Published online Nov 15, 2015. doi: 10.4291/wjgp.v6.i4.140
Peer-review started: June 27, 2015
First decision: September 17, 2015
Revised: September 28, 2015
Accepted: October 20, 2015
Article in press: October 27, 2015
Published online: November 15, 2015
Processing time: 143 Days and 16.6 Hours
Magnetic resonance imaging (MRI) is a well established technique that has revolutionized diagnostic radiology. Until recently, the impact that MRI has had in the assessment of gastrointestinal motor function and bowel fluid distribution in health and in disease has been more limited, despite the novel insights that MRI can provide along the entire gastrointestinal tract. MRI biomarkers include intestinal motility indices, small bowel water content and whole gut transit time. The present review discusses new developments and applications of MRI in the upper gastrointestinal tract, the small bowel and the colon reported in the literature in the last 5 years.
Core tip: Magnetic resonance imaging (MRI) of gastrointestinal motor function and fluids distribution is coming of age, with a range of MRI biomarkers that can be measured non-invasively. The novel MRI biomarkers include intestinal motility indexes, the small bowel water content and whole gut transit time. Future research directions will focus on small and large bowel motility and on gut transit. Further validation of the methods and automation of data analysis will finally translate the MRI biomarkers into clinical routine.
- Citation: Khalaf A, Hoad CL, Spiller RC, Gowland PA, Moran GW, Marciani L. Magnetic resonance imaging biomarkers of gastrointestinal motor function and fluid distribution. World J Gastrointest Pathophysiol 2015; 6(4): 140-149
- URL: https://www.wjgnet.com/2150-5330/full/v6/i4/140.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v6.i4.140
The first demonstrations of the use of dynamic, serial and cine magnetic resonance imaging (MRI) to investigate organ motor function and fluid distribution in the gastrointestinal (GI) tract were reported nearly three decades ago[1,2]. For a long period of time this niche field was explored in a handful of MRI research laboratories and dedicated researchers that put up with the very laborious and lengthy manual data processing, often carried out image by image. Recent advances in imaging methods and data analysis tools are now bringing MRI-based assessments of GI function and fluids into the clinical arena. The number of MRI biomarkers, as indicators of GI function that can be objectively measured, has broadened (Table 1). MRI is often perceived as an expensive technique; however the cost of a short MRI scan compares favorably with more invasive procedures such as, for example, manometric intubation. This review focuses only on the last 5 years of relevant literature using MRI to study gastrointestinal motor function and bowel fluid distribution in the upper GI tract, the small bowel and the colon in health and in disease. Previous years were covered by preceding reviews[3-5].
| Biomarker | Method | Ref. |
| Gastric emptying | Time courses of gastric volumes, ROI analysis | [18-21] |
| Gastric secretion volume | T1 mapping, dilution of a meal labeled with gadolinium contrast agent | [19,67] |
| Gastric motility | Cine-MRI | [23] |
| Small bowel motility | Cine-MRI, image registration, standard deviation of the Jacobian | [28,29] |
| Small bowel water content | Heavily T2 weighted imaging, ROI analysis using calibrated threshold | [61] |
| Oro-cecal transit time | Arrival of the head of a meal in the cecum | [65] |
| Colonic volumes | ROI analysis | [58,59] |
| Colon water content | Heavily T2 weighted imaging, ROI analysis using calibrated threshold | [74] |
| Colon motility | Cine-MRI, image registration, line ROI analysis | [28] |
| Whole gut transit | T1-weighted imaging, capsules filled with water and gadolinium contrast agent | [65] |
| Colonic chyme relaxometry | T1 and T2 measurements | [61,74] |
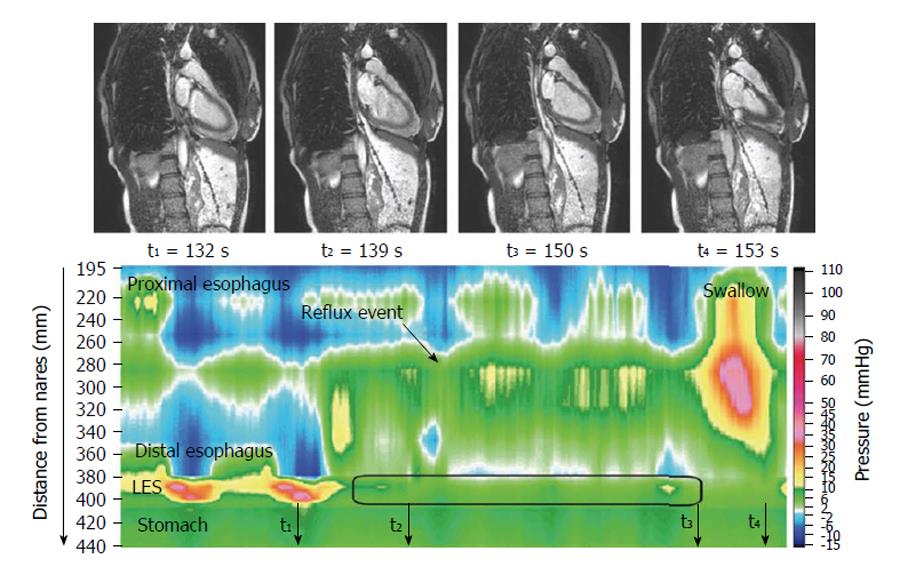
The dynamic of swallowing has been investigated with high temporal resolution MRI, providing functional information[6-8]. The images nicely delineate the motor action, and further work to validate these observations and establish clinical indications for “MR esophagography” would be welcome. One study showed a morpho-functional application to the study of achalasia[9] and another showed motility disturbances in some patients after Nissen fundoplication[10]. Gastroesophageal reflux was elegantly visualized using MRI and concomitant high resolution manometry[11] (Figure 1) with a view to improve understanding of reflux suppression by a raft-forming alginate, compared to a different antacid formulation. The same group provided a detailed biophysical analysis of the function and structure of the gastro-esophageal junction[12-14] hypothesizing that components of a “flap valve” contribute to reflux protection, and that this is impaired in patients with gastro esophageal reflux disease. These are unprecedented biomechanical insights into the function of the upper GI tract.
There has been continuing interest in the effect of manipulating the physical properties of food components on gastric motor function and appetite. Aerated foams were imaged for the first time in vivo demonstrating their effect on increasing gastric volumes and reducing appetite compared to isocaloric, non-aerated beverages[15]. It was also shown that fat emulsions of varying droplet size can modulate gastric emptying[16,17]. The data processing required to monitor gastric volumes and emptying can still be a burden. Developments were made in modeling the emptying curves including gastric secretion[18,19] and in automating the analysis[19-21], with a view to creating a protocol that would be acceptable in clinical practice. Gastric motility was evaluated by simple review of cine MRI series across the stomach after laparoscopic sleeve gastrectomy[22]. The sleeve was found to have little peristaltic function whilst the antrum showed accelerated propulsion. Comparison between manual and automated analysis of gastric motility[23], concluded that the semi-automated procedure for segmentation had comparable accuracy and much better efficiency than the manual method.
The MRI assessment of small bowel motility is the field that has seen some of the most interesting developments over the last 5 years. A number of publications reported developments towards increased automation of analysis and quantitation of small bowel motility biomarkers. The task is still challenging. Good bowel distention is generally required; this is achieved by either infusing a large amount of liquid contrast directly in the small bowel using a catheter (MR enteroclysis) or by ingesting it [magnetic resonance enterography (MRE)]. MRE has been more popular because it is less demanding on both staff and patients. There is however little consensus. Based on local preferences, different contrast media, prone or supine position as well as different acquisition protocols and analysis strategies are used.
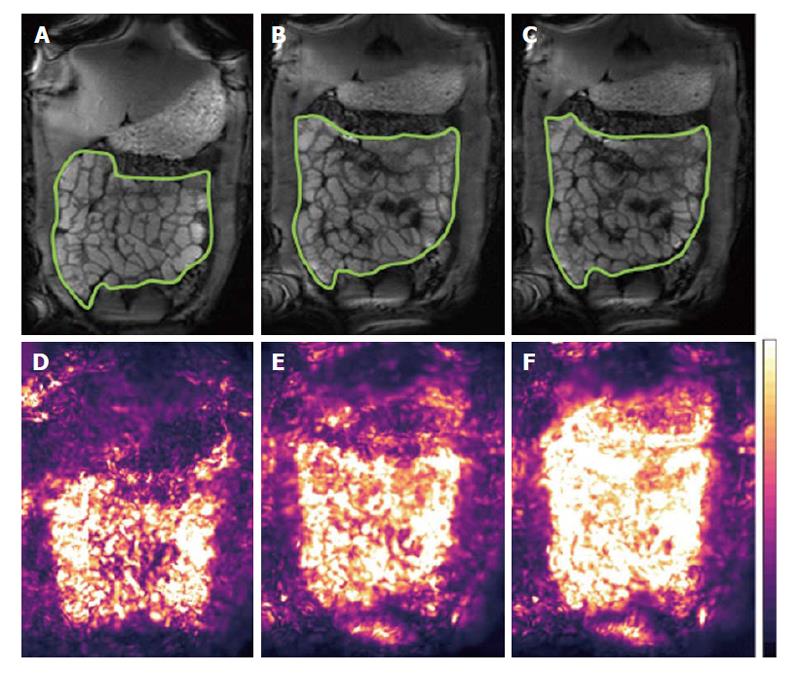
In terms of data acquisition, different MRI protocols have been proposed. Qualitatively, many MRI units nowadays add a short cine sequence to small bowel protocols, before injection of spasmolytics, for an overall visual assessment or operator’s grading of motility[24,25]. Robust biomarkers however require objective quantitation and their translation requires improvements in data processing. There are two distinct schools of thoughts: One prefers breath-hold acquisitions whilst the other favors acquiring data for longer periods of time, free-breathing. The former minimizes diaphragmatic displacement thus making the data analysis easier. Multiple breath-holds can be acquired to sample motility for longer periods. Displacement of the small bowel by abdominal or diaphragmatic movement can affect the analysis during prolonged observation; this was evaluated in the prone position finding that craniocaudal displacement is predominant but the amplitude of the displacement is modest[26]. The second school of thought seeks to acquire for longer periods of time with the patient breathing freely and gently. In this case respiratory motion affects the quantitation of motility substantially and techniques are needed to correct for this in the time series before analysis. Robust Data Decomposition Registration (RRDR)[27] was used as a pre-processing step to remove respiratory motion; after this step global small bowel motility[28] was determined using an optic flow registration method[29]. The motility biomarker is based on the standard deviation of the Jacobian calculated from the displacement fields of the image pixels. This biomarker is based on the pixel intensity changes that the software uses to derive the registration parameters; hence it is not exactly anchored “biomechanically” to the bowel walls. On the other hand, the method provides an elegant and operator-independent assessment of global motility from long, free breathing time-series and yields motility maps that are easy to interpret (Figure 2). Another automated approach based on the optic flow registration technique was implemented, without the dual registration pre-step, in studies in IBD patients[30,31]. An alternative MRI approach to monitor motility is the continuous tagging, as is common in cardiac MRI. A global tagging motility index biomarker was used[32] with the motility analysis subdivided in low, medium and high frequency bands[33]. The index was able to detect a decrease in motility due to intravenous anti-peristaltic agent. The tagging method is region of interest (ROI)-independent. Tagging may also depend less on bowel distension, as suggested by the authors suggest[32].
In terms of data analysis, there was a limited use of visual, consensus analysis[34], mean change in signal amplitude[35] and manual luminal caliber measurements[36]. Software assisted methods were applied to both breath-hold and free-breathing acquisitions[23,37,38] and performed better than manual measurements[39]. The choice of intra-segmental location for the software-assisted analysis did not influence substantially the measurements substantially[40]. Region of interest analysis of small bowel motility showed however inter-segmental variation and modest repeatability[41], which would favor global, operator independent methods[42]. The frequency band analysis of continuously tagged images was also assessed automatically[33].
The MRI assessment of motility has found interesting applications in Crohn’s disease (CD), a particularly vulnerable population. These patients are likely to undergo serial imaging examination over the course of their treatment and the cumulative radiation dose from repeated computed tomography is undesirable[43]. Reduced motility was associated with small bowel segments affected by CD[24], correlating well with histopathology[44] and inflammatory markers in the blood and stools[45]. Notably the MRI motility biomarker reflected disease activity. Motility scores were associated negatively with disease activity score[46,47], using a multivariate analysis based on mural thickness, mural T2 signal, perimural T2 signal and enhancement[48]. Another finding of great interest is the demonstration that small bowel motility is not only impaired at the site of the lesion but also proximally[49-51]. The availability of cine MRE images was shown to aid the reader’s evaluation of questionable segments in a less ordinary CD exam protocol without the use of anti-peristaltic agents[52].
Beyond specific CD applications, cine MRI of small bowel motility was used to compare intravenous and intramuscular delivery routes for anti-peristaltic agents[53]. The data showed that intravenous administration had a faster and more reliable onset, whilst a combination of different agents and delivery routes provided early onset and high degree, sustained spasmolysis. The effectiveness of sublingual hyoscyamine sulphate as an alternative to antiperistaltic intravenous agents was also investigated using cine MRI[54]. The treatment effect of the sublingual agent was modest. The oral glucose tolerance test was shown to accelerate intestinal motility after laparoscopic sleeve gastrectomy[25]. Another interesting application of cine MRI was in chronic intestinal pseudo-obstruction (CIPO), showing contractility impairments in the CIPO patients compared to healthy volunteers and patients with irritable bowel syndrome[55]. It is worth noting that MRI of small bowel motility has also found some applications in animal models[56,57] although those are beyond the scope of this review. MRI was also used to study postprandial colon volumes as another biomarker of function[58]. Manual colon segmentation is lengthy and methods to semi-automate the processing have been proposed recently[59].
Despite the lack of standardization and the need for some further validation, the emerging biomarkers of small bowel motility are very promising and the body of recent work demonstrates that cine MRI of small bowel motility is coming of age. The data acquisition can translate to the clinics relatively easily. The high-end image registration and data processing methods may however require implementation in the scanner viewing platforms or dedicated cloud computing services for the technique to move into routine use.
Despite the flourishing of MRI publications on small bowel motility, so far little attention has been given to colonic motility. One possible reason for this is that colonic motility is inherently erratic so that an observation based on a single breath-hold cine slab may not be very informative. A longer acquisition time of a cine MRI sequence would characterize motility better. However, the same respiratory motion problems detailed above for the small bowel will affect the data.
The published studies used a variety of approaches. Visual inspection of cine MRI stacks showed reduced or absent peristalsis in involved colonic segments of 3 patients with ulcerative colitis, compared to other bowel segments[34]. In one elegant study bisacodyl instillation was used to induce high amplitude propagated pressure waves in the (cleansed) descending colon of 10 healthy volunteers and motility was monitored by concomitant MRI and manometry[60]. Three perpendicular imaging planes were acquired at 4 s intervals at baseline and for 24 min post bisacodyl instillation. The MRI images in each plane were played as a cine loop identifying changes of 50% in the largest diameter of the haustras. Eleven of these larger amplitude contractions were detected and these had an excellent 100% correlation with the manometry readings.
In a different study a subjective colonic motility index score was assessed by an operator in response to an oral polyethylene glycol (PEG) stimulus that distended the ascending colon and stimulated motility in healthy volunteers[61]. A single sagittal slice was acquired every second for 2 min of free breathing. No motion correction was applied and the operator inspected the data by dividing the ascending colon in three regions, estimating for how long each region showed contractility. This applied to any visible contractility not just high amplitude propagated waves. Using this relatively basic method the authors showed a marked increase in motility upon ingestion of PEG and that the increase was dose-dependent.
More quantitative approaches can clearly benefit from the registration of abdominal motion as discussed for the small bowel. A recent study applied the optic flow and RRDR dual-registration method to MRI data from the ascending colon of 6 healthy volunteers who ingested an oral PEG stimulus[28]. A single sagittal slice was again acquired every second for 2 min of free breathing. The study then compared simple line ROIs analysis results with and without application of the motion correction and showed the importance of correcting for abdominal motion to remove ambiguity. Optic flow methods were also used to quantify effectively hypomotility of colonic segments affected by CD using the static images as guide to define regions of interest in global motility maps[30].
Work this area is likely to continue in the next few years and the focus for new developments will expand from the small bowel towards MRI of colonic motility.
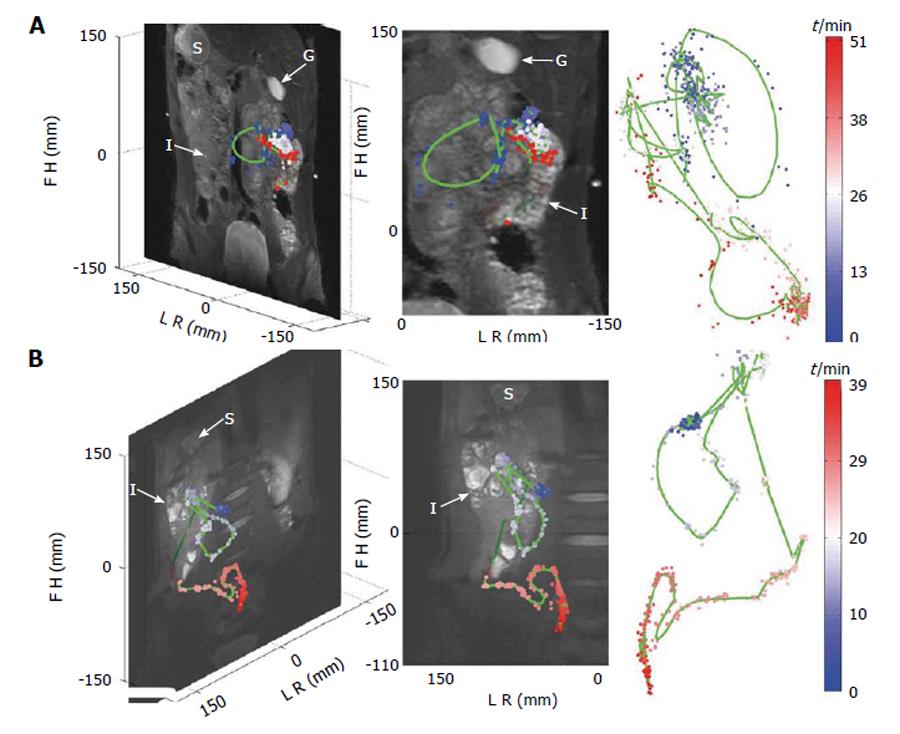
Bowel luminal flow has been overlooked whilst MRI of gastrointestinal transit has been the subject of a few new technical development studies. Three studies by Hahn et al[62] sought to use 19F imaging and MRI “transit capsule markers”. This is an interesting approach as there is basically no endogenous fluorine MRI signal in the human body, so any signal detected can be attributed to the capsules. Moreover the 19F nucleus has particularly good MRI visibility with 100% natural abundance and a gyromagnetic ratio close to the one of the hydrogen proton. The authors were able to show simultaneous, real-time tracking of one and two capsules in the GI tract of two healthy volunteers using 19F projection imaging superimposed to a proton anatomical reference[62] (Figure 3). In subsequent studies the “3D golden angle radial projection”19F imaging was deployed[63]. Using this acquisition they tracked capsules either embedded in a naso-gastric catheter (to enable tracking of the catheter) or ingested (to track the transit of the capsules in the GI tract) by one healthy volunteer. The 19F MRI catheter tracking methodology was further improved which allowed real time visualization and manipulation of the catheter[64]. The idea of using 19F to monitor GI transit is elegant; however there are significant barriers to translation including the need to use high field (≥ 3T), multinuclear transmit and receive hardware and a dedicated abdominal 19F transmit/receive coil, of which at the moment there are only few worldwide. The capsules are also relatively large (12 mm × 7 mm) and so unlikely to empty from the fed stomach. They are more likely to remain within the stomach until expelled by the migrating motor complex which will not develop until the fasting state is reached. Thus propulsion of these capsules along the GI tract is unlikely to mirror physiological transit of food. A different approach has been to use the proton MRI and MRI “transit capsule markers” filled with water doped with trace amounts of gadolinium contrast agent. Measurement of whole gut transit based on ingestion of 5 such markers and T1-weighted imaging was validated against standard radiopaque marker X-ray methods with repeated studies in 21 healthy volunteers[65]. The MRI method performed well against X-ray methodology and does not require high field or additional hardware. However the capsules are again relatively large (20 mm × 7 mm) and gastric sieving is likely to retain them during the fed state so they will only leave the stomach after the food has left. Furthermore their signal could be confused with high T1 food residue particularly at the terminal ileum/proximal colon. Within the same study, a simple method to measure oro-cecal transit time (OCTT) based on imaging the arrival of the “head of a meal” in the cecum was also evaluated against concomitant standard lactose ureide 13C breath test[65]. Correlation between the two methods was weak. Another major limitation of this MRI method is the need to continue imaging at intervals until the arrival of the “head of the meal” in the cecum is detected. This limits the time resolution of OCTT to the sampling frequency which is unsatisfactory. Furthermore the repeated scanning until detection is achieved would make its routine use expensive. Another study sought to evaluate OCTT by similar MRI methods comparing the results to concomitant standard lactulose hydrogen breath test[66]. The passing of the lactulose fluid bolus through the small bowel was followed visually on T2 weighted images until its arrival in the cecum was detected.
These studies show an increasing interest in developing non invasive MRI biomarkers for both oro-cecal and whole gut transit. Further work is needed to improve such methods and make them more physiological if they are to translate to the clinics effectively.
The investigation of fluids in the upper GI was predominantly focused on gastric secretion as measured by T1 mapping of a test meal doped with traces of a Gd-based contrast agent[19,67]. This showed a layer above the liquid meal in the stomach containing a lower concentration of contrast agent[68]. This is consistent with the concept of the “acid pocket” and could provide a target for gastroesophageal reflux treatments. Another study assessed the effect of pharmacologically enhanced gastric secretion on 13C-acetate breath test for gastric emptying[69]. There was new interest from the point of view of pharmaceutical sciences and drug dissolution. Two new studies investigated gastric fluid content under the standard fasting[70] and fed oral dosage form conditions[71] with a view to improving in vitro/in vivo correlation of drug dissolution modeling.
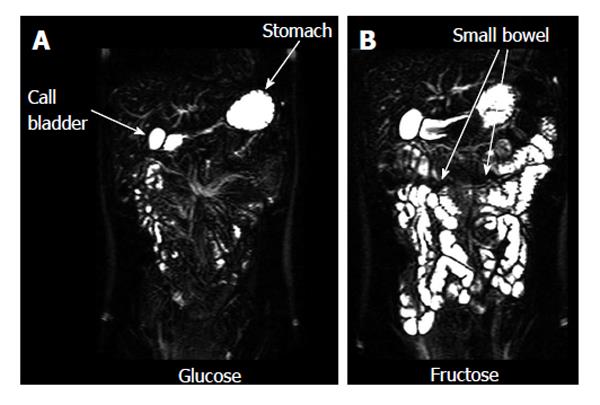
A number of studies evaluated the fluid content of the small bowel. Some monitored the effect of nutritional interventions[16,17,72,73]. These showed that the effect of physicochemical modifications in food microstructure (such as for example fat emulsion stability and droplet size) can markedly modulate small bowel postprandial fluid inflow. One study demonstrated the effect of a bowel preparation containing polyethylene glycol and electrolytes in generating inflow of fluid in the lumen[61]. By contrast another study showed the ability of a common anti-diarrheal agent, loperamide, to reduce the small bowel water content after a mannitol challenge model of secretory diarrhea[74]. Bowel fluid was also shown to be increased by an essential amino acid[75]. Other MRI studies showed that experimental stress reduced small bowel water content[76]. The effect of poorly absorbed and non absorbable carbohydrates on bowel fluid inflow and accumulation was also studied; these included fructose[77] (Figure 4) and lactulose[78]. The presence of separate small water pockets in the fasting small bowel was confirmed and the distribution and volume of the bowel pockets measured before and after ingestion of the standard fasting drug testing dose of 240 mL of water[70] with the same pharmaceutical sciences rationale as described above. The main finding was that the small bowel water pockets are discontinuous and their number and volume is small.
Two studies addressed colon fluid distribution using MRI. One study used an oral mannitol challenge and showed inflow of water from the small bowel into the ascending colon[74], quantifying the amount of freely mobile water in the ascending colon using similar methods as those used for the small bowel. The study found that there was only a small amount of freely mobile water detectable in the ascending colon. T2 relaxometry was also used in that study to characterize physicochemical changes in the chyme upon arrival of the fluid bolus, which showed an increase in T2 reflecting increased fluid mobility in the chyme. The other study showed that ingestion of a bowel preparation containing polyethylene glycol and electrolytes reached the colon rapidly increasing its size two-fold[61]. The study also used T1 relaxometry to characterize physicochemical changes in the chyme upon arrival of the fluid bolus. The relaxation time T1 of the ascending colon contents increased upon arrival of the fluid in the chyme as expected. Given the growing interest in bowel fluid dynamics and the work conducted so far more proximally, one can predict that MRI of colonic fluids will be an expanding field in the near future.
MRI of gastrointestinal function is coming of age. The development of more automated analysis methods will aid translation into clinical routine although further work on validating the MRI biomarkers is needed. The novel insights provided on bowel fluid volumes and distribution will improve understanding of disease and predictive models of drug dissolution. Further trials are needed to prove the value of the MRI biomarkers in clinical practice.
P- Reviewer: Landsman MJ, Lee SH, Sun XT S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | The British Society of Gastroenterology, 49th meeting. Sheffield, 14-16 September 1988. Abstracts. Gut. 1988;29:A1429-A1496. [PubMed] |
| 2. | Stehling MK, Evans DF, Lamont G, Ordidge RJ, Howseman AM, Chapman B, Coxon R, Mansfield P, Hardcastle JD, Coupland RE. Gastrointestinal tract: dynamic MR studies with echo-planar imaging. Radiology. 1989;171:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Schwizer W, Steingoetter A, Fox M. Magnetic resonance imaging for the assessment of gastrointestinal function. Scand J Gastroenterol. 2006;41:1245-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Schwizer W, Fox M, Steingötter A. Non-invasive investigation of gastrointestinal functions with magnetic resonance imaging: towards an “ideal” investigation of gastrointestinal function. Gut. 2003;52 Suppl 4:iv34-iv39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Marciani L. Assessment of gastrointestinal motor functions by MRI: a comprehensive review. Neurogastroenterol Motil. 2011;23:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Zhang S, Olthoff A, Frahm J. Real-time magnetic resonance imaging of normal swallowing. J Magn Reson Imaging. 2012;35:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Kulinna-Cosentini C, Schima W, Lenglinger J, Riegler M, Kölblinger C, Ba-Ssalamah A, Bischof G, Weber M, Kleinhansl P, Cosentini EP. Is there a role for dynamic swallowing MRI in the assessment of gastroesophageal reflux disease and oesophageal motility disorders? Eur Radiol. 2012;22:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Zhang J, Hu W, Zang L, Yao Y, Tang Y, Qian Z, Gao P, Wu X, Li S, Xie Z. Clinical investigation on application of water swallowing to MR esophagography. Eur J Radiol. 2012;81:1980-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Miyazaki Y, Nakajima K, Sumikawa M, Yamasaki M, Takahashi T, Miyata H, Takiguchi S, Kurokawa Y, Tomiyama N, Mori M. Magnetic resonance imaging for simultaneous morphological and functional evaluation of esophageal motility disorders. Surg Today. 2014;44:668-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Kulinna-Cosentini C, Schima W, Ba-Ssalamah A, Cosentini EP. MRI patterns of Nissen fundoplication: normal appearance and mechanisms of failure. Eur Radiol. 2014;24:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Sweis R, Kaufman E, Anggiansah A, Wong T, Dettmar P, Fried M, Schwizer W, Avvari RK, Pal A, Fox M. Post-prandial reflux suppression by a raft-forming alginate (Gaviscon Advance) compared to a simple antacid documented by magnetic resonance imaging and pH-impedance monitoring: mechanistic assessment in healthy volunteers and randomised, controlled, double-blind study in reflux patients. Aliment Pharmacol Ther. 2013;37:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Curcic J, Roy S, Schwizer A, Kaufman E, Forras-Kaufman Z, Menne D, Hebbard GS, Treier R, Boesiger P, Steingoetter A. Abnormal structure and function of the esophagogastric junction and proximal stomach in gastroesophageal reflux disease. Am J Gastroenterol. 2014;109:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Roy S, Fox MR, Curcic J, Schwizer W, Pal A. The gastro-esophageal reflux barrier: biophysical analysis on 3D models of anatomy from magnetic resonance imaging. Neurogastroenterol Motil. 2012;24:616-25, e269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Curcic J, Schwizer A, Kaufman E, Forras-Kaufman Z, Banerjee S, Pal A, Hebbard GS, Boesiger P, Fried M, Steingoetter A. Effects of baclofen on the functional anatomy of the oesophago-gastric junction and proximal stomach in healthy volunteers and patients with GERD assessed by magnetic resonance imaging and high-resolution manometry: a randomised controlled double-blind study. Aliment Pharmacol Ther. 2014;40:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Murray K, Placidi E, Schuring EA, Hoad CL, Koppenol W, Arnaudov LN, Blom WA, Pritchard SE, Stoyanov SD, Gowland PA. Aerated drinks increase gastric volume and reduce appetite as assessed by MRI: a randomized, balanced, crossover trial. Am J Clin Nutr. 2015;101:270-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Hussein MO, Hoad CL, Wright J, Singh G, Stephenson MC, Cox EF, Placidi E, Pritchard SE, Costigan C, Ribeiro H. Fat emulsion intragastric stability and droplet size modulate gastrointestinal responses and subsequent food intake in young adults. J Nutr. 2015;145:1170-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Steingoetter A, Radovic T, Buetikofer S, Curcic J, Menne D, Fried M, Schwizer W, Wooster TJ. Imaging gastric structuring of lipid emulsions and its effect on gastrointestinal function: a randomized trial in healthy subjects. Am J Clin Nutr. 2015;101:714-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Fruehauf H, Menne D, Kwiatek MA, Forras-Kaufman Z, Kaufman E, Goetze O, Fried M, Schwizer W, Fox M. Inter-observer reproducibility and analysis of gastric volume measurements and gastric emptying assessed with magnetic resonance imaging. Neurogastroenterol Motil. 2011;23:854-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Hoad CL, Parker H, Hudders N, Costigan C, Cox EF, Perkins AC, Blackshaw PE, Marciani L, Spiller RC, Fox MR. Measurement of gastric meal and secretion volumes using magnetic resonance imaging. Phys Med Biol. 2015;60:1367-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Banerjee S, Dixit S, Fox M, Pal A. Validation of a rapid, semiautomatic image analysis tool for measurement of gastric accommodation and emptying by magnetic resonance imaging. Am J Physiol Gastrointest Liver Physiol. 2015;308:G652-G663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Bharucha AE, Karwoski RA, Fidler J, Holmes DR, Robb RA, Riederer SJ, Zinsmeister AR. Comparison of manual and semiautomated techniques for analyzing gastric volumes with MRI in humans. Am J Physiol Gastrointest Liver Physiol. 2014;307:G582-G587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Baumann T, Kuesters S, Grueneberger J, Marjanovic G, Zimmermann L, Schaefer AO, Hopt UT, Langer M, Karcz WK. Time-resolved MRI after ingestion of liquids reveals motility changes after laparoscopic sleeve gastrectomy--preliminary results. Obes Surg. 2011;21:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Bickelhaupt S, Froehlich JM, Cattin R, Raible S, Bouquet H, Bill U, Patak MA. Software-supported evaluation of gastric motility in MRI: a feasibility study. J Med Imaging Radiat Oncol. 2014;58:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Guglielmo FF, Mitchell DG, O’Kane PL, Deshmukh SP, Roth CG, Burach I, Burns A, Dulka S, Parker L. Identifying decreased peristalsis of abnormal small bowel segments in Crohn’s disease using cine MR enterography: the frozen bowel sign. Abdom Imaging. 2015;40:1150-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Trung VN, Yamamoto H, Furukawa A, Yamaguchi T, Murata S, Yoshimura M, Murakami Y, Sato S, Otani H, Ugi S. Enhanced Intestinal Motility during Oral Glucose Tolerance Test after Laparoscopic Sleeve Gastrectomy: Preliminary Results Using Cine Magnetic Resonance Imaging. PLoS One. 2013;8:e65739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Bickelhaupt S, Froehlich JM, Wentz KU, von Weymarn C, Patak MA. Small-bowel dislocation during long-term MRI observation - insights in intestinal physiology. Clin Physiol Funct Imaging. 2015;35:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Hamy V, Dikaios N, Punwani S, Melbourne A, Latifoltojar A, Makanyanga J, Chouhan M, Helbren E, Menys A, Taylor S. Respiratory motion correction in dynamic MRI using robust data decomposition registration - application to DCE-MRI. Med Image Anal. 2014;18:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Menys A, Hamy V, Makanyanga J, Hoad C, Gowland P, Odille F, Taylor SA, Atkinson D. Dual registration of abdominal motion for motility assessment in free-breathing data sets acquired using dynamic MRI. Phys Med Biol. 2014;59:4603-4619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Odille F, Menys A, Ahmed A, Punwani S, Taylor SA, Atkinson D. Quantitative assessment of small bowel motility by nonrigid registration of dynamic MR images. Magn Reson Med. 2012;68:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Hahnemann ML, Nensa F, Kinner S, Gerken G, Lauenstein TC. Motility mapping as evaluation tool for bowel motility: initial results on the development of an automated color-coding algorithm in cine MRI. J Magn Reson Imaging. 2015;41:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Hahnemann ML, Nensa F, Kinner S, Maderwald S, Umutlu L, Gerken G, Lauenstein TC. Improved detection of inflammatory bowel disease by additional automated motility analysis in magnetic resonance imaging. Invest Radiol. 2015;50:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Sprengers AM, van der Paardt MP, Zijta FM, Caan MW, Lamerichs RM, Nederveen AJ, Stoker J. Use of continuously MR tagged imaging for automated motion assessment in the abdomen: a feasibility study. J Magn Reson Imaging. 2012;36:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | van der Paardt MP, Sprengers AM, Zijta FM, Lamerichs R, Nederveen AJ, Stoker J. Noninvasive automated motion assessment of intestinal motility by continuously tagged MR imaging. J Magn Reson Imaging. 2014;39:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Heye T, Stein D, Antolovic D, Dueck M, Kauczor HU, Hosch W. Evaluation of bowel peristalsis by dynamic cine MRI: detection of relevant functional disturbances--initial experience. J Magn Reson Imaging. 2012;35:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Farghal A, Kasmai B, Malcolm PN, Graves MJ, Toms AP. Developing a new measure of small bowel peristalsis with dynamic MR: a proof of concept study. Acta Radiol. 2012;53:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Wakamiya M, Furukawa A, Kanasaki S, Murata K. Assessment of small bowel motility function with cine-MRI using balanced steady-state free precession sequence. J Magn Reson Imaging. 2011;33:1235-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Bickelhaupt S, Froehlich JM, Cattin R, Raible S, Bouquet H, Bill U, Patak MA. Software-assisted small bowel motility analysis using free-breathing MRI: feasibility study. J Magn Reson Imaging. 2014;39:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Bickelhaupt S, Cattin R, Froehlich JM, Raible S, Bouquet H, Bill U, Patak MA. Automatic detection of small bowel contraction frequencies in motility plots using lomb-scargle periodogram and sinus-fitting method--initial experience. Magn Reson Med. 2014;71:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Bickelhaupt S, Froehlich JM, Cattin R, Raible S, Bouquet H, Bill U, Patak MA. Software-assisted quantitative analysis of small bowel motility compared to manual measurements. Clin Radiol. 2014;69:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Bickelhaupt S, Froehlich JM, Patak MA. Automated small bowel motility measurements in MRI using 2D coronal slices - does the intrasegmental location matter? A pilot study. Clin Imaging. 2015;39:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Menys A, Plumb A, Atkinson D, Taylor SA. The challenge of segmental small bowel motility quantitation using MR enterography. Br J Radiol. 2014;87:20140330. [PubMed] |
| 42. | Menys A, Taylor SA, Emmanuel A, Ahmed A, Plumb AA, Odille F, Alam A, Halligan S, Atkinson D. Global small bowel motility: assessment with dynamic MR imaging. Radiology. 2013;269:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Gee MS, Harisinghani MG. MRI in patients with inflammatory bowel disease. J Magn Reson Imaging. 2011;33:527-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Cullmann JL, Bickelhaupt S, Froehlich JM, Szucs-Farkas Z, Tutuian R, Patuto N, Dawson H, Patak MA. MR imaging in Crohn’s disease: correlation of MR motility measurement with histopathology in the terminal ileum. Neurogastroenterol Motil. 2013;25:749-e577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Bickelhaupt S, Pazahr S, Chuck N, Blume I, Froehlich JM, Cattin R, Raible S, Bouquet H, Bill U, Rogler G. Crohn’s disease: small bowel motility impairment correlates with inflammatory-related markers C-reactive protein and calprotectin. Neurogastroenterol Motil. 2013;25:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Menys A, Atkinson D, Odille F, Ahmed A, Novelli M, Rodriguez-Justo M, Proctor I, Punwani S, Halligan S, Taylor SA. Quantified terminal ileal motility during MR enterography as a potential biomarker of Crohn’s disease activity: a preliminary study. Eur Radiol. 2012;22:2494-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Hahnemann ML, Nensa F, Kinner S, Köhler J, Gerken G, Umutlu L, Lauenstein TC. Quantitative assessment of small bowel motility in patients with Crohn‘s disease using dynamic MRI. Neurogastroenterol Motil. 2015;27:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Steward MJ, Punwani S, Proctor I, Adjei-Gyamfi Y, Chatterjee F, Bloom S, Novelli M, Halligan S, Rodriguez-Justo M, Taylor SA. Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol. 2012;81:2080-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 49. | Menys A, Helbren E, Makanyanga J, Emmanuel A, Forbes A, Windsor A, Punwani S, Halligan S, Atkinson D, Taylor SA. Small bowel strictures in Crohn’s disease: a quantitative investigation of intestinal motility using MR enterography. Neurogastroenterol Motil. 2013;25:967-e775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Bickelhaupt S, Wurnig M, Boss A, Patak MA. Correlation between morphological expansion and impairment of intra- and prelesionary motility in inflammatory small bowel lesions in patients with Crohn’s disease - preliminary data. Eur J Radiol. 2014;83:1044-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Bickelhaupt S, Froehlich JM, Cattin R, Patuto N, Tutuian R, Wentz KU, Culmann JL, Raible S, Bouquet H, Bill U. Differentiation between active and chronic Crohn’s disease using MRI small-bowel motility examinations - initial experience. Clin Radiol. 2013;68:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Grand DJ, Beland MD, Machan JT, Mayo-Smith WW. Detection of Crohn’s disease: Comparison of CT and MR enterography without anti-peristaltic agents performed on the same day. Eur J Radiol. 2012;81:1735-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Gutzeit A, Binkert CA, Koh DM, Hergan K, von Weymarn C, Graf N, Patak MA, Roos JE, Horstmann M, Kos S. Evaluation of the anti-peristaltic effect of glucagon and hyoscine on the small bowel: comparison of intravenous and intramuscular drug administration. Eur Radiol. 2012;22:1186-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Ghobrial PM, Neuberger I, Guglielmo FF, Mitchell DG, Parker L, O’Kane PL, Roth CG, Deshmukh SP, Borowski A. Cine MR enterography grading of small bowel peristalsis: evaluation of the antiperistaltic effectiveness of sublingual hyoscyamine sulfate. Acad Radiol. 2014;21:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Ohkubo H, Kessoku T, Fuyuki A, Iida H, Inamori M, Fujii T, Kawamura H, Hata Y, Manabe N, Chiba T. Assessment of small bowel motility in patients with chronic intestinal pseudo-obstruction using cine-MRI. Am J Gastroenterol. 2013;108:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Ailiani AC, Neuberger T, Brasseur JG, Banco G, Wang Y, Smith NB, Webb AG. Quantifying the effects of inactin vs Isoflurane anesthesia on gastrointestinal motility in rats using dynamic magnetic resonance imaging and spatio-temporal maps. Neurogastroenterol Motil. 2014;26:1477-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Bickelhaupt S, Wurnig MC, Lesurtel M, Patak MA, Boss A. Quantitative in vivo analysis of small bowel motility using MRI examinations in mice--proof of concept study. Lab Anim. 2015;49:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Pritchard SE, Marciani L, Garsed KC, Hoad CL, Thongborisute W, Roberts E, Gowland PA, Spiller RC. Fasting and postprandial volumes of the undisturbed colon: normal values and changes in diarrhea-predominant irritable bowel syndrome measured using serial MRI. Neurogastroenterol Motil. 2014;26:124-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 59. | Sandberg TH, Nilsson M, Poulsen JL, Gram M, Frøkjær JB, Østergaard LR, Drewes AM. A novel semi-automatic segmentation method for volumetric assessment of the colon based on magnetic resonance imaging. Abdom Imaging. 2015;40:2232-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Kirchhoff S, Nicolaus M, Schirra J, Reiser MF, Göke B, Lienemann A. Assessment of colon motility using simultaneous manometric and functional cine-MRI analysis: preliminary results. Abdom Imaging. 2011;36:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Marciani L, Garsed KC, Hoad CL, Fields A, Fordham I, Pritchard SE, Placidi E, Murray K, Chaddock G, Costigan C. Stimulation of colonic motility by oral PEG electrolyte bowel preparation assessed by MRI: comparison of split vs single dose. Neurogastroenterol Motil. 2014;26:1426-1436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Hahn T, Kozerke S, Schwizer W, Fried M, Boesiger P, Steingoetter A. Visualization and quantification of intestinal transit and motor function by real-time tracking of 19F labeled capsules in humans. Magn Reson Med. 2011;66:812-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Hahn T, Kozerke S, Schwizer W, Fried M, Boesiger P, Steingoetter A. 19F MR imaging golden angle-based capsule tracking for intestinal transit and catheter tracking: initial in vivo experience. Radiology. 2012;265:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Hahn T, Kozerke S, Schwizer W, Fried M, Boesiger P, Steingoetter A. Real-time multipoint gastrointestinal 19-fluorine catheter tracking. Magn Reson Med. 2014;71:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 65. | Chaddock G, Lam C, Hoad CL, Costigan C, Cox EF, Placidi E, Thexton I, Wright J, Blackshaw PE, Perkins AC. Novel MRI tests of orocecal transit time and whole gut transit time: studies in normal subjects. Neurogastroenterol Motil. 2014;26:205-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Savarino E, Savarino V, Fox M, Di Leo G, Furnari M, Marabotto E, Gemignani L, Bruzzone L, Moscatelli A, De Cassan C. Measurement of oro-caecal transit time by magnetic resonance imaging. Eur Radiol. 2015;25:1579-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Curcic J, Sauter M, Schwizer W, Fried M, Boesiger P, Steingoetter A. Validation of a golden angle radial sequence (GOLD) for abdominal T1 mapping during free breathing: demonstrating clinical feasibility for quantifying gastric secretion and emptying. J Magn Reson Imaging. 2015;41:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Sauter M, Curcic J, Menne D, Goetze O, Fried M, Schwizer W, Steingoetter A. Measuring the interaction of meal and gastric secretion: a combined quantitative magnetic resonance imaging and pharmacokinetic modeling approach. Neurogastroenterol Motil. 2012;24:632-638, e272-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Kuyumcu S, Goetze O, Menne D, Treier R, Boesiger P, Fox M, Fried M, Schwizer W, Steingoetter A. Gastric secretion does not affect the reliability of the 13C-acetate breath test: A validation of the 13C-acetate breath test by magnetic resonance imaging. Neurogastroenterol Motil. 2013;25:176-e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Mudie DM, Murray K, Hoad CL, Pritchard SE, Garnett MC, Amidon GL, Gowland PA, Spiller RC, Amidon GE, Marciani L. Quantification of gastrointestinal liquid volumes and distribution following a 240 mL dose of water in the fasted state. Mol Pharm. 2014;11:3039-3047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 371] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 71. | Koziolek M, Grimm M, Garbacz G, Kühn JP, Weitschies W. Intragastric volume changes after intake of a high-caloric, high-fat standard breakfast in healthy human subjects investigated by MRI. Mol Pharm. 2014;11:1632-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 72. | Marciani L, Hall N, Pritchard SE, Cox EF, Totman JJ, Lad M, Hoad CL, Foster TJ, Gowland PA, Spiller RC. Preventing gastric sieving by blending a solid/water meal enhances satiation in healthy humans. J Nutr. 2012;142:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 73. | Marciani L, Pritchard SE, Hellier-Woods C, Costigan C, Hoad CL, Gowland PA, Spiller RC. Delayed gastric emptying and reduced postprandial small bowel water content of equicaloric whole meal bread versus rice meals in healthy subjects: novel MRI insights. Eur J Clin Nutr. 2013;67:754-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 74. | Placidi E, Marciani L, Hoad CL, Napolitano A, Garsed KC, Pritchard SE, Cox EF, Costigan C, Spiller RC, Gowland PA. The effects of loperamide, or loperamide plus simethicone, on the distribution of gut water as assessed by MRI in a mannitol model of secretory diarrhoea. Aliment Pharmacol Ther. 2012;36:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Baruffol C, Jordi J, Camargo S, Radovic T, Herzog B, Fried M, Schwizer W, Verrey F, Lutz TA, Steingoetter A. L-lysine dose dependently delays gastric emptying and increases intestinal fluid volume in humans and rats. Neurogastroenterol Motil. 2014;26:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Pritchard SE, Garsed KC, Hoad CL, Lingaya M, Banwait R, Thongborisute W, Roberts E, Costigan C, Marciani L, Gowland PA. Effect of experimental stress on the small bowel and colon in healthy humans. Neurogastroenterol Motil. 2015;27:542-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Murray K, Wilkinson-Smith V, Hoad C, Costigan C, Cox E, Lam C, Marciani L, Gowland P, Spiller RC. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. 2014;109:110-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 262] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 78. | Undseth R, Berstad A, Kløw NE, Arnljot K, Moi KS, Valeur J. Abnormal accumulation of intestinal fluid following ingestion of an unabsorbable carbohydrate in patients with irritable bowel syndrome: an MRI study. Neurogastroenterol Motil. 2014;26:1686-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |