Published online May 15, 2017. doi: 10.4291/wjgp.v8.i2.96
Peer-review started: October 27, 2016
First decision: December 1, 2016
Revised: January 3, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: May 15, 2017
Processing time: 203 Days and 4.8 Hours
According to current guidelines, follow-up of patients with colorectal cancer is ended after five years. Also, chest X-ray is not part of standard investigation during follow-up. We describe a case of a 74-year-old patient, more than ten years after a sigmoid resection because of carcinoma of the sigmoid. No recurrence was detected during intensive follow-up. However, ten years after resection of the sigmoid adenocarcinoma, complaints of coughing induced further examination with as result the detection of a solitary metastasis in the left lung of the patient. Within half-a-year after metastasectomy of the lung metastasis, she presented herself with thoracic pain and dyspnea resulting in discovering diffuse metastasis on pulmonary, pleural, costal and muscular level. Five year follow-up of colorectal carcinoma without chest X-ray can be questioned to be efficient. The growing knowledge of tumor biology might in future adjust the duration and frequency of diagnostic follow-up to prevent (late) recurrence in patients with colorectal carcinoma.
Core tip: This case report presents a case of a woman with pulmonary metastases 10 years after a sigmoid resection. No recurrence of the primary intestinal tumor was detected during follow-up. Upcoming knowledge on tumor behavior based on its biology might give new insights in late-onset metastases. Therefore, follow-up protocols and current therapy guidelines might have to be re-evaluated.
- Citation: Daniels AM, Vogelaar JFJ. Late onset pulmonary metastasis more than 10 years after primary sigmoid carcinoma. World J Gastrointest Pathophysiol 2017; 8(2): 96-99
- URL: https://www.wjgnet.com/2150-5330/full/v8/i2/96.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v8.i2.96
Colorectal carcinoma (CRC) is the most common type of gastrointestinal cancer. The current screening methods enable early detection, for example adenomatous polyps, to prevent the development of invasive carcinoma[1]. Etiology of CRC comprises genetic involvement, molecular events, environmental factors and inflammatory conditions[2]. CRC usually develops over a period of 10-15 years[3], most often affecting people aged over 50[4]. After a curative resection, prognosis of CRC is worse in poorly differentiated, mucinous carcinomas with large invasion depth and in the presence of lymph node metastases[5]. Follow-up of patients with CRC according to the national guidelines consists of echography or computed tomography (CT) of the abdomen, colonoscopy and determination of carcinoembryonic antigen (CEA) until five years after surgical resection[6].
CRC can spread by lymphatic and hematogenous dissemination, as well as by contiguous and transperitoneal routes. Metastasis, at initially diagnosis present in 20% of all patients, is the main cause of mortality in patients with CRC[7,8]. When cancer spreads to one single organ, the malignancy presents itself with a specific biological profile and clinical characteristics. The site of the metastatic disease influences the prognosis and the possibilities for treatment. Organs most often affected by CRC are the liver and the lungs[9]. For pulmonary lesions, the criteria for resectability of the metastatic lesion comprise; control of the primary tumor, possible complete resection and adequate pulmonary reserve to tolerate the planned resection[10].
Ten years ago, a 63-year-old woman was admitted to our hospital with changes in bowel habits. At that time, she had no medical history of abdominal complaints or surgical operations. Her father was familiar with lung carcinoma, her mother and both of her sisters suffered from breast carcinoma.
A colonoscopy reveals a sigmoid colon stricture with unknown length due to the impossibility of endoscopically passing the stricture. Biopsy of the sigmoid shows a poorly differentiated adenocarcinoma. Computed tomography and echography of the liver, spleen and of the pelvic, cardio-thoracic, para-aortic and paracaval region show no lesions suspected for malignancy. An uncomplicated surgical resection of the sigmoid took place. The tumor, with a maximum diameter of five centimeters, including five surrounding lymph nodes and infiltrated fat tissue were removed in totality. No lymph node metastases were detected.
One year after the surgical resection, biopsy of a polyp of the ascending colon showed a tubular adenoma with low grade dysplasia. During further follow up of this patients no malignancies or recurrent lesions were disclosed. The CEA was tested every three months with a maximum value of 2.2 mcg/L. The intensive follow-up period was completed five years after sigmoid resection.
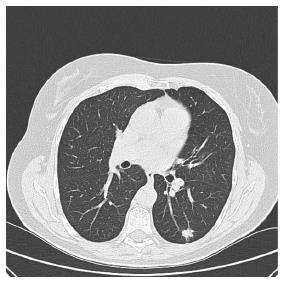
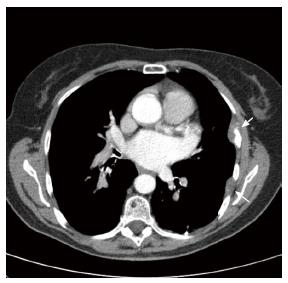
Ten years after resection of the adenocarcinoma located in the sigmoid without recurrent carcinoma’s during follow up, our patient presented herself with persistent coughing. Imaging of the thorax shows a lesion of 1.5 centimeters located in the inferior lobe of the left lung (Figure 1). Biopsy reveals an adenocarcinoma with positive CDX-2 staining, corresponding with intestinal origin of the cells. The pulmonary tumor was removed by video assisted thoracic surgery (VATS), presumably in totality. Colonoscopy shows no metachronous neoplasia. Within half a year, she was admitted to our emergency department with complaints of pain on the left side of her chest resulting in dyspnea, the pain was coherent with breathing movement and pain radiation towards her spine. CT-thorax shows extensive pleural, costal and muscular metastases (Figure 2). To reduce pain, palliative chemotherapy and additional radiotherapy were started.
Early detection of relapse or metastatic disease can be effectuated by intensive follow up during five years as recorded in the directive of Oncoline[6]. This follow-up comprises echography and optional CT-abdomen twice a year during 1-2 years, followed by once a year up to 5 years after the surgical resection. CEA should be determined every 3-6 mo during 3 years, followed by every 6 mo until 5 years after treatment. Colonoscopy is performed three, six and twelve months after surgical resection. Based on the number, localization and size of the lesions, repetition of colonoscopy is indicated after 3 or 5 years[6].
However, several clinical and experimental observations suggest that metastases can develop even in the absence of a detectable primary tumor. Both large and small tumors have the capability to metastasize[11,12]. Dissemination of tumor cells can occur in pre-invasive stages of tumor progression resulting in the development of early dormant disseminated tumor cells (DTCs). Early DTCs can remain dormant for a long period with manifestation of metastatic growth as result[13,14]. Metastases may thus be initiated by and evolve from dormant DTCs, rather than established primary tumors. These DTCs can generate metastases with different characteristics from those of the primary tumor. The discriminating characteristics may explain the lack of success treating metastases with therapies based on primary tumor characteristics[14]. Clinical evidence supports that the vast majority of early DTCs seem to be dormant[13].
In conclusion, five year follow-up of colorectal carcinoma without chest X-ray can be questioned to be efficient with the upcoming knowledge of early DTC’s. Adjustment of the duration and frequency of diagnostic follow-up and adjustment of treatment might be effective for early detection of metastatic disease and prevention of late recurrence in patients with colorectal carcinoma.
The authors wish to thank Simons P, MD, PhD for providing the CT-scan figures.
A 63-year-old woman presented with a poorly differentiated adenocarcinoma of the sigmoid as primary tumor. No recurrence of the primary intestinal tumor was detected during follow-up. Ten years after resection of the primary tumor, computed tomography reveals a pulmonary metastasis in the left lung of the patient.
Persistent cough.
The most common causes of chronic cough are postnasal drip, asthma, and acid reflux from the stomach. Less common causes include infections, medications, and lung diseases (e.g., primary/secondary malignancy).
No raised CRP or ESR. Normal leucocyte count. Carcinoembryonic antigen 1.7 mcg/L.
Computed tomography (CT) shows a lesion of 1.5 centimeters located in the inferior lobe of the left lung.
Pulmonary metastasis of intestinal origin; a poorly differentiated adenocarcinoma.
Metastasectomy by video assisted thoracic surgery.
Follow-up of colorectal carcinoma consists of echography, optional CT-abdomen, CEA determination and colonoscopy. Without recurrence of the tumor within five years after primary resection, intensive follow-up is ended. However, several clinical and experimental observations suggest that metastases can develop even in the absence of a detectable primary tumor, based on the principle of dormant disseminated tumor cells.
Late onset pulmonary metastases more than ten years after primary colorectal cancer, without recurrence of the primary tumor, is very rare.
Most recent guidelines recommend follow-up until five years after treatment of colorectal cancer. A pulmonary mass on imaging of the thorax, even more than 10 years after curative resection, is suspicious for metastatic disease. In general, more insights in the behavior of cancer (tumor biology) might change follow-up regimens in future.
The authors presented a case of a woman with pulmonary metastases 10 years after a sigmoid resection. This is clinically important and the paper is well written.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Akiho H, Deng CX, Peinado MA S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL
| 1. | Desch CE, Benson AB, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS, Petrelli NJ. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512-8519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 381] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 2. | Dragovich T, Tsikitis VL, Talavera F, Espat NJ, Schulman P. Colon Cancer (Medscape 2016). Available from: http://emedicine.medscape.com/article/277496-overview. |
| 3. | Kelloff GJ, Schilsky RL, Alberts DS, Day RW, Guyton KZ, Pearce HL, Peck JC, Phillips R, Sigman CC. Colorectal adenomas: a prototype for the use of surrogate end points in the development of cancer prevention drugs. Clin Cancer Res. 2004;10:3908-3918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Bethesda, MD, United States. Available from: http://seer.cancer.gov/csr/1975_2012/. |
| 5. | Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran Pathologic Basis of Disease, Professional Edition. Elsevier Health Sciences;. 2014;. |
| 6. | Comprehensive Cancer Centre The Netherlands. Dutch guideline “colorectal carcinoma”. 2016;. |
| 7. | Van Cutsem E, Oliveira J. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [PubMed] |
| 9. | Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: Metastases to a single organ. World J Gastroenterol. 2015;21:11767-11776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 232] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (2)] |
| 10. | Warwick R, Page R. Resection of pulmonary metastases from colorectal carcinoma. Eur J Surg Oncol. 2007;33 Suppl 2:S59-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | van de Wouw AJ, Janssen-Heijnen ML, Coebergh JW, Hillen HF. Epidemiology of unknown primary tumours; incidence and population-based survival of 1285 patients in Southeast Netherlands, 1984-1992. Eur J Cancer. 2002;38:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Engel J, Eckel R, Kerr J, Schmidt M, Fürstenberger G, Richter R, Sauer H, Senn HJ, Hölzel D. The process of metastasisation for breast cancer. Eur J Cancer. 2003;39:1794-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 886] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 14. | Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 844] [Article Influence: 76.7] [Reference Citation Analysis (0)] |