Published online Nov 15, 2015. doi: 10.4291/wjgp.v6.i4.219
Peer-review started: June 22, 2015
First decision: August 16, 2015
Revised: September 2, 2015
Accepted: October 23, 2015
Article in press: October 28, 2015
Published online: November 15, 2015
Processing time: 148 Days and 1 Hours
Ulcerative colitis (UC) is a chronic lifelong condition characterized by alternating flare-ups and remission. There is no single known unifying cause, and the pathogenesis is multifactorial, with genetics, environmental factors, microbiota, and the immune system all playing roles. Current treatment modalities for UC include 5-aminosalicylates, corticosteroids, immunosuppressants (including purine antimetabolites, cyclosporine, and tacrolimus), and surgery. Therapeutic goals for UC are evolving. Medical treatment aims to induce remission and prevent relapse of disease activity. Infliximab, an anti-tumor necrosis factor (TNF)-α monoclonal antibody, is the first biological agent for the treatment of UC. Over the last decade, infliximab and adalimumab (anti-TNF-α agents) have been used for moderate to severe UC, and have been shown to be effective in inducing and maintaining remission. Recent studies have indicated that golimumab (another anti-TNF-α agent), tofacitinib (a Janus kinase inhibitor), and vedolizumab and etrolizumab (integrin antagonists), achieved good clinical remission and response rates in UC. Recently, golimumab and vedolizumab have been approved for UC by the United States Food and Drug Administration. Vedolizumab may be used as a first-line alternative to anti-TNF-α therapy in patients with an inadequate response to corticosteroids and/or immunosuppressants. Here, we provide updated information on various biological agents in the treatment of UC.
Core tip: Ulcerative colitis (UC) is a chronic lifelong condition characterized by alternating flare-ups and remission. Current treatment modalities for UC include 5-aminosalicylates, corticosteroids, immunosuppressants (e.g., cyclosporine, tacrolimus), and surgery. Medical treatment aims to induce remission and prevent relapse of disease activity. Infliximab and adalimumab have been used for moderate to severe UC, and are effective in inducing and maintaining remission in UC. Recent studies have indicated that golimumab, tofacitinib, vedolizumab and etrolizumab achieved good clinical remission and response rates in UC. In this review, we provide updated information on various biological agents in the treatment of UC.
- Citation: Akiho H, Yokoyama A, Abe S, Nakazono Y, Murakami M, Otsuka Y, Fukawa K, Esaki M, Niina Y, Ogino H. Promising biological therapies for ulcerative colitis: A review of the literature. World J Gastrointest Pathophysiol 2015; 6(4): 219-227
- URL: https://www.wjgnet.com/2150-5330/full/v6/i4/219.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v6.i4.219
Ulcerative colitis (UC) is an inflammatory disorder of the gastrointestinal tract that affects the colon and rectum. The symptoms of UC are rectal bleeding, diarrhea, and abdominal pain. It is a chronic lifelong condition characterized by alternating flare-ups and remission. There is no single known unifying cause, and the pathogenesis is multifactorial, with genetics, environmental factors, microbiota, and immune system all playing roles[1,2].
Medical treatment aims to induce remission and prevent relapse of disease activity, thereby minimizing the impact on quality of life. Current treatment modalities for UC include 5-aminosalicylates, corticosteroids, immunosuppressants (including purine antimetabolites, cyclosporine, and tacrolimus), and surgery. Therapeutic goals for the treatment of UC are evolving. Over the past decade there has been increasing evidence in favor of more objective measures of biological disease activity, including biomarkers such as C-reactive protein, faecal calprotectin, and the histological resolution of active inflammation in UC[3,4].
Infliximab, an anti-tumor necrosis factor (TNF)-α monoclonal antibody, is the first biological agent to have received United States Food and Drug Administration (FDA) approval. Over the last decade, infliximab has been used for moderate to severe UC, and has been shown to be effective in inducing and maintaining remission in UC[5]. Recently the TNF-α antagonists adalimumab and golimumab have shown a significant effect on UC[6,7].
In 2014, integrin receptor antagonist vedolizumab was approved for UC by the United States FDA and European Commission. In this review, we provide updated information on various biological agents in the treatment of UC.
TNF has been known to play a pivotal role in the pathogenesis of inflammatory bowel disease (IBD)[8]. When released by active macrophages and T lymphocytes, TNF initiates multiple biological reactions like modulates immune cell function, drives adaptive immune responses, triggers epithelium apoptosis and breaks epithelial barrier[9,10]. Anti-TNF-α agents have changed the treatment paradigm in the management of patients with UC.
As the first monoclonal TNF antibody approved for human treatment, infliximab is a purified, recombinant DNA-derived chimeric human-mouse IgG monoclonal antibody and contains murine heavy and light chain variable regions, ligated to genomic human heavy and light chain constant regions[11,12]. Infliximab can quickly form stable complexes with the human soluble or the membrane form of TNF and terminate the biological activity and signals of TNF[13]. With a serum half-life of 9.5 d and still detectable in serum of IBD patients 8 wk after infusion treatment, infliximab provides a useful strategy to neutralize TNF and to inhibit immune responses of IBD[14]. Infliximab is administered intravenously, and has been found to be effective for the treatment of moderate to severe UC in clinical trials[5,15]. Two randomized, double-blind, placebo-controlled studies-the Active UC Trials 1 and 2 (ACT 1 and ACT 2, respectively)-evaluated the efficacy of infliximab for induction and maintenance therapy in adults with UC[5]. Clinical response was defined as a decrease from baseline in the total Mayo score of ≥ 3 points and ≥ 30%, with an accompanying decrease in the subscore for rectal bleeding of ≥ 1 point or an absolute subscore for rectal bleeding of 0 or 1. Clinical remission was defined as a total Mayo score of ≤ 2 points, with no individual subscore exceeding 1 point. In ACT 1, 69.4% of patients who received 5 mg infliximab and 61.5% of those who received 10 mg had a clinical response at week 8, as compared with 37.2% of those who received placebo (P < 0.001 for both comparisons with placebo). In ACT 2, 64.5% of patients who received 5 mg infliximab and 69.2% of those who received 10 mg had a clinical response at week 8, as compared with 29.3% of those who received placebo (P < 0.001 for both comparisons with placebo). In both studies, patients who received infliximab were more likely to have a clinical response at week 30 (P≤ 0.002 for all comparisons). In ACT 1, more patients who received 5 or 10 mg infliximab had a clinical response at week 54 (45.5% and 44.3%, respectively) than did those who received placebo[5]. The results of ACT 1 and ACT 2 showed that infliximab had superior clinical efficacy compared with placebo, both in induction and maintenance phases.
Adalimumab is a complete human IgG1 anti-TNF-α monoclonal Ab that has been generated through repertoire cloning. It binds to the soluble and transmembrane forms of TNF-α with high affinity, thereby preventing TNF-α from binding to its receptors. In vitro studies have also demonstrated its effect on the induction of cell lysis and apoptosis[16]. It is generally administered at a dose of 40 mg subcutaneously every 2 wk, or at higher doses administered once a week. It is indicated for use in rheumatoid arthritis, psoriasis, ankylosing spondylitis, and moderate to severe Crohn’s disease. Adalimumab can be self-administered by patients at home. Two randomized, double-blind, placebo-controlled studies-UC long-term remission and maintenance with adalimumab 1 and 2 (ULTRA 1 and ULTRA 2, respectively)-evaluated the efficacy of adalimumab for induction and maintenance therapy in UC patients[6,17]. ULTRA 1 was an 8-wk clinical trial investigating the use of adalimumab as induction therapy in patients with moderate to severe UC despite conventional therapy[17]. In this trial, 576 patients were divided into 160/80 mg and 80/40 mg groups, based on the loading dose, and then compared with the placebo group. At the end of 8 wk, the clinical remission rate of patients receiving adalimumab was twice that of the placebo group (P = 0.031). There was no significant difference in remission rates between patients receiving adalimumab 80/40 mg and placebo (P = 0.833). In ULTRA 2, a 52-wk randomized controlled study investigating the use of adalimumab as maintenance therapy, 494 patients were divided into 160/80 mg adalimumab and placebo groups. Overall rates of clinical remission at week 8 were 16.5% on adalimumab and 9.3% on placebo (P = 0.019); corresponding values for week 52 were 17.3% and 8.5% (P = 0.004). Among anti-TNF-α-naïve patients, rates of remission at week 8 were 21.3% on adalimumab and 11% on placebo (P = 0.017); corresponding values for week 52 were 22% and 12.4% (P = 0.029). Among patients who had previously received anti-TNF-α agents, rates of remission at week 8 were 9.2% on adalimumab and 6.9% on placebo (P = 0.559); corresponding values for week 52 were 10.2% and 3% (P = 0.039). Importantly, on sub-analysis, it was observed that the anti-TNF-α-naïve group exhibited approximately two times higher clinical remission rates at week 8 and week 52, compared with the placebo group. Though it is not direct comparison, infliximab is more likely to induce a favorable clinical outcome than adalimumab. The dose of adalimumab trough level might not enough to induce remission and maintenance for UC. More date are needed for dose escalation of adalimumab.
Up to 4 years of data for adalimumab-treated patients from ULTRA 1 and 2, and the open-label extension ULTRA 3 have been presented[18]. A total of 600/1094 patients enrolled in ULTRA 1 or 2 were randomized to receive adalimumab and induced in the intent to treat analyses. Of these, 199 patients remained on adalimumab after 4 years follow-up. Rates of remission according to partial Mayo score, remission according to inflammatory bowel disease questionnaire score, mucosal healing, and corticosteroid discontinuation at week 208 were 24.7%, 26.3%, 27.7% (nonresponder imputation), and 59.2% (observed), respectively. Of the patients who were followed up in ULTRA 3 (588/1094), a total of 360 patients remained on adalimumab 3 years later. Remission according to partial Mayo score and mucosal healing after ULTRA 1 or 2 to year 3 of ULTRA 3 were maintained by 63.6% and 59.9% of patients, respectively (nonresponder imputation). Nonresponder imputation method is used for dichotomous (“yes or no”) or categorical variables, if a subject drops out of a study, that subject is assumed to be a non-responder, regardless of whether or not the subject was responding to treatment at the time of dropout.
Golimumab is a fully human IgG1 monoclonal antibody that targets TNF-α. It is subcutaneously administered and approved for use in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. The affinity of golimumab for soluble TNF-α was similar to that of etanercept and greater than those of infliximab and adalimumab (2.4-fold and 7.1-fold, respectively). A similar pattern was observed regarding golimumab neutralization of soluble TNF-α in the cytotoxicity and endothelial cell activation assays. The IC50 values for golimumab were comparable to those for etanercept and ranged from 2.5- to 5.7-fold lower than those for infliximab and adalimumab. These in vitro bioassays suggest that a lower serum concentration of golimumab, compared with infliximab or adalimumab, would provide similar pharmacological effects in patients[19]. Two large, double-blinded, randomized, controlled trials have been conducted-the Program of UC Research Studies Utilizing an Investigation Treatment, which was divided into Subcutaneous and Maintenance phases (PURSUIT-SC, PURSUIT-M, respectively)[7,20]. In PURSUIT-SC, 774 patients were randomized to receive golimumab at week 6. The clinical response and remission rates showed a significant change in both the golimumab 200/100 mg and 400/200 mg groups (P < 0.0001)[10]. In PURSUIT-M, 464 patients who had responded to golimumab induction therapy in PURSUIT-SC were randomized to receive placebo or golimumab 50/100 mg every 4 wk for 52 wk. Clinical response was maintained through week 54 in 47.0% of patients receiving 50 mg golimumab, 49.7% of patients receiving 100 mg golimumab, and 31.2% of patients receiving placebo (P = 0.010 and P < 0.001, respectively). At weeks 30 and 54, a higher percentage of patients who received 100 mg golimumab were in clinical remission and had mucosal healing (27.8% and 42.4%) than patients given placebo (15.6% and 26.6%; P= 0.004 and P = 0.002, respectively) or 50 mg golimumab (23.2% and 41.7%, respectively)[7]. Though PURSUIT-M had included only persons who responded to induction in its maintenance phase, golimumab is more likely to induce a favorable clinical outcome than adalimumab (Table 1).
| Drug | Trial | Study population | Protocol | Follow-up (wk) | Outcome |
| Infliximab | ACT 1 Rutgeerts et al[5] | 121 | 5 mg/kg iv at 0, 2, 6, | 54 | 69.4% (P < 0.001) clinical response at week 8 |
| and every 8 wk | 45.5% (P < 0.001) clinical response at week 54 | ||||
| 38.8% (P < 0.001) clinical remission at week 8 | |||||
| 34.7% (P = 0.001) clinical remission at week 54 | |||||
| 122 | 10 mg/kg iv at 0, 2, 6, | 54 | 61.5% (P < 0.001) clinical response at week 8 | ||
| and every 8 wk | 44.3% (P < 0.001) clinical response at week 54 | ||||
| 32.0% (P = 0.002) clinical remission at week 8 | |||||
| 34.4% (P = 0.001) clinical remission at week 54 | |||||
| ACT 2 Rutgeerts et al[5] | 121 | 5 mg/kg iv at 0, 2, 6, | 30 | 64.5% (P < 0.001) clinical response at week 8 | |
| and every 8 wk | 47.1% (P < 0.001) clinical response at week 30 | ||||
| 33.9% (P < 0.001) clinical remission at week 8 | |||||
| 25.6% (P = 0.003) clinical remission at week 30 | |||||
| 120 | 10 mg/kg iv at 0, 2, 6, | 30 | 69.2% (P < 0.001) clinical response at week 8 | ||
| and every 8 wk | 60.0% (P < 0.001) clinical response at week 30 | ||||
| 27.5% (P < 0.001) clinical remission at week 8 | |||||
| 35.8% (P < 0.001) clinical remission at week 30 | |||||
| Adalimumab | ULTRA1 Reinisch et al[17] | 130 | 80/40 mg sc | 8 | 51.5% clinical response at week 8 |
| 80 mg at week 0, 40 mg at week 2, 4 and 6 | 10.0% (P = 0.833) clinical remission at week 8 | ||||
| 130 | 160/80 mg sc | 54.6% clinical response at week 8 | |||
| 160 mg at week 0, 80 mg at week 2, 40 mg at week 4 and 6 | 18.5% (P = 0.031) clinical remission at week 8 | ||||
| ULTRA2 Sandborn et al[6] | 248 | 160/80 mg sc | 52 | 16.5% (P = 0.019) clinical remission at week 8 | |
| 160 mg at week 0, 80 mg at week 2, and then 40 mg every other week | 17.3% (P = 0.004) clinical remission at week 52 | ||||
| ULTRA3 Colombel et al[18] | 360 | 40 mg sc every other week | 208 | 63.6% remission per partial Mayo score at week 208 | |
| Golimumab | PURSUIT-SC[20] | 253 | 200/100 mg sc 2 wk apart | 6 | 51.6% (P < 0.0001) clinical response at week 6 |
| 17.8% (P < 0.0001) clinical remission at week 6 | |||||
| 257 | 400/200 mg sc 2 wk apart | 6 | 54.9% (P < 0.0001) clinical response at week 6 | ||
| 17.9% (P < 0.0001) clinical remission at week 6 | |||||
| PURSUIT-M[7] | 151 | 50 mg sc every 4 wk | 54 | 47% (P = 0.010) clinical response at week 54 | |
| 23.2% clinical remission at week 54 | |||||
| 151 | 100 mg sc every 4 wk | 54 | 49.7% (P < 0.001) clinical response at week 54 | ||
| 27.8% (P = 0.004) clinical remission at week 54 |
Janus kinase inhibitor: Various cytokines and intracellular messengers play a key role in pathogenesis of UC. Tyrosine kinases, such as Janus kinase 1 (JAK1) and JAK3, are intracellular molecules for the signal transmission of interleukins.
Tofacitinib (CP-690,550) is an oral inhibitor of JAK 1, 2 and 3 (with in vitro functional specificity for JAK1 and JAK3 over JAK2), which is expected to block signaling involving gamma-chain-containing cytokines including interleukins 2, 4, 7, 9, 15 and 21. In a double-blind, placebo-controlled, phase 2 trial, it was evaluated the efficacy of tofacitinib in 194 adults with moderate to severe active UC. Patients were randomly assigned to receive tofacitinib at a dose of 0.5, 3, 10 or 15 mg or placebo twice daily for 8 wk[21]. The primary outcome, clinical response at 8 wk, occurred in 32%, 48%, 61% and 78% of patients receiving tofacitinib at a dose of 0.5 mg (P = 0.39), 3 mg (P = 0.55), 10 mg (P = 0.10), and 15 mg (P < 0.001), respectively, as compared with 42% of patients receiving placebo. Clinical remission at 8 wk occurred in 13%, 33%, 48% and 41% of patients receiving tofacitinib at a dose of 0.5 mg (P = 0.76), 3 mg (P = 0.01), 10 mg (P < 0.001), and 15 mg (P < 0.001), respectively, as compared with 10% of patients receiving placebo[21]. Though the study population is small, 15 mg of tofacitinib showed most superior clinical response rate in induction phase than the other biological agents for UC.
The integrin inhibitors are currently under development and have shown promising results to date. This group of drugs targets the leukocyte adhesion and trafficking systems, thereby reducing inflammation.
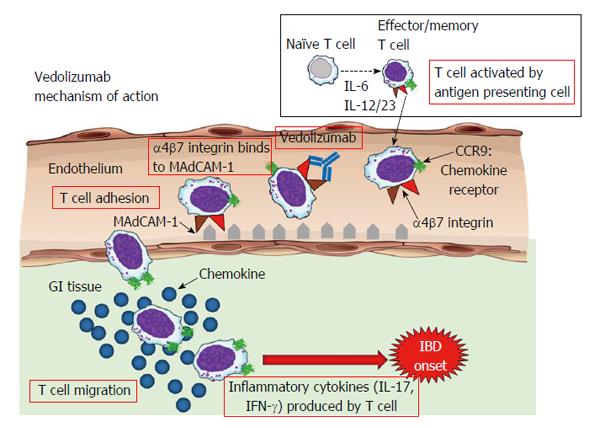
The α4β7 integrin[22], a cell surface glycoprotein variably expressed on circulating B and T lymphocytes, interacts with mucosal addressin-cell adhesion molecule 1 (MAdCAM-1)[23] on the intestinal vasculature[24,25]. Vedolizumab, a humanized monoclonal antibody that specifically recognizes the α4β7 heterodimer, selectively blocks gut lymphocyte trafficking without interfering with trafficking to the central nervous system[26-28] (Figure 1).
A predecessor molecule (MLN02) showed proof-of-concept in a phase 2 trial[29]. Natalizumab, a monoclonal antibody with efficacy in multiple sclerosis and Crohn’s disease, inhibits both α4β1 and α4β7 integrins and is associated with progressive multifocal leukoencephalopathy; a serious brain infection. Natalizumab and vedolizumab differ in that natalizumab blocks lymphocyte trafficking to multiple organs, including the brain and gut[30,31].
Randomized, double-blinded, placebo-controlled trials of vedolizumab in patients with active UC have been conducted[32]. In a trial of induction therapy, 374 patients (Cohort 1) received vedolizumab (300 mg) or placebo intravenously at weeks 0 and 2, and 521 patients (Cohort 2) received open-label vedolizumab at weeks 0 and 2, with disease evaluation at week 6. In a trial of maintenance therapy, patients in either cohort who had a response to vedolizumab at week 6 were randomly assigned to continue receiving vedolizumab every 8 or 4 wk or to switch to placebo for up to 52 wk.
Response rates at week 6 were 47.1% and 25.5% among patients in the vedolizumab and placebo groups, respectively (difference with adjustment for stratification factors, 21.7% points; 95%CI: 11.6-31.7; P < 0.001). At week 52, 41.8% of patients who continued to receive vedolizumab every 8 wk and 44.8% of patients who continued to receive vedolizumab every 4 wk were in clinical remission (Mayo Clinic score ≤ 2 and no subscore > 1), as compared with 15.9% of patients who switched to placebo [adjusted difference, 26.1% points for vedolizumab every 8 wk vs placebo (95%CI: 14.9-37.2; P < 0.001) and 29.1% points for vedolizumab every 4 wk vs placebo (95%CI: 17.9-40.4; P < 0.001)]. The frequency of adverse events was similar between the vedolizumab and placebo groups.
A network meta-analysis showed that in patients with moderate to severe active UC naïve to biological therapy, vedolizumab has similar efficacy to the anti-TNF-α antibodies, infliximab, adalimumab, and golimumab for induction of response and remission, and for maintenance of response and remission, but only vedolizumab had an incidence of serious adverse events lower than that of placebo[33]. Thus, in UC, vedolizumab may be used as a first-line alternative to anti-TNF-α therapy in patients with an inadequate response to corticosteroids and/or immunosuppressants. Vedolizumab may also be used in patients with UC not responding to anti-TNF therapy (primary nonresponders and secondary loss of response), because the drug has shown efficacy for this particular subpopulation[32].
The United States FDA and European Commission approved vedolizumab (Entyvio) for treatment of adults with moderate to severe active UC or CD in 2014. Up to 2015, vedolizumab for UC is approved in the United States, European Union, Canada, Israel, Switzerland, Puerto Rico, and Bosnia and Herzegovina. A phase 3, multicenter, randomized, double-blinded, placebo-controlled, parallel-group study to examine the efficacy, safety, and pharmacokinetics of MLN0002 (vedolizumab) in induction and maintenance therapy in Japanese patients with moderate or severe active UC is ongoing.
Etrolizumab is an IgG1 humanized monoclonal antibody that selectively binds the subunit of the α4β7 and the αEβ7 integrin heterodimers in the intestine. Etrolizumab antagonizes α4β7/MAdCAM-1-mediated leukocyte recruitment in the intestinal vasculature and αEβ7/E-cadherin interactions, which are believed to be involved in retention of α4β7 cells in the intraepithelial compartment and in the migration and function of retinoic acid-producing CD103+ dendritic cells expressing β7. The safety and pharmacology of etrolizumab were evaluated in a randomized phase 1 study in patients with moderate to severe UC. In the single ascending-dose stage, etrolizumab up to 10 mg/kg intravenously or 3.0 mg/kg subcutaneously showed no dose-limiting toxicity[34]. In a subsequent phase 2 study, patients with moderate to severe active UC were treated with three monthly doses of etrolizumab at 100 mg, a loading dose of etrolizumab at 420 mg and then 300 mg, or placebo[35]. Clinical remission occurred at week 10 in 20.5% of patients in the etrolizumab 100 mg group (P = 0.004), 10.3% of patients in the etrolizumab 420 mg loading dose group (P = 0.048), and no patients in the placebo group. The study population is so small, more studies are needed to confirm these data (Table 2).
| Drug | Trial | Study population | Protocol | Follow-up (wk) | Outcome |
| Tofacitinib | Sandborn et al[21] | 31 | 0.5 mg po twice daily | 8 | 32% (P = 0.39) clinical response at week 8 |
| 13% (P = 0.76) clinical remission at week 8 | |||||
| 33 | 3 mg po twice daily | 8 | 48% (P = 0.55) clinical response at week 8 | ||
| 33% (P = 0.01) clinical remission at week 8 | |||||
| 33 | 10 mg po twice daily | 8 | 61% (P = 0.10) clinical response at week 8 | ||
| 48% (P < 0.001) clinical remission at week 8 | |||||
| 49 | 15 mg po twice daily | 8 | 78% (P < 0.001) clinical response at week 8 | ||
| 41% (P < 0.001) clinical remission at week 8 | |||||
| Vedolizumab | GEMINI 1[32] | 225 | 300 mg iv at weeks 0, 2 and 6 | 6 | 47.1% (P < 0.001) clinical response at week 6 |
| 16.9% (P = 0.00) clinical remission at week 6 | |||||
| 122 | 300 mg iv at week 0, 2, 6 and every 4 wk | 52 | 44.8% (P < 0.001) clinical remission at week 52 | ||
| 125 | 300 mg iv at week 0, 2, 6 and every 8 wk | 52 | 41.8% (P < 0.001) clinical remission at week 52 | ||
| Etrolizumab | Vermeire et al[35] | 39 | 100 mg sc at week 0, 4 and 8 | 10 | 21% (P = 0.0040) clinical remission at week 10 |
| 39 | 420 mg sc loading dose then 300 mg at week 2, 4, and 8 | 10 | 10% (P = 0.048) clinical remission at week 10 |
Safety: Recent studies have shown that a few patients experience adverse events with biological agents. For adverse events, such as infections, neoplasms are related to the immunosuppressive effects of biological agents. Patients who are administered biological agents frequently develop antibodies against these drugs. This problem is more frequent with chimeric agents like infliximab than fully humanized agents like adalimumab.
Infliximab: Infliximab is a chimeric monoclonal antibody with a protein sequence that is 75% human and 25% mouse; therefore, human antichimeric antibody formation can occur in the blood. The presence of human antichimeric antibody is associated with an increased risk of infusion reactions during administration and reduced clinical efficacy. The common adverse events of infliximab are acute infusion reaction, and infection such as reactivation of tuberculosis.
As with other immunomodulatory drugs, infliximab therapy increases the risk of developing non-serious infections (RR approximately equal to 2); however, the data on serious infections are inconsistent[36]. Examples of reported serious infections include sepsis, pneumonia, cellulitis and intra-abdominal abscess[37]. Thus, infliximab should not be administered to a patient who has a clinically active infection. Patients who are at a high risk of chronic hepatitis B infection should be screened before the initiation of infliximab therapy.
Approximately 10% of infliximab infusions are associated with mild reactions such as headache, dizziness, fever, chills, chest pain, cough dyspnea or pruritus. These reactions occur within 1-2 h after infusion and can be alleviated by reducing the rate of infusion or by pretreatment with an H1-receptor antagonist[36,37]. In the ACT 1 and ACT 2 trials, 11.4% of the patients receiving infliximab experienced infusion reactions (44 of 484), compared with 9.4% of those receiving a placebo (23 of 244)[5].
For reasons that are unclear, 1 in 1000 infliximab infusions results in a serious reaction[37]. Delayed hypersensitivity-like reactions (serum sickness-like disorders) can occur 3-14 d after episodic infliximab infusions and include, but are not limited to, myalgia, fever, rash, pruritus, dysphagia, urticaria and headache[37]. In the ACT 1 and ACT 2 trials, three patients who received either 5 or 10 mg/kg infliximab had delayed hypersensitivity reactions (n = 484), as compared with two patients in the placebo study group (n = 244)[5].
Cases of aplastic anemia, pancytopenia, vasculitis, hepatitis, reversible mono/polyneuropathy and demyelination have been attributed to infliximab therapy[38].
At present, there is no consensus regarding the estimated lymphoma risk for patients treated with infliximab[36]. However, most experts believe that immunosuppression does impart some small cumulative risk of malignancy. The development of hepatosplenic T-cell lymphoma, a rare malignancy, has been reported in pediatric patients receiving infliximab treatment for Crohn’s disease in the United States[38,39].
Adalimumab: A total of 1010 patients received at least one dose of adalimumab in the ULTRA 1, 2 and 3 trials. The most frequently reported serious adverse event was worsening or flare of UC. Two serious events of cytomegalovirus colitis were reported. After the double-blind study period, one serious infection of tuberculosis and two treatment-emergent fatal adverse events were reported. Three events of B-cell lymphoma occurred during ULTRA 3. All three patients had a history of smoking and either previous or concomitant azathioprine use[18].
Golimumab: The most commonly observed adverse events in golimumab- and placebo-treated patients were headache and nasopharyngitis. Overall, the incidences of serious adverse events (3.0% vs 6.1%), including serious infections (0.5% vs 1.8%), were also similar, respectively, for golimumab- and placebo-treated patients. The most common serious adverse event was the exacerbation of UC, reported by eight (1.1%) golimumab-treated and eight (2.4%) placebo-treated patients. The only serious infection reported by more than one patient was pneumonia (one receiving 200/100 mg golimumab and one placebo patient). One patient (400/200 mg) died from peritonitis and sepsis after surgical complications related to an ischiorectal abscess and subsequent bowel perforation after surgery; this patient was receiving concomitant 20 mg prednisolone. One patient (400/200 mg) had a demyelinating disorder reported after the patient completed PURSUIT-SC induction and subsequently was randomized to placebo in the maintenance study. Two opportunistic infections were reported up to week 6: Esophageal candidiasis (400/200 mg golimumab) and cytomegalovirus infection (placebo). Neither event was reported as serious. No patient developed active tuberculosis[20].
Tofacitinib: The most commonly reported adverse events related to infection were influenza and nasopharyngitis (in six patients each). During the study period, the absolute neutrophil count was < 1500 cells/mm3 in three patients receiving tofacitinib (one at a dose of 10 mg twice daily and two at a dose of 15 mg twice daily); it was < 1000 cells/mm3 in none of the patients[21].
Vedolizumab: In the large GEMINI I study, no significant difference was observed among the study groups for the most commonly reported adverse events: Namely, flare of UC, headache, nasopharyngitis and arthralgia. Serious infections were no more common with vedolizumab than with placebo. No cases of progressive multifocal leukoencephalopathy occurred[32].
Etrolizumab: Patients in the 100 mg etrolizumab group had higher rates of rash, influenza-like illness, and arthralgia than did those in the placebo or 300 mg etrolizumab plus loading dose (LD) groups; all of these events were regarded as mild to moderate in severity. Serious adverse events were reported in 12 patients; five of these were related to UC (two in the 100 mg etrolizumab group; one in the 300 mg etrolizumab plus LD group; and two in the placebo group; Appendix)[35].
A number of biological agents are currently available for treatment of UC. These agents serve as another appropriate treatment option for gastrointestinal clinicians in patients with moderate to severe UC who may not be effectively treated with conventional agents. Various cytokines and intracellular messengers are involved in the pathogenesis of UC; thus, further discovery and development of new agents are required.
P- Reviewer: Hillman LC, Hokama A, Huerta-Franco MR S- Editor: Gong ZM L- Editor: A E- Editor: Liu SQ
| 1. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1511] [Article Influence: 83.9] [Reference Citation Analysis (2)] |
| 2. | Engel MA, Neurath MF. New pathophysiological insights and modern treatment of IBD. J Gastroenterol. 2010;45:571-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Hanauer SB, Kirsner JB. Treat the patient or treat the disease? Dig Dis. 2012;30:400-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 5. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2886] [Article Influence: 144.3] [Reference Citation Analysis (2)] |
| 6. | Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, Kron M, Tighe MB, Lazar A, Thakkar RB. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257-65.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 943] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 7. | Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Colombel JF, Reinisch W. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96-109.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 517] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 8. | Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 547] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 9. | Apostolaki M, Armaka M, Victoratos P, Kollias G. Cellular mechanisms of TNF function in models of inflammation and autoimmunity. Curr Dir Autoimmun. 2010;11:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2019] [Cited by in RCA: 1883] [Article Influence: 134.5] [Reference Citation Analysis (2)] |
| 11. | Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 12. | Danese S. Mechanisms of action of infliximab in inflammatory bowel disease: an anti-inflammatory multitasker. Dig Liver Dis. 2008;40 Suppl 2:S225-S228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Rutgeerts P, Van Assche G, Vermeire S. Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology. 2004;126:1593-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 281] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 14. | Cornillie F, Shealy D, D’Haens G, Geboes K, Van Assche G, Ceuppens J, Wagner C, Schaible T, Plevy SE, Targan SR. Infliximab induces potent anti-inflammatory and local immunomodulatory activity but no systemic immune suppression in patients with Crohn’s disease. Aliment Pharmacol Ther. 2001;15:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, van Hoogstraten HJ, Chen AC, Zheng H, Danese S. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392-400.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 695] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 16. | Shen C, Assche GV, Colpaert S, Maerten P, Geboes K, Rutgeerts P, Ceuppens JL. Adalimumab induces apoptosis of human monocytes: a comparative study with infliximab and etanercept. Aliment Pharmacol Ther. 2005;21:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, Panaccione R, Fedorak RN, Tighe MB, Huang B. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 671] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 18. | Colombel JF, Sandborn WJ, Ghosh S, Wolf DC, Panaccione R, Feagan B, Reinisch W, Robinson AM, Lazar A, Kron M. Four-year maintenance treatment with adalimumab in patients with moderately to severely active ulcerative colitis: Data from ULTRA 1, 2, and 3. Am J Gastroenterol. 2014;109:1771-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Shealy DJ, Cai A, Staquet K, Baker A, Lacy ER, Johns L, Vafa O, Gunn G, Tam S, Sague S. Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor α. MAbs. 2010;2:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 20. | Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Colombel JF, Reinisch W. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85-95; quiz e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 683] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 21. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 631] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 22. | von Andrian UH, Engelhardt B. Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med. 2003;348:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153:517-528. [PubMed] |
| 24. | Arihiro S, Ohtani H, Suzuki M, Murata M, Ejima C, Oki M, Kinouchi Y, Fukushima K, Sasaki I, Nakamura S. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn’s disease. Pathol Int. 2002;52:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97-110. [PubMed] |
| 26. | Fedyk ER, Wyant T, Yang LL, Csizmadia V, Burke K, Yang H, Kadambi VJ. Exclusive antagonism of the α4 β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm Bowel Dis. 2012;18:2107-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Haanstra KG, Hofman SO, Lopes Estêvão DM, Blezer EL, Bauer J, Yang LL, Wyant T, Csizmadia V, ‘t Hart BA, Fedyk ER. Antagonizing the α4β1 integrin, but not α4β7, inhibits leukocytic infiltration of the central nervous system in rhesus monkey experimental autoimmune encephalomyelitis. J Immunol. 2013;190:1961-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 346] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 29. | Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Dubé R, Cohen A, Steinhart AH, Landau S. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 530] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 31. | Ransohoff RM. Natalizumab and PML. Nat Neurosci. 2005;8:1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1866] [Article Influence: 155.5] [Reference Citation Analysis (1)] |
| 33. | Danese S, Fiorino G, Peyrin-Biroulet L, Lucenteforte E, Virgili G, Moja L, Bonovas S. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med. 2014;160:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 34. | Rutgeerts PJ, Fedorak RN, Hommes DW, Sturm A, Baumgart DC, Bressler B, Schreiber S, Mansfield JC, Williams M, Tang M. A randomised phase I study of etrolizumab (rhuMAb β7) in moderate to severe ulcerative colitis. Gut. 2013;62:1122-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 35. | Vermeire S, O’Byrne S, Keir M, Williams M, Lu TT, Mansfield JC, Lamb CA, Feagan BG, Panes J, Salas A. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 354] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 36. | Desai SB, Furst DE. Problems encountered during anti-tumour necrosis factor therapy. Best Pract Res Clin Rheumatol. 2006;20:757-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:940-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 344] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 38. | Panaccione R, Fedorak RN, Aumais G, Bernard EJ, Bernstein CN, Bitton A, Croitoru K, Dieleman LA, Enns R, Feagan BG. Review and clinical perspectives for the use of infliximab in ulcerative colitis. Can J Gastroenterol. 2008;22:261-272. [PubMed] |
| 39. | Mackey AC, Green L, Liang LC, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:265-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 321] [Article Influence: 17.8] [Reference Citation Analysis (0)] |