Published online May 15, 2017. doi: 10.4291/wjgp.v8.i2.87
Peer-review started: November 24, 2016
First decision: December 15, 2016
Revised: March 11, 2017
Accepted: April 6, 2017
Article in press: April 10, 2017
Published online: May 15, 2017
Processing time: 174 Days and 14.1 Hours
Chronic granulomatous disease (CGD) is a primary immune deficiency that is commonly diagnosed under the age of 5 years (95%) and is rarely seen in adulthood. CGD may manifest as inflammatory bowel disease (IBD) in childhood. Without proper diagnosis, these patients may be monitored for years as IBD; some may even be regarded as steroid-resistant ulcerative colitis (UC) and end up having a colectomy. In this case report, we described a patient who had been followed-up for years as UC and subsequently underwent colectomy, but was finally diagnosed in adulthood as primary immune deficiency.
Core tip: In the context of current knowledge, monogenetic diseases are at the basis of early-onset inflammatory bowel disease (IBD). Genetic and immunological studies on this subject have gained momentum in recent years. Chronic granulomatous disease (CGD) is an immune deficiency and may manifest itself as IBD. In an adolescent patient, CGD may present with clinical signs of IBD without evidence of immune deficiency. It should be kept in mind that CGD in adolescents may extend to adulthood.
- Citation: Kotlarz D, Egritas Gurkan O, Haskologlu ZS, Ekinci O, Aksu Unlusoy A, Gürcan Kaya N, Puchalka J, Klein C, Dalgic B. Differential diagnosis in ulcerative colitis in an adolescent: Chronic granulomatous disease needs extra attention. World J Gastrointest Pathophysiol 2017; 8(2): 87-92
- URL: https://www.wjgnet.com/2150-5330/full/v8/i2/87.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v8.i2.87
Chronic granulomatous disease (CGD) is a primary immunodeficiency seen in approximately 1 in 200-250000 individuals[1]. CGD is caused by abnormalities in the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex resulting in defective production of reactive oxygen species by phagocytic cells. The NADPH oxidase enzyme complex consists of at least five subunits: gp91phox, p22phox, p47phox, p67phox, and p40phox. Approximately, 66% of all CGD cases result from mutations within the X-linked [CYBB gene (encoding gp91phox)], followed by the autosomal recessive forms of CGD (%30) with defects in the (NCF1) gene coding for p47phox. Only 5% of the cases are due to mutations in CYBA, NCF-2 or NCF-4 which encode for p22phox, p67phox and p40phox respectively[2,3].
As a result of the failure to activate the respiratory burst, patients with CGD present with severe recurrent intracellular bacterial and fungal infections such as pneumonia, lymphadenitis, cutaneous and hepatic abscesses, osteomyelitis and septicemia[3]. These severe infections are caused predominantly by Staphylococcus aureus Aspergillus species, Candida, enteric gram negative bacteria, Serratia marcescens, Burkholderia cepacia complex, or Mycobacterium tuberculosis. CGD patients may also present with diffuse granulomas that can become large enough to cause obstructive or painful symptoms in the esophagus, stomach, biliary, intestinal, urogenital, or pulmonary systems[1-3].
Colitis is an important gastrointestinal manifestation of CGD, typically seen more commonly, but not exclusively in the X-linked form of the disease[4]. The clinical presentation of colitis is similar to that of inflammatory bowel disease (IBD), either ulcerative colitis (UC) or Crohn’s disease, and includes diarrhea, abdominal pain and rectal bleeding. A diagnosis of CGD is usually made by gastroenterologists who have previously labeled patients as therapy-resistant IBD[1-3].
A 15-year-old male patient was admitted to a medical center with persistent mucoid, bloody diarrhea. A diagnosis of UC had been made and the patient was followed for six months after discharge. In the interim, treatment comprised pulsed steroids, 5-aminosalicylic acid, and azathioprine. The patient was referred to our center due to persisted symptoms. Medical history revealed absence of recurrent bacterial and fungal infections or frequent antibiotic use. His parents were non-consanguineous.
On physical examination body weight was 51 kg (25th percentile), and the height was 156 cm (10th percentile). Systemic examination was normal. Perianal modifier was not observed. Laboratory results showed hemoglobin of 11.4 g/dL, white blood cell count of 8900/mm³, neutrophils count of 7900/mm³, lymphocyte count of 2200/mm³ and platelet count of 448000/mm³. Stool microscopy showed the presence of occult blood and leukocytes. Total protein was 6.2 g/dL, albumin was 3.8 g/dL, erythrocyte sedimentation rate was 68 mm/h, C-reactive protein was 38 mg/dL, and PANCA was positive. IgA: 68 mg/dL, IgG: 1100 mg/dL, IgM: 79.7 mg/dL, IgE: 38.5 mg/dL, IgA: 217 mg/dL, total C3: 128 mg/dL, total C4: 26.3 mg/dL. Liver and kidney function tests were normal. Colonoscopy was compatible with the diagnosis of pancolitis, but the terminal ileum was normal. Upper gastrointestinal endoscopy revealed pangastritis. Histopathologic evaluation showed cryptitis, crypt abscesses, and pseudopolyps of the entire colonic mucosa; granulomas were not detected.
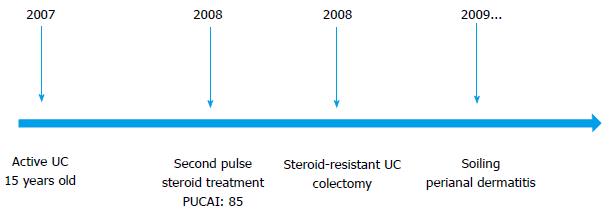
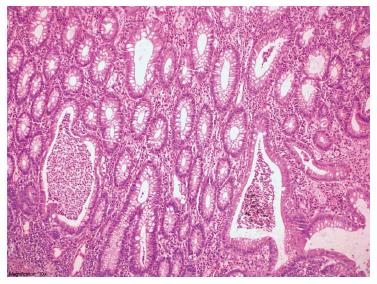
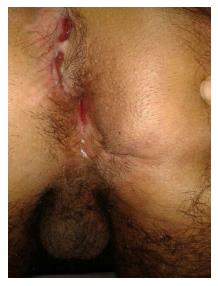
The subsequent medical treatment and follow-up of the patient are shown in Figure 1. Due to the refractory course of the patient, familial Mediterranean fever (MEVF mutation) and Behcet’s disease (Pathergy test, HLA B5 test) were considered and excluded. The patient had a steroid-resistant course and total colectomy was performed. Light microscopic examination of colectomy specimen revealed crypt distortion, cryptitis, crypt loss and crypt abscesses throughout whole colonic mucosa. Epithelial ulcerations, regenerative changes and pseudopolyps were also noted. Neutrophils were present in lamina propria, dense and mixed type inflammatory cell infiltration was seen. These changes were limited thorough mucosa and submucosa. Granulomas and dysplasia were not encountered. The surgically resected specimen was compatible with UC (Figure 2). Acute phase proteins remained slightly increased and soiling was the major problem in the postoperative period. Anorectal manometry showed increased rectal sensitivity, significantly low resting anal sphincter pressure, normal rectoanal inhibitor reflex, and adequate pressure increase in voluntary sphincter contraction. However, perianal dermatitis and impaired perianal wound healing persisted (Figure 3).
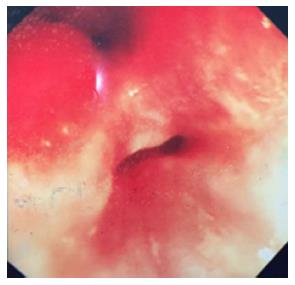
Ileoscopy performed 8 mo after colectomy operation revealed a second lumen layer outside the lumen. Both lumen openings were hyperemic, edematous, and eroded (Figure 4). Ileal biopsies revealed backwash ileitis, without signs of Crohn’s disease.
At the age of 18 years, the patient was referred to adult gastroenterology section. Pelvic magnetic resonance imaging and fistulography revealed an ileoileal fistula. At the age of 23 years, there was persistence of the perianal lesions and diarrhea despite treatment.
To screen for monogenetic forms of IBD, we have performed whole exome sequencing of the patient sample using an exome enrichment kit (SureSelect V4UTR; Agilent) and next-generation sequencing device (Illumina HiSeq 2500). Analysis of rare genetic variants that affected protein sequence or interfered with mRNA transcription revealed a mutation in the NCF2 gene (c.326A>G, p.Y109Y>C). Bioinformatic analysis of this sequence variant predicted that the amino acid exchange that was deleterious to the protein function.
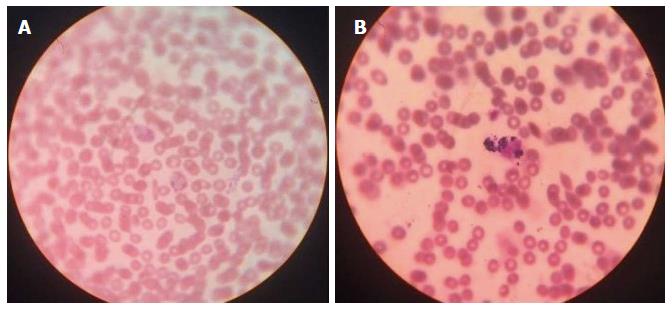
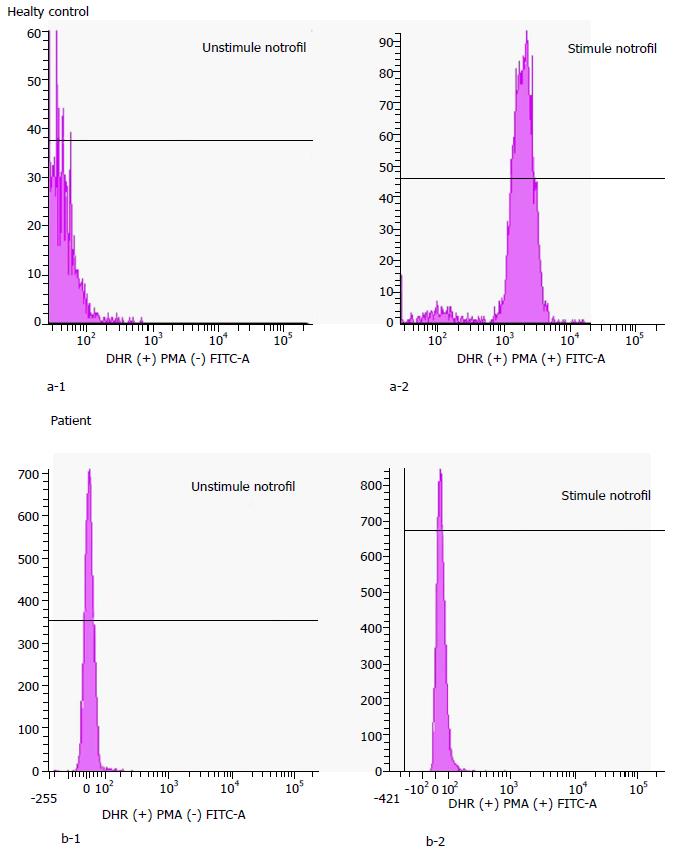
A diagnosis of CGD was confirmed biologically by a nitroblue tetrazolium test (NBT) and flow cytometry-based dihydrorhodamine (DHR) or 2′7′-dichlorofluorescein diacetate assays. An NBT slide test with Escherichia coli lipopolysaccharide (840 W Sigma-Aldrich) was used to stimulate respiratory burst in phagocytic cells. Normally the percentage of blue-stained neutrophils in an NBT test (Figure 5) is close to 100 - but this percentage is close to 0 in CGD patients. In this patient, the NBT test confirmed a defective respiratory burst in the neutrophils. DHR test (Figure 6) uses flow cytometry to measure the oxidation of dihydrorhodamine 123 to rhodamine 123 in phorbol myrisate acetate-stimulated neutrophils, a marker for cellular NADPH oxidase activity. In this test the generation of hydrogen peroxide oxidizes the dye, leading to the emission of fluorescence. Mean fluorescence intensity of the activated cells correlates directly with (and thus serves as a reliable surrogate for) superoxide production. In this patient, the diagnosis of CGD was confirmed by using the NBT test and the DHR assay.
Based on the treatment-refractory course and the underlying primary immunodeficiency in the patient, we considered hematopoietic stem cell transplant (HSCT) for cure. The patient had been referred to the Department of Hematology and is currently being prepared for HSCT.
CGD is a primary immunodeficiency caused by a defect in a superoxide formation. More than 95% of cases are diagnosed before the age of 5 years. However, some patients may be diagnosed only in adulthood[1]. During evaluation of very early onset IBD under six years of age, one can easily consider the presence of underlying immune deficiencies. However, it will be tough to consider this point in an adolescent patient with UC alone, normal physical development and negative history of recurrent infections like our patient.
Gastrointestinal tract involvement in CGD patients causes significant morbidity and mortality. Its phenotype might be similar in presentation-with IBD. Intestinal motility dysfunction, obstruction, ulceration and recurrent infection may occur along the entire tract[5]. Gastrointestinal involvement was reported in 32% to 48% of patients with CGD[1,6-8]. A study by van den Berg et al[6] found that 48% of CGD patients had experienced at least one episode of gastrointestinal manifestation. Colitis, perianal abscess and fistula formation may be the first symptoms for gastrointestinal involvement[9,10]. In our patient, the multiple colonoscopies revealed only large bowel involvement and normal terminal ileum. Therefore, the patient was monitored for years with a diagnosis of UC and eventually underwent colectomy due to the absence of response to medical therapy.
The endoscopic and pathological findings of patients with bowel involvement might be indistinguishable from that of IBD[10]. Cryptitis, crypt abscesses, eosinophilic infiltrates and granulomas may be detected in affected intestinal segments of CGD patients. Although presence of large pigment laden histiocytes in involved organs of CGD patients is significant, these findings are not specific or sensitive[11]. In our patient evaluation of colectomy specimens revealed no granulomas, therefore Crohn’s disease or Crohn’s-alike diseases were not considered. In addition, there were no recurrent bacterial and fungal infections or frequent antibiotic use. Immunologic tests for primary immunodeficiency were not considered due to the late onset of IBD. Eventually, unbiased whole exome sequencing revealed a homozygous missense mutation in NCF2, which was indicative of CGD diagnosis that was confirmed by NBT assay. Genetic studies showed NCF2 mutation for CGD which shows an autosomal recessive trend. Consanguineous marriage was not noted in family history, but coming from same village and the CGD disease with an autosomal recessive trait made us think of a distant relativity between parents.
Very early onset-IBD (VEO-IBD) (below-six years of age) has been shown to be associated with monogenetic etiologies in particular immune deficiencies[12]. Accordingly immunologic tests and genetic analysis are recommended in VEO-IBD patients. In IBD patients over the age of 6 years, initial search for immunologic parameters is recommended for the following reasons: (1) presence of lesions in the perianal region; (2) consanguineous parents; (3) unresponsive to treatment or steroid dependent; (4) family history of early-onset IBD; and (5) the presence of skin, nail, or hair abnormalities. With the rise in genetic studies, we learned that similar to IBD, immunodeficiencies like CGD may occur in after adolescence. Our experience on this case would make one wonder whether immunologic tests are necessary for every IBD patient, regardless of age.
In literature, basic immunologic tests as the first step in IBD patients were not recommended regardless of the presence of risk factors. However, considering the prolonged follow-up required of this chronic disease, we recommend otherwise.
Main symptoms were bloody mucoid diarrhea.
Main clinical findings were perianal modifiers after colectomy operation.
Crohn’s disease should be kept in mind in patients with perianal modifiers.
Elevated acute phase reactants and mutation in the NCF2 gene (c.326A>G, p.Y109Y>C) were seen.
Colonoscopic appearance was compatible with pancolitis.
Histopathologic evaluation showed cryptitis, crypt abscesses, and pseudopolyps of the entire colonic mucosa; granulomas were not detected.
Treatment was consisted of steroids and immunosuppressive drugs.
Chronic granulomatous disease may present itself as ulcerative colitis in adulthood.
This case report was well organized and well investigated. This paper will give us a new information especially in the field of inflammatory bowel disease.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Freeman HJ, Ma TY, Jamali R, Naito Y, Ruffolo C, Sipos F S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Winkelstein JA, Marino MC, Johnston RB, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore). 2000;79:155-169. [PubMed] |
| 2. | Meischl C, Roos D. The molecular basis of chronic granulomatous disease. Springer Semin Immunopathol. 1998;19:417-434. [PubMed] |
| 3. | Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore). 2000;79:170-200. [PubMed] |
| 4. | Muise AM, Xu W, Guo CH, Walters TD, Wolters VM, Fattouh R, Lam GY, Hu P, Murchie R, Sherlock M. NADPH oxidase complex and IBD candidate gene studies: identification of a rare variant in NCF2 that results in reduced binding to RAC2. Gut. 2012;61:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Barton LL, Moussa SL, Villar RG, Hulett RL. Gastrointestinal complications of chronic granulomatous disease: case report and literature review. Clin Pediatr (Phila). 1998;37:231-236. [PubMed] |
| 6. | van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Español T, Fischer A, Kurenko-Deptuch M, Mouy R. Chronic granulomatous disease: the European experience. PLoS One. 2009;4:e5234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 485] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 7. | Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 8. | Marciano BE, Rosenzweig SD, Kleiner DE, Anderson VL, Darnell DN, Anaya-O’Brien S, Hilligoss DM, Malech HL, Gallin JI, Holland SM. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics. 2004;114:462-468. [PubMed] |
| 9. | Glocker E, Grimbacher B. Inflammatory bowel disease: is it a primary immunodeficiency? Cell Mol Life Sci. 2012;69:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Alimchandani M, Lai JP, Aung PP, Khangura S, Kamal N, Gallin JI, Holland SM, Malech HL, Heller T, Miettinen M. Gastrointestinal histopathology in chronic granulomatous disease: a study of 87 patients. Am J Surg Pathol. 2013;37:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Barbato M, Ragusa G, Civitelli F, Marcheggiano A, Di Nardo G, Iacobini M, Melengu T, Cucchiara S, Duse M. Chronic granulomatous disease mimicking early-onset Crohn’s disease with cutaneous manifestations. BMC Pediatr. 2014;14:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Uhlig HH. Monogenic diseases associated with intestinal inflammation: implications for the understanding of inflammatory bowel disease. Gut. 2013;62:1795-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |