha (TNF-α) were measured in duplicate using a commercially available bead-based multiplex assay (Milliplex; MilliporeSigma; St. Louis, MO, United States) and an automated analyzer (Luminex MagPix; Austin, TX, United States). Raw data files were used to calculate unknowns from standards using Milliplex Analyst software (MilliporeSigma).
Prior to formal statistical testing data were assessed for normality. Non-normal data was log-transformed to stabilize this assumption prior to formal testing. Data were analyzed using a condition (placebo or probiotic) × experiment time (baseline and 30-d post) × meal time (pre, 3, and 5-h post) analysis of variance (ANOVA) with repeated measurements on the 2nd and 3rd factors. P-values were adjusted using the Huynh-Feldt method to account for the repeated measures design. Significance was set at P < 0.05. Location of significant effects was determined using separate t-tests with a Bonferroni correction for multiple comparisons.
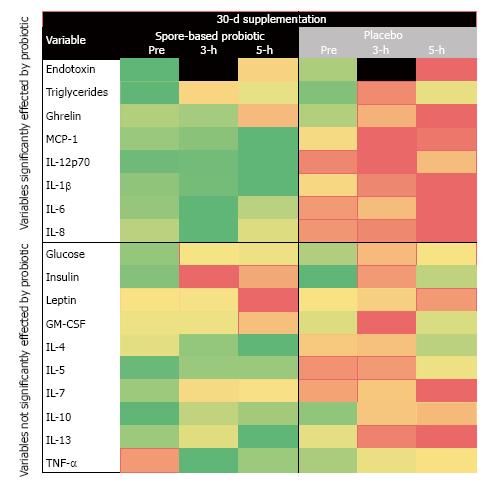
In order to visualize the responses collectively, we log transformed all the responses to normalize the various biomarkers to a similar scale. We then created three radar plots (one for each sampling time point). Each plot contained the log transformed variable response at baseline and 30-d post and a third line for the fold-change from pre-meal response). Heat maps were generated for variables that showed similarity to endotoxin responses using a three-color approach: Red (large increase from pre-meal), yellow (intermediate response), and green (large decrease from pre-meal) (Figure 2). We have used a similar approach to data visualization in past manuscripts and this is an effective and accepted method[19,28].
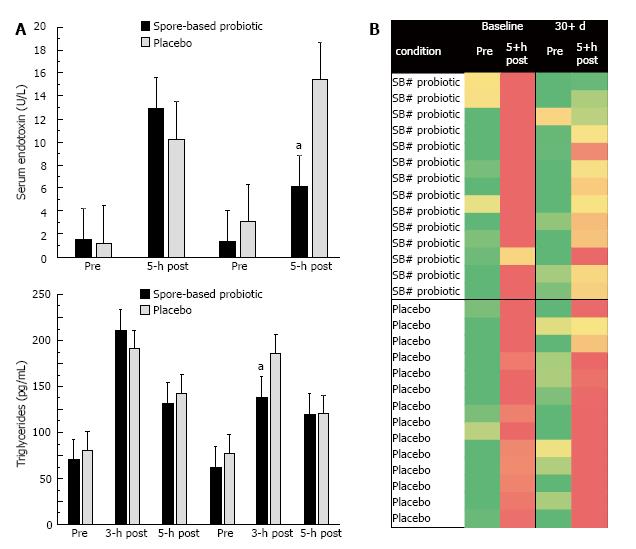
We found significant three-way interaction effects for both serum endotoxin (P = 0.011; Figure 3A) and triglycerides (P = 0.004; Figure 3B). In each instance, there was no difference between the post-prandial response between the two treatment groups (i.e., placebo vs spore-based probiotic) at baseline; however, the significant differences were apparent at post-supplementation. Specifically, spore-based probiotic supplementation was associated with a 42% reduction in serum endotoxin at 5-h post-prandial compared to a 36% increase in placebo at the same time point. Spore-based probiotic supplementation was associated with a 24% reduction in serum triglycerides at 3-h post-prandial compared to a 5% reduction in placebo at the same time point.
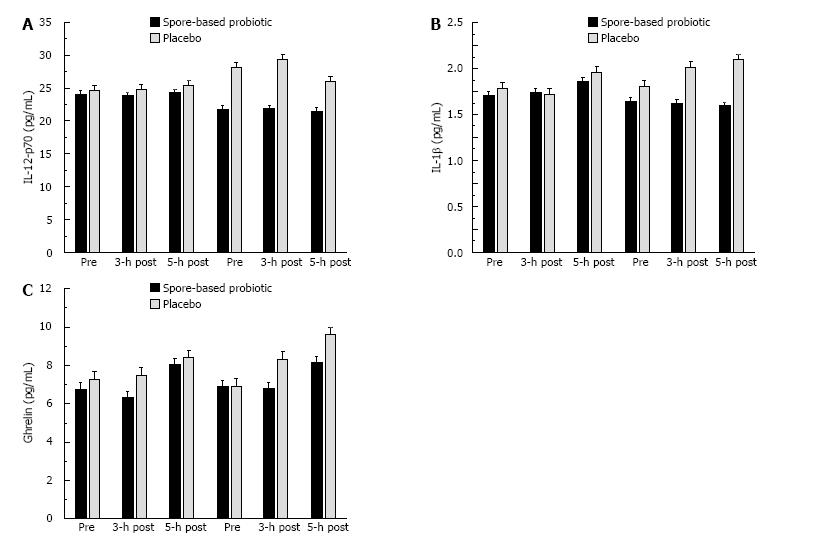
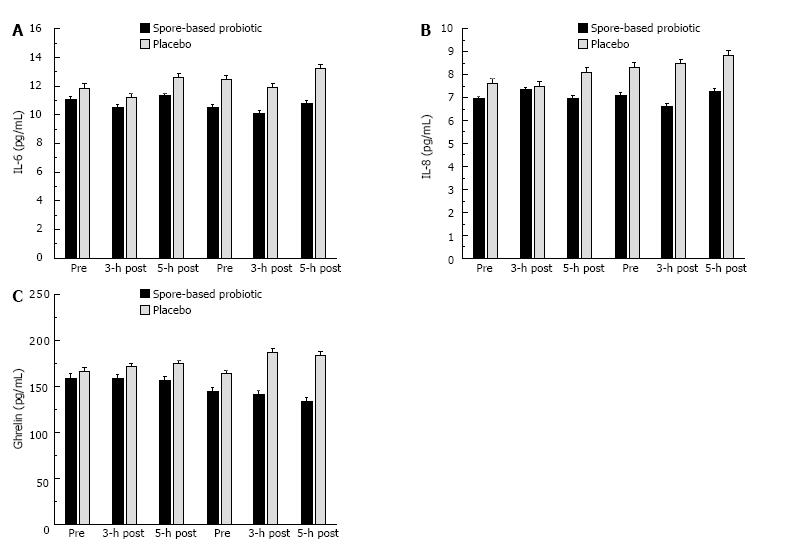
We found significant trial × condition interactions for IL-12p70 (P = 0.017; Figure 4A), IL-1β (P = 0.020; Figure 4B), and ghrelin (P = 0.017; Figure 4C). We also found potentially interesting trends for IL-6 (P = 0.154; Figure 5A), IL-8 (P = 0.284; Figure 5B), and MCP-1 (P = 0.141; Figure 5C). These effects were consistent with the pattern observed for serum endotoxin in that spore-based probiotic intervention was associated with a reduction in a given biomarker at post-supplementation compared to pre-supplementation and placebo.
An a priori review of the existing literature[5,29], lead our team to speculate that there may be an ideal subject phenotype that was “responsive” to spore-based probiotic treatment. Thus, we designed and implemented a screening protocol for the present study to identify individuals who presented with post-prandial endotoxemia at baseline, which may be a hallmark sign of intestinal permeability and “leaky gut” syndrome[14,15,22,23]. We believe our approach to subject selection increased the efficacy and applicability of our key findings. Within our “responder” population (who likely had a non-protective microbiome), we were able to demonstrate that 30-d of oral supplementation with a viable, spore-based probiotic was associated with a significant reduction in post-prandial endotoxin and triglycerides. Further, we found that several of our exploratory biomarkers were either significantly reduced (IL-12p70, IL-1β, and ghrelin) or trended toward reduction (IL-6, IL-8, and MCP-1) with spore-based probiotic supplementation. It is reasonable to speculate that the spore-based probiotic supplement may have exerted its effect by altering the gut microbial profile, altering intestinal permeability, or a combination of the two effects. The present study was designed to assess systemic changes rather than focus on intestinal measures that are invasive or impossible to make accurately in human subjects. The reductions observed in the present study with spore-based probiotic supplementation were consistent with a transient reduction in chronic disease risk. It is also important to note that the reported changes were observed while the college-aged subjects continued to lead their habitual life with no directed modification. They continued to be exposed to many of the stressors that are known to negatively affect gut permeability in college-aged individuals (i.e., consumption of microwaved and other processed food, fast foods, soft drinks with their excess of sugars, including artificial sugars, colorings and flavorings, energy drinks, alcohol consumption, lack of sleep, exam anxiety, etc.).
Previous authors consistently speculate that the onset and progression of chronic disease results from the accumulation of transient changes in ones’ health that result from lifestyle choices[16,18,19,22,28,30-32]. Unfortunately, the current literature has yet to define the quantity of transient change that must be accumulated to cause disease onset. Instead, previous studies have attempted to use lifestyle modifications (i.e., nutrition, physical activity, etc.) to minimize negative changes in health. One such problem, especially in western cultures, is the wide accessibility to high-fat, high-calorie meals, creating an environment where excessive, low-quality nutritional habits are the norm. In these diets elevated post-prandial endotoxin and triglyceride are consistently reported as problematic changes. Our observed baseline responses mirror previous reports[22,31-33]. Recently a review article touted the potential of probiotic supplementation to prevent metabolic or dietary endotoxemia[24], but to our knowledge no published study has yet to demonstrate this outcome. Thus, our finding of a 42% reduction in metabolic endotoxemia is novel and unique. Further interpretation of our finding does reveal a potentially interesting effect, while 30-d of supplementation reduced metabolic endotoxemia by 42%, it did not completely prevent metabolic endotoxemia. It is plausible to speculate than a longer period of supplementation may result in greater reductions in metabolic endotoxemia. Cani et al[14,15] previously reported in rodents, that the only viable method to “reprogram” the gut microbial response was to initially treat animals with a broad-spectrum antibiotic. For obvious ethical reasons treating human subjects with antibiotics is likely not a viable experimental design consideration, but perhaps the same effect could be achieved with a longer period of probiotic supplementation. In addition to probiotic effects, we also observed an interesting response in placebo subjects. Specifically, the placebo subjects presented with an even greater metabolic endotoxemia response following a 30-d period. We do not believe that this observation is due to the experimental treatment, but is rather likely due to a diurnal fluctuation in metabolic endotoxemia responses. Thus, placebo subjects trended toward increased metabolic endotoxemia, while probiotic intervention reversed that effect. Since the present 30-d probiotic intervention did not completely prevent metabolic endotoxemia, it is reasonable to speculate that an intervention longer than 30-d may be necessary to completely prevent metabolic endotoxemia.
We have previously demonstrated that the consumption of a high-fat meal causes transient biological changes that were consistent with a transient increase in risk of atherosclerosis[16,18,21,32]. These changes combined with a post-prandial increase in serum triglycerides creates a milieu that favors foam cell formation and the development of atherosclerotic plaques[19,31,33,34]. In the present study, the baseline post-prandial meal response presented outcomes that were consistent with published data from our laboratory and others[16,18,19,22,31-33,35]. Thus, the present study presented an opportunity to assess if a probiotic intervention would change disease risk biomarkers in a similar manner as endotoxin and triglycerides. We found significance across the entire meal combined between conditions, but were unable to tease apart specific time point differences. A post hoc sample size analysis revealed that we would have needed to enroll approximately 20 “responder” subjects in each group to delineate specific time point changes for biomarkers. Regardless, we found significant reductions in IL-12p70, IL-1β, and ghrelin. Previous research has indicated that obese subjects do not have as great of a post-prandial suppression ghrelin than normal weight subjects[36]. The authors do not explain the nature of the change, but given the observations of the present study, it is reasonable to speculate that obesity status may very well effect the gut microbiome[36]. It is plausible that in the present study, without changing body weight, we were able to create the microbiome of a normal weight individual thus restoring normal post-prandial ghrelin responses.
Given the pro-inflammatory actions of IL-1β, the observed reduction with probiotic supplementation was consistent with reductions in post-prandial systemic inflammation. Reduced ghrelin may be indicative of better post-prandial hunger/satiety control with probiotic. IL-12p70 has a variety of metabolic actions, the chief action in the present study is the ability to modulate the release of TNF-α or related inflammatory cytokines following antigenic challenge[37,38]. In the case of the present study, reduced IL-12p70 with probiotic supplementation may reflect a reduction in systemic inflammatory capacity. In addition to the biomarkers that reached significance, we also found similar numerical trends for IL-6, IL-8, and MCP-1, which are all released by adipose tissues and commonly elevated in obese individuals[27,31,39]. The biomarkers observed to change in the present study following the probiotic intervention are involved in the accumulation of systemic inflammation[38,40-43]. The existing literature has linked elevated systemic inflammation to the pathophysiology of cardiovascular and metabolic diseases, thus even a transient reduction in systemic inflammation biomarkers may be associated with reduced disease risk[2,24]. The biomarkers measured in the present study are most often measured in the context of long-term weight loss (> 12 wk) interventions. In those weight loss models, it can take up to 16-wk to reduce body weight enough that biomarkers change. It is interesting that we demonstrated similar reductions in inflammatory biomarkers in 1/4 the time, but also in the absence of weight loss. We have presented novel results concerning the ability of probiotic supplementation to elicit transient effects.
In summary, the key findings of the present study demonstrate that 30-d of spore-based probiotic supplementation resulted in a blunting of dietary endotoxin, triglycerides, and potentially systemic inflammation. To our knowledge, the present study is the first to report that a short-term spore-based probiotic intervention altered dietary endotoxemia in human subjects, although the effect has been widely reported in mice[1,14]. Due to limitations associated with using human subjects, it was not possible to directly measure gut permeability in the present study. Despite this, it is reasonable to speculate that the underlying cause of the observed reductions in post-prandial endotoxemia may be due to changes in the gut microbiome, gut permeability, or a combination of the two. Future research is needed to determine if a longer course of treatment with a spore-based probiotic results in additional health improvements.
The present study was funded in part by a competitive research grant from Microbiome Labs, LLC (Glenview, IL, United States) to the University of North Texas. The UNT team did not receive direct funding associated with the completion of the present study. The funding agency was not involved in the data collection, analysis, interpretation, and manuscript preparation. Double blind procedures and confidentially were used to conduct the present study in a sound and unbiased manner. As such, the authors report no conflict of interest associated with completing the present study.
Dietary or metabolic endotoxemia is a condition that affects approximately 1/3 of individuals living in Western society. It is characterized by increased serum endotoxin concentration during the first five hours of the post-prandial period following consumption of a meal with a high-fat, high-calorie content. Long-term repeated dietary endotoxemia may increase the risk of developing a variety of chronic diseases via an inflammatory etiology. Of the available treatments, oral probiotic supplementation has been purported to reduce gastrointestinal (GI) permeability to endotoxin, which in theory should suppress the dietary endotoxin response.
GI health is a hot topic and there is great interest in the study of natural substances that have the potential to improve GI health. Probiotics have been studied with inconsistent results, the present study was designed to specifically address previous limitations
For the purposes of this study the authors validated a new method for identifying subjects that may be “responders” to probiotic intervention. This screening method included only enrolling subjects that had at least a 5-fold increase in serum endotoxin at 5-h post prandial. Using this method of subject screening, the authors believe that the present study is the first published account demonstrating a significant reduction in post-prandial endotoxemia. It is also significant that the authors also found reductions in disease risk biomarkers after only 30-d of probiotic supplementation. The approach to subject screening for probiotic studies is a novel approach that the authors hope will become a standard for future studies in the area.
To the knowledge the present study is the first published report that has conclusively documented that short-term probiotic supplementation can reduce the incidence of leaky gut syndrome. The authors believe that the lessons learned from this study that will be critical to future projects is that: (1) detailed screening is needed to qualify subjects who are certain to respond; and (2) the type of probiotic used should be carefully selected. The present study used a spore-based probiotic that is known to have greater than 90% survivability after exposure to stomach acid. Survivability after exposure to stomach acid is a critical factor in the assessment of commercial probiotics that is often overlooked in the selection and study design.
Dietary or metabolic endotoxemia is defined as a rise in blood serum endotoxin concentration during the first five hours after eating a meal. This post-meal period is also known as the post-prandial period. The most common cause of dietary endotoxemia is a disruption of gut barrier function. There is currently no accepted clinical test in humans to measure for gut barrier function. Disrupted gut barrier function cannot be predicted by any combination of baseline measures. Thus, to the knowledge the only means by which to assess gut barrier function was to complete a dietary endotoxemia test used in the present study. The endotoxin measured in the blood comes from bacteria that populate the GI track.
The article is interesting and maybe beneficial to the clinical physician.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lee HC S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Generoso SV, Viana ML, Santos RG, Arantes RM, Martins FS, Nicoli JR, Machado JA, Correia MI, Cardoso VN. Protection against increased intestinal permeability and bacterial translocation induced by intestinal obstruction in mice treated with viable and heat-killed Saccharomyces boulardii. Eur J Nutr. 2011;50:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Sanz Y, Santacruz A, Gauffin P. Gut microbiota in obesity and metabolic disorders. Proc Nutr Soc. 2010;69:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 194] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 3. | Berni Canani R, Cucchiara S, Cuomo R, Pace F, Papale F. Saccharomyces boulardii: a summary of the evidence for gastroenterology clinical practice in adults and children. Eur Rev Med Pharmacol Sci. 2011;15:809-822. [PubMed] |
| 4. | Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104:1033-1049; quiz 1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Anhê FF, Marette A. A microbial protein that alleviates metabolic syndrome. Nat Med. 2017;23:11-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Wolvers D, Antoine JM, Myllyluoma E, Schrezenmeir J, Szajewska H, Rijkers GT. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of infections by probiotics. J Nutr. 2010;140:698S-712S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 7. | McKean J, Naug H, Nikbakht E, Amiet B, Colson N. Probiotics and Subclinical Psychological Symptoms in Healthy Participants: A Systematic Review and Meta-Analysis. J Altern Complement Med. 2017;23:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Samah S, Ramasamy K, Lim SM, Neoh CF. Probiotics for the management of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;118:172-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Coghetto CC, Brinques GB, Ayub MA. Probiotics production and alternative encapsulation methodologies to improve their viabilities under adverse environmental conditions. Int J Food Sci Nutr. 2016;67:929-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Lewis ZT, Shani G, Masarweh CF, Popovic M, Frese SA, Sela DA, Underwood MA, Mills DA. Validating bifidobacterial species and subspecies identity in commercial probiotic products. Pediatr Res. 2016;79:445-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Hong HA, To E, Fakhry S, Baccigalupi L, Ricca E, Cutting SM. Defining the natural habitat of Bacillus spore-formers. Res Microbiol. 2009;160:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 12. | Cutting SM. Bacillus probiotics. Food Microbiol. 2011;28:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 509] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 13. | Derikx JP, Luyer MD, Heineman E, Buurman WA. Non-invasive markers of gut wall integrity in health and disease. World J Gastroenterol. 2010;16:5272-5279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3224] [Cited by in RCA: 3531] [Article Influence: 207.7] [Reference Citation Analysis (0)] |
| 15. | Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1735] [Cited by in RCA: 1884] [Article Influence: 117.8] [Reference Citation Analysis (1)] |
| 16. | Henning AL, Venable AS, Vingren JL, Hill DW, McFarlin BK. Consumption of a high-fat meal was associated with an increase in monocyte adhesion molecules, scavenger receptors, and Propensity to Form Foam Cells. Cytometry B Clin Cytom. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Henning AL, Venable AS, Prado EA, McFarlin BK. Using Image-Based Flow Cytometry to Assess Monocyte Oxidized LDL Phagocytosis Capacity. Methods Mol Biol. 2016;1389:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Henning AL, McFarlin BK. Consumption of a high-fat, high-calorie meal is associated with an increase in intracellular co-localization of PPAR-γ mRNA and protein in monocytes. Methods. 2017;112:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | McFarlin BK, Carpenter KC, Venable AS, Prado EA, Henning AL. Consumption of a high-fat breakfast on consecutive days alters area-under-the-curve for selected cardiovascular disease biomarkers. J Mol Pathophysiol. 2015;4:6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Spielmann G, McFarlin BK, O’Connor DP, Smith PJ, Pircher H, Simpson RJ. Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav Immun. 2011;25:1521-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Teeman CS, Kurti SP, Cull BJ, Emerson SR, Haub MD, Rosenkranz SK. The effect of moderate intensity exercise in the postprandial period on the inflammatory response to a high-fat meal: an experimental study. Nutr J. 2016;15:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286-1292. [PubMed] |
| 23. | Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775-2786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 821] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 24. | Le Barz M, Anhê FF, Varin TV, Desjardins Y, Levy E, Roy D, Urdaci MC, Marette A. Probiotics as Complementary Treatment for Metabolic Disorders. Diabetes Metab J. 2015;39:291-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | McFarlin BK, Venable AS. Measurement of Low Concentration Human Serum Cytokines using a Millipore High-Sensitivity Milliplex Assay. J Vis Exp. 2014; Available from: https://www.jove.com/video/5088/measurement-low-concentration-human-serum-cytokines-using-millipore. |
| 26. | Carpenter KC, Breslin WL, Davidson T, Adams A, McFarlin BK. Baker’s yeast β-glucan supplementation increases monocytes and cytokines post-exercise: implications for infection risk? Br J Nutr. 2013;109:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Breslin WL, Johnston CA, Strohacker K, Carpenter KC, Davidson TR, Moreno JP, Foreyt JP, McFarlin BK. Obese Mexican American children have elevated MCP-1, TNF-α, monocyte concentration, and dyslipidemia. Pediatrics. 2012;129:e1180-e1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | McFarlin BK, Venable AS, Henning AL, Prado EA, Best Sampson JN, Vingren JL, Hill DW. Natural cocoa consumption: Potential to reduce atherogenic factors? J Nutr Biochem. 2015;26:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferriéres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219-1223. [PubMed] |
| 30. | Perez-Martinez P, Alcala-Diaz JF, Delgado-Lista J, Garcia-Rios A, Gomez-Delgado F, Marin-Hinojosa C, Rodriguez-Cantalejo F, Delgado-Casado N, Perez-Caballero AI, Fuentes-Jimenez FJ. Metabolic phenotypes of obesity influence triglyceride and inflammation homoeostasis. Eur J Clin Invest. 2014;44:1053-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Herieka M, Erridge C. High-fat meal induced postprandial inflammation. Mol Nutr Food Res. 2014;58:136-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 32. | Strohacker K, Breslin WL, Carpenter KC, Davidson TR, Agha NH, McFarlin BK. Moderate-intensity, premeal cycling blunts postprandial increases in monocyte cell surface CD18 and CD11a and endothelial microparticles following a high-fat meal in young adults. Appl Physiol Nutr Metab. 2012;37:530-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Raz O, Steinvil A, Berliner S, Rosenzweig T, Justo D, Shapira I. The effect of two iso-caloric meals containing equal amounts of fats with a different fat composition on the inflammatory and metabolic markers in apparently healthy volunteers. J Inflamm (Lond). 2013;10:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Aoi W, Yamauchi H, Iwasa M, Mune K, Furuta K, Tanimura Y, Wada S, Higashi A. Combined light exercise after meal intake suppresses postprandial serum triglyceride. Med Sci Sports Exerc. 2013;45:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Bladbjerg EM, Larsen TM, Due A, Jespersen J, Stender S, Astrup A. Postprandial coagulation activation in overweight individuals after weight loss: acute and long-term effects of a high-monounsaturated fat diet and a low-fat diet. Thromb Res. 2014;133:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab. 2005;90:1068-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 37. | Cazzola M, Tompkins TA, Matera MG. Immunomodulatory impact of a synbiotic in T(h)1 and T(h)2 models of infection. Ther Adv Respir Dis. 2010;4:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Li CY, Lin HC, Lai CH, Lu JJ, Wu SF, Fang SH. Immunomodulatory effects of lactobacillus and Bifidobacterium on both murine and human mitogen-activated T cells. Int Arch Allergy Immunol. 2011;156:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | McFarlin BK, Johnson CA, Moreno JP, Foreyt JP. Mexican American children have differential elevation of metabolic biomarkers proportional to obesity status. J Pediatr Gastroenterol Nutr. 2013;57:718-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Malago JJ, Tooten PC, Koninkx JF. Anti-inflammatory properties of probiotic bacteria on Salmonella-induced IL-8 synthesis in enterocyte-like Caco-2 cells. Benef Microbes. 2010;1:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Periti P, Tonelli F. Preclinical and clinical pharmacology of biotherapeutic agents: Saccharomyces boulardii. J Chemother. 2001;13:473-493. [PubMed] |
| 42. | Pothoulakis C. Review article: anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment Pharmacol Ther. 2009;30:826-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Thomas S, Metzke D, Schmitz J, Dörffel Y, Baumgart DC. Anti-inflammatory effects of Saccharomyces boulardii mediated by myeloid dendritic cells from patients with Crohn’s disease and ulcerative colitis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1083-G1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |