Published online Nov 15, 2015. doi: 10.4291/wjgp.v6.i4.203
Peer-review started: July 5, 2015
First decision: July 31, 2015
Revised: September 11, 2015
Accepted: October 23, 2015
Article in press: October 27, 2015
Published online: November 15, 2015
Processing time: 140 Days and 6 Hours
Inflammatory bowel disease (IBD) is a chronic and relapsing disorder which leads to an inflammation of the gastrointestinal tract. A tailored therapy to achieve mucosal healing with the less adverse events has become a key issue in the management of IBD. In the past, the clinical remission was the most important factor to consider for adapting diagnostic procedures and therapeutic strategies. However, there is no a good correlation between symptoms and intestinal lesions, so currently the goals of treatment are to achieve not only the control of symptoms, but deep remission, which is related with a favourable prognosis. Thus, the determination of biological markers or biomarkers of intestinal inflammation play a crucial role. Many biomarkers have been extensively evaluated in IBD showing significant correlation with endoscopic lesions, risk of recurrence and response to treatment. One of the most important markers is faecal calprotectin (FC). Despite calprotectin limitations, this biomarker represents a reliable and noninvasive alternative to reduce the need for endoscopic procedures. FC has demonstrated its performance for regular monitoring of IBD patients, not only to the diagnosis for discriminating IBD from non-IBD diagnosis, but for assessing disease activity, relapse prediction and response to therapy. Although, FC provides better results than other biomarkers such as C-reactive protein and erythrocyte sedimentation rate, these surrogate markers of intestinal inflammation should not be used isolation but in combination with other clinical, endoscopic, radiological or/and histological parameters enabling a comprehensive assessment of IBD patients.
Core tip: The surveillance of inflammatory bowel disease (IBD) course is needed to select the patients with worse prognosis and to adapt an early therapeutic strategy. Faecal calprotectin constitutes a surrogate marker of intestinal inflammation and a robust alternative to invasive procedures as endoscopy. This biomarker has been demonstrated reliable and accuracy in different aspects of IBD such as diagnosis of IBD, activity assessment, response to treatment and relapse prediction. Although a cut-off level of calprotectin has not been fully established, the combination with other biomarkers allows an appropriate management of the patient.
- Citation: Benítez JM, García-Sánchez V. Faecal calprotectin: Management in inflammatory bowel disease. World J Gastrointest Pathophysiol 2015; 6(4): 203-209
- URL: https://www.wjgnet.com/2150-5330/full/v6/i4/203.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v6.i4.203
The main forms of inflammatory bowel disease (IBD) are the ulcerative colitis (UC) and Crohn’s disease (CD). Both are chronic inflammatory disorders characterized by a relapsing-remitting clinical behavior. The course of IBD is unpredictable and can lead to cumulative intestinal tissue damage and complications which affect quality of life of patients[1]. The chronic nature of the disease requires a continuous assessment of activity to adapt the therapeutic strategy. Thus, physicians need reliable tools which allow to evaluate the disease activity and relapses risk.
Initially, the aim of therapy was to reach clinical remission, but this way is not enough to change the natural history of the disease. In recent years, the goal of treatment in IBD has changed and it is guided towards the mucosal healing, considered as a good predictor of the disease course, and associated with better patient outcomes[2,3].
Diagnosis and monitoring of IBD activity is based on a combination of clinical assessment, serologic and fecal markers of inflammation, cross-sectional imaging and endoscopy. Although endoscopy remains the gold standard for assessing IBD activity and mucosal healing, it has some risks and limitations: It is an invasive procedure, usually with low acceptance by the patient and potentially harmful, relatively high cost, it does not give information of the transmural inflammation, and finally is not well-known the timing of endoscopic evaluation. For this reason, numerous biomarkers have been proposed as surrogate markers of intestinal inflammation, and therefore also as potential markers of IBD activity. The biomarkers most extensively studied and commonly employed in clinical practice are C-reactive protein (CRP) and faecal calprotectin (FC).
This review offers a practical overview of the role of FC in several scenarios of clinical practice such as diagnosis of IBD, disease activity measurement, therapy response assessment and disease relapse prediction, describing its advantages and limitations (Table 1).
| Advantages | Disadvantages |
| Relatively good acceptance | Not always well accepted by patients (faecal samples) |
| Non-invasive | Subject to non-specific variations |
| Relatively low cost | Predictive threshold values not fully established |
| May be combined to improve prediction | Imperfect correlation with mucosal healing and transmural healing |
| Can be repeated as a longitudinal monitoring tool | |
| Predictive value for | |
| Disease relapse | |
| Response to anti-TNF therapy | |
| Mucosal healing |
Calprotectin is a calcium and zinc-binding protein which constitutes 60% of neutrophil cytosolic proteins[4,5], and that has functions such as antibacterial activity and induction of apoptosis[6]. Granulocytes produce FC at the site of mucosal inflammation increasing levels of this protein in faeces[7].
The FC level is a marker more specific of mucosal inflammation than CRP or erythrocyte sedimentation rate (ESR), which is less influenced by other non-intestinal conditions[8]. FC determination can be performed by enzyme-linked immunosorbent assay[5], and shows great stability at room temperature for a week[7]. This easy and inexpensive determination becomes calprotectin in a useful tool for monitoring of IBD patients.
Calprotectin presents some limitations in clinical practice. FC concentrations can be increase in non-IBD disorders; a cut-off level has not been well-established, and some authors described significant variability in a same patient[9]. Although a concentration < 50 μg/g may be considered upper limit of normal[10], an optimal cut-off for distinguishing IBD from other entities has not been fully described. The cut-off level of FC most commonly used varies from 50 to 200 μg/g[11]. von Roon et al[12] evaluated the diagnostic accuracy of FC for IBD and demonstrated that a cut-off level of 100 μg/g had better accuracy than 50 μg/g. Even, others authors increased the cut-off up to 150 μg/g[13].
The diagnosis of IBD is based not only on clinical data, because symptoms are unspecific and present in other organic or functional disorders, but also, endoscopic, radiological and histological criteria are needed to confirm or exclude the diagnosis. The use of biological markers capable to differentiate between organic and functional diseases, would select those patients with suspected IBD which needs further invasive procedures such as colonoscopy. The role of biomarkers in this setting is variable.
FC has a great diagnostic accuracy for discriminating IBD from non-organic entities like has been reported in the literature[14] and evaluated in multiple studies[15-19].
Gisbert et al[14] reported an overall sensitivity of 80% and specificity of 76% for the diagnosis of IBD, reaching a higher accuracy for CD (sensitivity 83%, specificity 85%) than for UC (sensitivity 72%; specificity 74%). In a meta-analysis, von Roon et al[12] assessed the diagnostic precision of FC for IBD, and showed higher FC levels than non-IBD patients with a sensitivity of 95% and a specificity of 91%. Similar results have been published by other meta-analysis which included adult and pediatric studies with patients suspected to have IBD, with sensitivity and specificity of FC for distinction between IBD and irritable bowel syndrome of 93% and 96%, respectively. In pediatric population, this accuracy is lower reaching a sensitivity of FC of 0.92 (95%CI: 0.84-0.96) and specificity slightly lower 0.76 (95%CI: 0.62-0.86), probably due to the higher FC levels in healthy children up to 9 years of age[20].
This diagnostic accuracy of FC would decrease the numbers of endoscopies needed up to 3-fold in adults and 35% in children[21] and, therefore, significantly reduces costs[22].
Therefore, FC is a reliable marker for organic gastrointestinal disorders, however, it is not specific for IBD, and other process can increase it such as neoplasms (colorectal cancer, polyps), gastrointestinal infections, other inflammatory entities (microscopic colitis, diverticulitis) and NSAID-induced enterocolitis[21]. A high value of FC constitutes a solid reason for performing a colonoscopy and confirming the diagnosis.
Although there is no established cut-off level to predict IBD, it is widely accepted that 50 μg/g is an accurate FC level to exclude organic intestinal disease with a high negative predictive value (NPV)[23]. Higher levels are not recommended because they would result in more false negative results and in this setting, the predictive negative value needs to be high in order to prevent delays in diagnosis. A normal value of FC makes unlikely the diagnosis of intestinal organic disease. The performance of FC with a cutoff of 50 μg/g as the first step to exclude organic disease seems reasonable, if the suspicion of IBD is not too high.
The diagnostic accuracy of FC for the diagnosis of IBD has been shown higher than other biomarkers such as CRP, ESR, anti-neutrophil cytoplasmic antibodies and anti-saccharomyces cerevisiae antibodies.
The role of faecal calprotectin to evaluate disease activity: The identification of inflammatory activity in a symptomatic IBD patient is crucial before changing the therapeutic strategy. Most of clinical indices employed to assess disease activity in IBD are based on patient symptoms and, therefore, subjective and poorly correlated with mucosal inflammation. The availability of biomarkers with a good correlation with clinical, endoscopic and histological activity is of capital relevance in daily clinical practice avoiding repeating invasive procedures. Moreover, fecal biomarkers are cheaper and easier, providing an important alternative to endoscopic procedures.
FC levels have shown a good correlation with the degree of inflammatory activity in IBD[24-26]. In CD, the median Pearson r correlation between the CD Endoscopic Index of Severity (CDEIS) and FC was 0.49, and for lactoferrin was 0.77[27-29]; the correlation with the simple endoscopic score for CD was similar for both FC (0.53) and lactoferrin (0.62)[27,30]. A meta-analysis with 550 patients evaluated the accuracy of CRP, FC and endoscopic scores, and it showed that in symptomatic patients (CDAI > 220), the sensitivity and specificity of CRP ≤ 5 mg/L or FC ≤ 200 μg/g to anticipate a CDEIS ≤ 6 was 83% and 71%, respectively[31].
In UC, FC levels show a better association with disease activity than in CD, and its correlation with endoscopic May score[28,32], Rachmilevitz index, and modified Baron score[33] was 0.72 (0.49-0.83).
Although no cut-off level has been validated, a FC > 200-250 μg/g has shown to have good accuracy in predicting endoscopic activity[28]. However, in CD with exclusively small bowel location, the sensitivity of FC to detect endoscopic lesions might be lower[34].
CRP has shown a sensitivity and specificity lower than FC for endoscopic disease activity both in UC and CD, so FC constitutes a more valuable marker than CRP in this context. In an appropriate scenario, the performance of FC could prevent the need for colonoscopy to confirm or exclude endoscopy activity in a symptomatic patient.
The role of faecal calprotectin to confirm mucosal healing and predict disease relapse: The course of IBD varies over time and while some patients have a favourable course with long periods of remission, others have a more aggressive disease, with unpredictable activity flare-up. Predicting the course along with the risk of relapse is useful because would allow to clinicians to individualize the management of each patient, conducting a more personalized approach and optimizing therapeutic strategies, minimizing adverse effects. The prediction of relapses would allow an early and intensive treatment in patients with worse prognosis. Studies examining this issue prospectively are limited and with inconclusive results regarding the frequency of determination of these biomarkers.
The capability to predict IBD relapse is one of the potential of the FC[25,35]. High levels of calprotectin in remission are associated with an increased risk of clinical relapse, with a sensitivity of 90% and specificity of 83%[18]. So, patients in clinical remission with high concentrations of FC had a risk of relapse of 2 and 14 times higher in CD and UC, respectively, compared to patients without elevated calprotectin[36]. However, CRP and ESR are not as helpful to predict disease’s relapse, probably because theses biomarkers estimate intestinal inflammation indirectly.
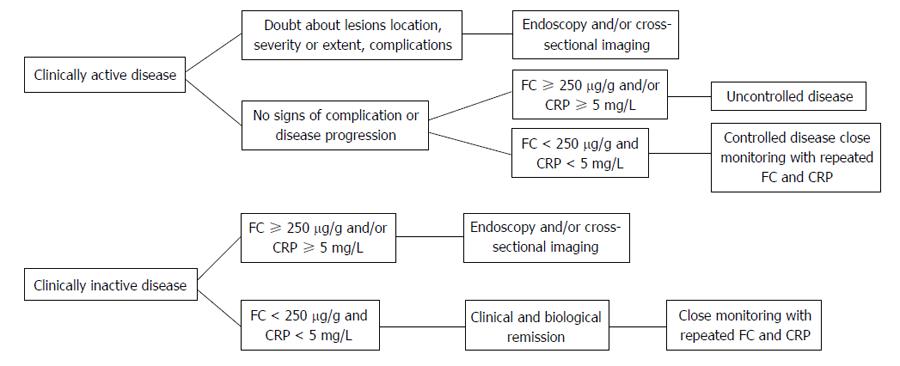
It is necessary to clarify the predictive value of FC in UC and CD, its chronological relationship with the occurrence of relapse and the best cut-off point to determine relapse risk. D’Haens et al[28] showed that CD patients with a level of FC > 250 μg/g predicted the presence of large ulcers with a sensitivity of 60% and specificity of 80%, while a concentration of < 250 μg/g predicted mucosal healing (CDEIS < 3) with a sensitivity and specificity of 94% and 62%, respectively. A recent subanalysis of STORI study[37] suggested that the combination of FC (with threshold < 250 μg/g) and PCR (with threshold < 5 mg/L) can improve the capacity to predict mucosal healing with reasonably good sensitivity and specificity, around 70%. When considering inactive CD patients (CDAI ≤ 150), the association of a PCR ≤ 10 mg/L and a calprotectin ≤ 200 μg/g has a sensitivity of 78% and a specificity of 58% for predicting no significant endoscopic activity (CDEIS ≤ 3), with a positive predictive value between 65%-88% and 40%-70% NPV. So, if a colonoscopy is performed to 100 patients with CD in clinical remission with both biomarkers below this threshold, 30-40 colonoscopies could have been avoided. Patients with higher calprotectin or CRP levels should be considered holders of active intestinal lesions (Figure 1).
García-Sánchez et al[35] showed that the predictive value of FC was similar in UC and CD with colon involvement, and considering FC > 120 μg/g as a predictor of relapse risk with a sensitivity of 80% and a specificity of 60%. This predictive value is lower in patients with ileal CD. Although the appropriate frequency of determination of these markers is not well-established to date, data from the GETAID-STORI cohort indicates that both CRP and FC begin to increase their concentrations 4-6 mo before clinical relapse, so determinations every 3-4 mo should be sufficient to detect high levels and allow to clinicians to tailor therapeutic strategies[38].
The FC determination could also be useful to assess diseases activity evolution. Thus, Casellas et al[39] studied patients with clinically quiescent UC for 1 year or until clinical relapse; and they observed that FC values remains stable in patients with inactive UC, and increased in relapsing patients.
Ho et al[40] reported as FC levels could predict the colectomy risk in patients with acute severe UC. They evaluated 90 patients hospitalized for acute severe colitis and showed as very high levels of FC on admission were associated with an increased risk of colectomy. An initial calprotectin over 1900 μg/g predicted colectomy of 87% patients in the first year.
The role of faecal calprotectin to evaluate response to treatment: Another feature of faecal biomarkers is the rapid confirmation of drug efficacy after initiation of therapy. Usually, the evaluation of the response to treatment is based on clinical assessment, while endoscopy is rarely performed. It would be of great interest to have markers that reliably estimate the probability of response to different therapies. Thus, it is possible to identify subgroups of patients who would benefit from a particular therapeutic strategy as well as patients will have a poor response to the treatment being able to avoid exposure to them and the risk of adverse events. The lack of response to treatment may affect the quality of life of patients and increase their mortality.
Nowadays, the goal of treatment in IBD is to achieve mucosal healing, which has been associated with better outcomes and fewer relapses. However, to confirm absence of endoscopic lesions would be needed repeated endoscopic procedures. Therefore, biomarkers able to indirectly estimate this healing are imperative.
FC has been suggested as surrogate faecal marker of response to therapy. Several studies have demonstrated that normalization of calprotectin levels in IBD patients after medical treatment is a marker that predicts the endoscopic healing. Decreased levels of FC after therapy are associated with clinical, endoscopic and histological improvement[41]. The normalization of calprotectin (< 50 mg/g) is more difficult to reach than the CRP normalization, so a significant decrease of FC could represent a deeper remission and a higher tissue healing[42,43].
When a steroid-free remission is achieved, de-escalation therapy may be tried to optimize benefit/risk. The combination of CRP and FC represents a good option to predict the risk of relapse after infliximab withdrawal[43].
For de-escalation of any drug or cessation of corticosteroids or mesalamine, a confirmation of biological remission with biomarkers such as CRP or FC can be sufficient. However, if we are willing to stop immunosuppressants or anti-TNF drugs, a confirmation of mucosal healing by endoscopy seems desirable[44].
The role of faecal calprotectin in postoperative recurrence assessment: There are scarce and conflicting data regarding the value of biomarkers in the postoperative setting to predict disease recurrence. FC usually returns to normal level by 2 mo postoperatively and any increase of its concentrations are associated with inflammatory recurrence[45].
Lobatón et al[34] suggested that FC is a more accurate and better surrogate marker of endoscopy activity in recurrent CD than clinical or serological markers, allowing to distinguish between postoperative recurrence patients (Rutgeert’s score 2-4) and patients without recurrence (Rutgeert’s score 0-1). In this study, using a cut-off value of FC of 203 μg/g reached a sensitivity of 75% and a specificity of 72%.
Beltrán et al[46] reported that FC is a useful early noninvasive marker for assessing recurrence of CD. A cut-off of 175 μg/g for FC is proposed.
The availability of biomarkers as FC represents a complementary tool to the clinical, endoscopic, radiological and histological procedures in the management of IBD patients. This surrogate marker is non-invasive, objective and non-expensive, and has a high accuracy for assessing different scenarios in IBD (to distinguish organic and functional disease, to evaluate disease activity, to predict risk of relapse, response to treatment and postoperative recurrence risk). FC can help to clinicians to avoid repeating invasive techniques selecting patients and to guide therapeutic decision. FC could be determined during follow-up allowing an early detection rather than just prediction of relapses. A combination of serological and faecal markers and endoscopy allow to the overall understanding of intestinal inflammation.
P- Reviewer: Francesco C, Tsai HH S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
| 1. | Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 755] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 2. | Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15:1295-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 532] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 3. | Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, Stokkers P, Hommes D, Rutgeerts P, Vermeire S. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463-468; quiz e10-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 644] [Article Influence: 42.9] [Reference Citation Analysis (35)] |
| 4. | Nacken W, Roth J, Sorg C, Kerkhoff C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech. 2003;60:569-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 275] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 492] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 6. | Sutherland AD, Gearry RB, Frizelle FA. Review of fecal biomarkers in inflammatory bowel disease. Dis Colon Rectum. 2008;51:1283-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Angriman I, Scarpa M, D’Incà R, Basso D, Ruffolo C, Polese L, Sturniolo GC, D’Amico DF, Plebani M. Enzymes in feces: useful markers of chronic inflammatory bowel disease. Clin Chim Acta. 2007;381:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 301] [Article Influence: 13.1] [Reference Citation Analysis (2)] |
| 9. | Husebye E, Tøn H, Johne B. Biological variability of fecal calprotectin in patients referred for colonoscopy without colonic inflammation or neoplasm. Am J Gastroenterol. 2001;96:2683-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Tøn H, Brandsnes S, Holtlund J, Skuibina E, Schjønsby H, Johne B. Improved assay for fecal calprotectin. Clin Chim Acta. 2000;292:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Abraham BP, Kane S. Fecal markers: calprotectin and lactoferrin. Gastroenterol Clin North Am. 2012;41:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | von Roon AC, Karamountzos L, Purkayastha S, Reese GE, Darzi AW, Teare JP, Paraskeva P, Tekkis PP. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (2)] |
| 13. | Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol M, Bjarnason I. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 351] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 14. | Gisbert JP, McNicholl AG. Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis. 2009;41:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Otten CM, Kok L, Witteman BJ, Baumgarten R, Kampman E, Moons KG, de Wit NJ. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med. 2008;46:1275-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Dai J, Liu WZ, Zhao YP, Hu YB, Ge ZZ. Relationship between fecal lactoferrin and inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1440-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Schoepfer AM, Trummler M, Seeholzer P, Criblez DH, Seibold F. Accuracy of four fecal assays in the diagnosis of colitis. Dis Colon Rectum. 2007;50:1697-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 542] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 19. | Wang S, Wang Z, Shi H, Heng L, Juan W, Yuan B, Wu X, Wang F. Faecal calprotectin concentrations in gastrointestinal diseases. J Int Med Res. 2013;41:1357-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 487] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 21. | Kopylov U, Rosenfeld G, Bressler B, Seidman E. Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:742-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 22. | Kim MK, Chae YN, Kim HD, Yang EK, Cho EJ, Choi SH, Cheong YH, Kim HS, Kim HJ, Jo YW. DA-1229, a novel and potent DPP4 inhibitor, improves insulin resistance and delays the onset of diabetes. Life Sci. 2012;90:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Yang Z, Clark N, Park KT. Effectiveness and cost-effectiveness of measuring fecal calprotectin in diagnosis of inflammatory bowel disease in adults and children. Clin Gastroenterol Hepatol. 2014;12:253-262.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Tibble JA, Bjarnason I. Fecal calprotectin as an index of intestinal inflammation. Drugs Today (Barc). 2001;37:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | García Sánchez Mdel V, González R, Iglesias Flores E, Gómez Camacho F, Casais Juanena L, Cerezo Ruiz A, Montero Pérez-Barquero M, Muntané J, de Dios Vega JF. [Diagnostic value of fecal calprotectin in predicting an abnormal colonoscopy]. Med Clin (Barc). 2006;127:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Langhorst J, Elsenbruch S, Mueller T, Rueffer A, Spahn G, Michalsen A, Dobos GJ. Comparison of 4 neutrophil-derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm Bowel Dis. 2005;11:1085-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Sipponen T, Kärkkäinen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | D’Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 621] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 29. | Meuwis MA, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Piver E, Seidel L, Colombel JF, Louis E. Serum calprotectin as a biomarker for Crohn’s disease. J Crohns Colitis. 2013;7:e678-e683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 359] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 31. | Bondjemah V, Mary JY, Jose J, Sandborn W, Schoepfer A, Louis E, Sipponen T, Vieira A, Colombel JF, Allez M. Fecal calprotectin and CRP as biomarkers of endoscopic activity in Crohn’s disease: a meta-study. J Crohn Colitis. 2012;6:P133. [DOI] [Full Text] |
| 32. | Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162-169. [PubMed] |
| 33. | Schoepfer AM, Beglinger C, Straumann A, Safroneeva E, Romero Y, Armstrong D, Schmidt C, Trummler M, Pittet V, Vavricka SR. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis. 2013;19:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 34. | Lobatón T, López-García A, Rodríguez-Moranta F, Ruiz A, Rodríguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn’s disease. J Crohns Colitis. 2013;7:e641-e651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 35. | García-Sánchez V, Iglesias-Flores E, González R, Gisbert JP, Gallardo-Valverde JM, González-Galilea A, Naranjo-Rodríguez A, de Dios-Vega JF, Muntané J, Gómez-Camacho F. Does fecal calprotectin predict relapse in patients with Crohn’s disease and ulcerative colitis? J Crohns Colitis. 2010;4:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 36. | Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 37. | Lemann M, Colombel J-F, Grimaud J-C. Fecal calprotectin and high sensitivity c-reactive protein levels to predict mucosal healing in patients with Crohn’s disease. A subanalysis of the STORI study. Gut. 2010;59 Suppl III:A80. |
| 38. | De Suray N, Salleron J, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M. Close monitoring of CRP and fecal calprotectin levels to predict relapse in Crohn’s disease patients. A sub-analysis of the STORI study. J Crohn Colitis. 2012;6:P274. [DOI] [Full Text] |
| 39. | Casellas F, Borruel N, Antolín M, Varela E, Torrejón A, Armadans L, Guarner F, Malagelada JR. Fecal excretion of deoxyribonucleic acid in long-term follow-up of patients with inactive ulcerative colitis. Inflamm Bowel Dis. 2007;13:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Ho GT, Lee HM, Brydon G, Ting T, Hare N, Drummond H, Shand AG, Bartolo DC, Wilson RG, Dunlop MG. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am J Gastroenterol. 2009;104:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 41. | Røseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol. 2004;39:1017-1020. [PubMed] |
| 42. | Benitez JM, Meuwis MA, Reenaers C, Van Kemseke C, Meunier P, Louis E. Role of endoscopy, cross-sectional imaging and biomarkers in Crohn’s disease monitoring. Gut. 2013;62:1806-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Louis E, Mary JY, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63-70.e5; quiz e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 459] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 44. | Pittet V, Froehlich F, Maillard MH, Mottet C, Gonvers JJ, Felley C, Vader JP, Burnand B, Michetti P, Schoepfer A. When do we dare to stop biological or immunomodulatory therapy for Crohn’s disease? Results of a multidisciplinary European expert panel. J Crohns Colitis. 2013;7:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Lamb CA, Mohiuddin MK, Gicquel J, Neely D, Bergin FG, Hanson JM, Mansfield JC. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn’s disease. Br J Surg. 2009;96:663-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 46. | Beltrán B, Cerrillo E, Iborra M. Fecal calprotectin is a useful early predictive marker for postoperative recurrence in Crohn’s disease. Gastroenterol. 2012;142:S659. |