Published online Aug 15, 2015. doi: 10.4291/wjgp.v6.i3.51
Peer-review started: February 1, 2015
First decision: March 6, 2015
Revised: March 27, 2015
Accepted: June 1, 2015
Article in press: June 2, 2015
Published online: August 15, 2015
Processing time: 200 Days and 22.9 Hours
Chronic alcohol abuse damages nearly every organ in the body. The harmful effects of ethanol on the brain, the liver and the pancreas are well documented. Although chronic alcohol consumption causes serious impairments also in the gastrointestinal tract like altered motility, mucosal damage, impaired absorption of nutrients and inflammation, the effects of chronically consumed ethanol on the enteric nervous system are less detailed. While the nitrergic myenteric neurons play an essential role in the regulation of gastrointestinal peristalsis, it was hypothesised, that these neurons are the first targets of consumed ethanol or its metabolites generated in the different gastrointestinal segments. To reinforce this hypothesis the effects of ethanol on the gastrointestinal tract was investigated in different rodent models with quantitative immunohistochemistry, in vivo and in vitro motility measurements, western blot analysis, evaluation of nitric oxide synthase enzyme activity and bio-imaging of nitric oxide synthesis. These results suggest that chronic alcohol consumption did not result significant neural loss, but primarily impaired the nitrergic pathways in gut region-dependent way leading to disturbed gastrointestinal motility. The gut segment-specific differences in the effects of chronic alcohol consumption highlight the significance the ethanol-induced neuronal microenvironment involving oxidative stress and intestinal microbiota.
Core tip: Chronic ethanol administration causes neurodegeneration in the central nervous system. In the enteric nervous system neurodegeneration was not demonstrated, however alcohol-induced quantitative, functional and neurochemical changes of nitrergic myenteric neurons were observed in gut region-dependent way. These suggest that disturbed gastrointestinal transit characteristic to alcoholic patients due to an impairment of a nitric oxide-mediated descending inhibition during peristalsis. The better understanding of the effects of chronic ethanol administration on enteric neurons may reveal new targets for therapy.
- Citation: Bagyánszki M, Bódi N. Gut region-dependent alterations of nitrergic myenteric neurons after chronic alcohol consumption. World J Gastrointest Pathophysiol 2015; 6(3): 51-57
- URL: https://www.wjgnet.com/2150-5330/full/v6/i3/51.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v6.i3.51
Alcoholism is one of the world’s leading risk factor for morbidity, disability and mortality. In 2012, 5.9% of all global deaths in the world were attributable to alcohol abuse. Chronic alcohol consumption is a component cause of more than 200 disease and injury conditions[1].
The majority of absorbed ethanol is metabolized in the liver, but disassembly of ethanol also occurs in the whole length of the gastrointestinal (GI) tract, including the oral cavity, the esophagus, the stomach, and the small and large intestines. Ethanol is metabolized oxidatively into acetaldehyde by alcohol dehydrogenase, by the microsomal ethanol oxidizing system cytochrome P4502E1, and by catalase in the peroxisomes[2,3].
Ethanol and its oxidative and non-oxidative metabolites can be found throughout the GI tract, where they can interfere with several functions, including the intestinal barrier[2], GI motility and absorption of nutrients[4-6].
Animal models are available to investigate alcohol-related diseases[7-20], however there is still a need for animal models resembling more the human condition. It is well documented that alcohol ingestion results neuroinflammation and neurodegeneration in humans and animals[19]. In the induction of neuronal apoptosis, oxidative stress plays an important role[21]. Toxic and metabolic effects of ethanol vary in brain regions, the most affected regions are the frontal lobes, the cortical limbic-circuits and the cerebellum. Skeletal muscle, and peripheral nerves are also important targets of chronic alcohol-related metabolic injury and degeneration[22,23].
In this review the effects of chronic ethanol consumption on the enteric nervous system (ENS) are highlighted, particular the changes in the quantitative properties of nitrergic myenteric neurons and related motility disturbances in the different parts of the GI tract.
In the ENS, nitric oxide (NO) plays a critical role in mediating non-adrenergic, non-cholinergic relaxation of the intestinal smooth muscle in a gut regionally different way[24-26]. High concentrations of ethanol are reached only in the duodenum and jejunum[5,27,28], however the concentration of ethanol reached in the ileum is not significantly different to the levels in the blood[27]. Therefore, the neuronal NO may be altered directly by the ethanol in the duodenum, while by the different oxidative and reductive metabolites in the different intestinal segments after chronic ethanol consumption. More findings provide evidence that effects of ethanol on NO system of intestinal relaxation[6,29] is responsible to the impaired motor function leading gut motility disorders[5,30,31]. NO is synthesized by the neuronal (n), endothelial (e) and inducible (i) nitric oxide synthases (NOSs)[32], and now, numerous investigations have already confirmed that all the NOSs are constitutively expressed in the myenteric neurons[33,34]. However, the effects of chronic alcohol intake on the density of nitrergic myenteric neurons, the amount of the three NOSs and/or their activity in different parts of the GI tract have been poorly investigated.
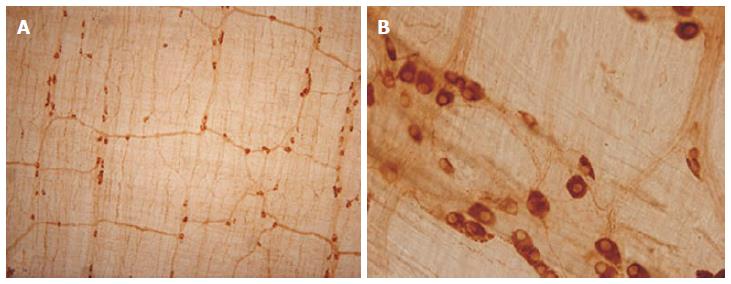
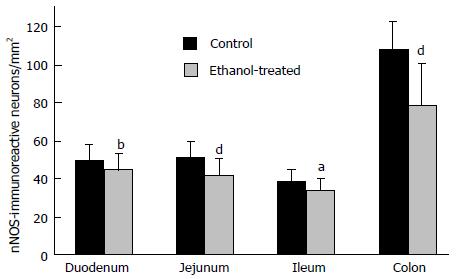
Therefore, in the last ten years we concentrated our research on the alcohol-induced alterations of nitrergic myenteric neurons in different gut regions[6,31,35,36]. We established a model suitable to study the NOS activities, protein content and the number of total and nNOS-immunoreactive myenteric neurons (Figure 1) in the duodenum, jejunum, ileum and colon of control and ethanol-exposed animals[35]. The activity of constitutive NOS (cNOS, both neuronal and endothelial) was 20 times higher in the proximal colon than in any part of the small intestine in control animals. Except of duodenum cNOS activity decreased significantly after chronic ethanol consumption. Under physiological conditions, the iNOS activity was also higher in the distal gut segments, but it did not change by the effects of ethanol. Similar results were observed in NOS protein content of tissue samples; the nNOS density of colonic fractions was more than twice as high as those of the samples prepared from the other gut segments and it also decreased after chronic ethanol consumption. The densities of eNOS-fractions were very weak and differences were not revealed in different intestinal samples and conditions. In intestinal whole-mount preparations from control rats, the number of nNOS-immunoreactive neurons was the highest in the colon. After ethanol exposure the decrease in the nitrergic cell number was significant in the whole length of the gut (Figure 2), however the greatest decrease in density of nitrergic neurons was observed in the colon[35]. The total number of myenteric neurons labelled with HuC/HuD pan-neuronal marker did not differ between controls and ethanol-drinking rats which suggest that chronic alcohol administration did not result in significant cell loss, but primarily impaired the nitrergic pathways in regionally different way.
Reduced number of nNOS-expressing neurons after chronic alcohol intake was also demonstrated in the murine jejunum[31] without changing in the total neuronal number. Both results indicate that chronic alcohol consumption leads to reduced nNOS expression resulting in motility disorders. In another study[6], the bio-imaging of basal NO synthesis of individual myenteric neurons was validated by loading the whole-mount preparations with the fluorescent indicator DAF-FM[37,38]. Based on DAF-FM recordings, chronic alcohol consumption induced a markedly increased basal NO synthesis in myenteric neurons as well as in glial cells or smooth muscle cells, indicates that chronic alcohol intake caused a general overproduction of NO in the jejunal gut wall. They confirmed reduced proportion of nNOS-expressing myenteric neurons and an increase of the proportion of iNOS-immunoreactive neurons was also revealed in murine small intestine[6]. Interestingly, the percentage of iNOS-containing neurons is in reasonable agreement with the measured percentages of neurons that produced NO but were not immunoreactive for nNOS. Others has also demonstrated that ethanol increased the amount of intestinal iNOS[36,39] and content of NO[39] in rats. Ethanol-induced NO overproduction appears to be relevant to the intestinal barrier dysfunction and alcoholic gut leakiness[40].
Besides myenteric neurons, the presence of the three NOSs shows characteristic cell type-specific distribution in enteric smooth muscle cells and capillary endothelium[34]. In accord with recent studies[41,42], we hypothesized that the presence of the three NOSs with similar functions in the same type of cells, the gut wall is able to adapt to different pathological conditions. To evaluate the possible rearrangement of the cellular and subcellular NOS compartments in response to chronic ethanol treatment post-embedding immuno-electron microscopy was used in different gut segments and cell types. Counting gold particles labelling different NOSs, the nNOS labels were in general the most numerous under normal conditions[36] which is in agreement with the finding of an earlier study[43] and strengthen that in the GI tract, nNOS is the main source of NO. However in the different intestinal segments and cellular compartments, well-pronounced differences were observed in the number of nNOS labels under physiological and alcoholic conditions. After chronic ethanol consumption, the numbers of nNOS labels are decreased in one intestinal segment and increased in another suggest significant differences in the microenvironment in different gut regions. Interestingly, the quantitative features of eNOS labels were changed in the opposite way to those in nNOS signing after ethanol intake. For example, while the number of nNOS gold particles decreased by more than 50% in the ganglia of duodenum, the eNOS labels approximately doubled here[36]. Depending on the investigated gut segment and type of NOS, a pronounced subcellular realignment of NOS labels was also found in ethanol-treated rats. The opposite alterations of eNOS and nNOS and subcellular rearrangement of NOS compartments may reflect a functional plasticity, in which different NOSs can replace each other to help maintenance the optimum NO level even under pathological condition.
Although the effects of ethanol consumption on gastrointestinal motility is well documented[44-49], even the opposite effects of acute and chronic administration of alcohol on GI transit have demonstrated, the mechanisms underlying impaired smooth muscle contractility are poorly understood and several conflicting data are present.
To reinforce the pathogenic role of NO in the ENS during chronic alcohol treatment, we investigated possible changes in the proportion of nitrergic myenteric neurons in relation to GI motility disturbances observed after chronic alcohol consumption in a murine model[31].
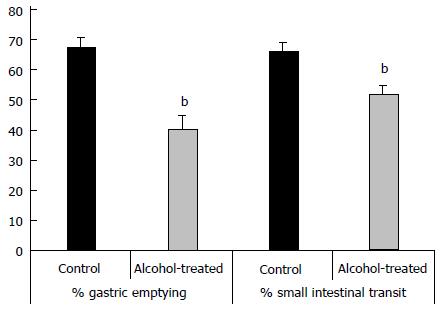
We demonstrated that chronic alcohol consumption affects gastric emptying and small intestinal transit in vivo (Figure 3). Migration of an Evans blue bolus throughout the stomach and small intestine was significantly delayed in chronic alcohol-treated mice when compared with controls receiving tap water. These findings point to an effect of chronic alcohol treatment on both stomach and small intestinal motility.
To elucidate whether this delay in intestinal transit could be associated with altered nitrergic relaxation of smooth muscle, we performed in vitro organ bath experiments. Jejunal muscle strips relaxed to electrical field stimulation (EFS) and these relaxations were mimicked by exogenous NO. Relaxations to EFS were blocked by the NOS inhibitor L-NNA, confirming that they are mediated by NO[50]. In chronic alcohol-treated mice, the nitrergic relaxations to EFS were significantly decreased, whereas those to increasing concentrations of exogenous NO did not differ between chronic alcohol-treated and control mice. This finding indicates that the effect exerted by chronic alcohol consumption on smooth muscle relaxation is not because of a defective responsiveness of the smooth muscle to NO but appears to originate from impaired nitrergic neuronal activity[31].
Recently Yazir et al[51] found that chronic alcohol consumption impairs relaxant and contractile responses of both esophageal tunica muscularis mucosae and lower esophageal sphincter smooth muscle. Similarly to our results they found decreased nNOS immunoreactivity in esophageal myenteric plexus in alcohol-exposed group compared to control groups[51].
It is well documented that the anatomical, functional, and pathological regionality of the gastrointestinal tract develops under strict genetic control[52,53], which itself result in the unique susceptibility of the neurons to pathological conditions in different intestinal segments. The gut region-specific neuronal damage demonstrated in rats with chronic ethanol consumption[6,31,35,36] indicates the importance of the molecular differences in the microenvironment of nitrergic neurons located in different gut segments[36]. It has recently evidenced that after chronic ethanol consumption, the three NOS isoforms were affected differentially not only in the myenteric neurons but also in mesenteric capillaries running in the vicinity of myenteric ganglia and smooth muscle cells[36]. Among the many factors that are implicated in this regionally distinct pathologic microenvironment of neurons in different gut segments, the intestinal microbiota got recently in the focus of research gastrointestinal diseases[54,55].
The composition of gut microbiome and the amount of bacteria is also unique along the oro-anal gut axis. The upper gastrointestinal tract does not harbour a rich of microbial concentration due to gastric acid, biliary and pancreatic secretion, while in the colon the highest density of bacterial community is found[56,57] with potential role in prevention, several metabolic activities and gut permeability[58-60]. Alcohol has been shown to increase in total number of bacteria in jejunum[61] and also result in duodenal bacterial overgrowth[62,63]. Others[64] also demonstrated that chronic ethanol feeding caused an increase in the abundance of the gram negative Proteobacteria including several pathogenic species and gram positive Actinobacteria, as well as resulted in a decline of Bacteroidetes and Firmicutes phyla. The balance in the composition of microbiome is critical to maintain gut homeostasis, therefore the breakdown of it associated with endotoxemia, lipopolysaccharides translocation and several immunological reactions[65]. Elevation of the growth of gram negative bacteria results in augmentation of lipopolysaccharides like endotoxins, a component of gram negative bacterial wall. Endotoxins release several pro-inflammatory cytokines from activated macrophages[66,67] which lead the alteration of intestinal barrier through disruption of tight junctions[68] and contribute to the progression of alcoholic liver disease, cirrhosis or alcoholic pancreatitis[55,69]. Increased gut permeability allows to endotoxins passing into the bloodstream creating harmful cycles[70]. However, Zhong et al[71] found that the gut leakiness after chronic alcohol exposure occurs in the ileum but not in the duodenum or jejunum. They also observed that alcohol exposure caused ROS accumulation in the small intestine with strongest labelling in the ileum. In parallel with oxidative stress, the zinc dyshomeostasis was also found gut region-specific as a consequence of ethanol exposure; the zinc status (an important trace element of all the major cell functions) was not affected in the duodenum and jejunum but significantly decreased in the ileum[71]. Besides oxidative stress, alcohol reactive metabolites also have been suggested to critically mediate alcohol-induced intestinal barrier dysfunction[72,73]. Acetaldehyde is produced in a high concentration through ethanol metabolism by bacterial alcohol dehydrogenase[74,75] mediated mainly by aerobic or facultative anaerobic bacteria in the colon[76-78]. Based on these findings, further investigations on the region-specific composition of gut microbiome and alcohol-related alterations of intestinal microbiota in different gut segments should be performed to reveal the underlying events.
Endogenous NO is largely involved in the regulation of gut motility, secretion and blood flow[79-81]. More findings provide evidence that nitrergic subpopulation of the myenteric neurons is especially susceptible to different pathological conditions[35,36,81-85]. Furthermore, the NOS neurons are more sensitive to damage than other enteric neurons[86]. Among the possible reasons involving NOS neurons in enteric neuropathies, intracellular Ca+ concentration is thought to be critical[87]. In stress, neurons cannot maintain the optimal intracellular Ca+ level. Elevated Ca+ activates NOSs result in excessive production of free radicals as NO or peroxynitrite which lead cytotoxicity of neurons[86,87]. It has also been demonstrated that impairment of nitrergic myenteric neurons after chronic ethanol consumption is strictly gut region-dependent[35,36], which emphasize the importance of neuronal microenvironment. Therefore, to reveal the region-specific structural and molecular differences along the whole length of GI tract is essential to outline new directions in the diagnosis and the therapies GI diseases in chronic alcoholism.
We thank our colleague and teacher, Professor Éva Fekete for her valuable comments on the manuscript.
P- Reviewer: Boros M, Chiba T S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 2. | Elamin EE, Masclee AA, Dekker J, Jonkers DM. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr Rev. 2013;71:483-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Seth D, D’Souza El-Guindy NB, Apte M, Mari M, Dooley S, Neuman M, Haber PS, Kundu GC, Darwanto A, de Villiers WJ. Alcohol, signaling, and ECM turnover. Alcohol Clin Exp Res. 2010;34:4-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Papa A, Tursi A, Cammarota G, Certo M, Cuoco L, Montalto M, Cianci R, Papa V, Fedeli P, Fedeli G. Effect of moderate and heavy alcohol consumption on intestinal transit time. Panminerva Med. 1998;40:183-185. [PubMed] |
| 5. | Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 261] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Bagyánszki M, Krecsmarik M, De Winter BY, De Man JG, Fekete E, Pelckmans PA, Adriaensen D, Kroese AB, Van Nassauw L, Timmermans JP. Chronic alcohol consumption affects gastrointestinal motility and reduces the proportion of neuronal NOS-immunoreactive myenteric neurons in the murine jejunum. Anat Rec (Hoboken). 2010;293:1536-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Siegmund S, Haas S, Schneider A, Singer MV. Animal models in gastrointestinal alcohol research-a short appraisal of the different models and their results. Best Pract Res Clin Gastroenterol. 2003;17:519-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Siegmund SV, Haas S, Singer MV. Animal models and their results in gastrointestinal alcohol research. Dig Dis. 2005;23:181-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Patten AR, Fontaine CJ, Christie BR. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front Pediatr. 2014;2:93. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Exp Clin Psychopharmacol. 2002;10:193-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 241] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 358] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 12. | Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1842] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 13. | Ding J, Eigenbrodt ML, Mosley TH, Hutchinson RG, Folsom AR, Harris TB, Nieto FJ. Alcohol intake and cerebral abnormalities on magnetic resonance imaging in a community-based population of middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2004;35:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Harper C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009;44:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 15. | Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 16. | Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-κB and proinflammatory cytokines. Alcohol Clin Exp Res. 2010;34:777-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 162] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 18. | Ponomarev I. Epigenetic control of gene expression in the alcoholic brain. Alcohol Res. 2013;35:69-76. [PubMed] |
| 19. | Szabo G, Lippai D. Converging actions of alcohol on liver and brain immune signaling. Int Rev Neurobiol. 2014;118:359-80. [RCA] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | González-Reimers E, Santolaria-Fernández F, Martín-González MC, Fernández-Rodríguez CM, Quintero-Platt G. Alcoholism: a systemic proinflammatory condition. World J Gastroenterol. 2014;20:14660-14671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 118] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 21. | Zou JY, Crews FT. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS One. 2014;9:e87915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | de la Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta Neuropathol. 2014;127:71-90. [RCA] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 23. | Bernardin F, Maheut-Bosser A, Paille F. Cognitive impairments in alcohol-dependent subjects. Front Psychiatry. 2014;5:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 24. | Takahashi T, Owyang C. Vagal control of nitric oxide and vasoactive intestinal polypeptide release in the regulation of gastric relaxation in rat. J Physiol. 1995;484:481-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Takahashi T, Owyang C. Regional differences in the nitrergic innervation between the proximal and the distal colon in rats. Gastroenterology. 1998;115:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Nakao K, Takahashi T, Utsunomiya J, Owyang C. Extrinsic neural control of nitric oxide synthase expression in the myenteric plexus of rat jejunum. J Physiol. 1998;507:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Bode JC. Alcohol and the gastrointestinal tract. Ergeb Inn Med Kinderheilkd. 1980;45:1-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Mezey E. Effect of ethanol on intestinal morphology, metabolism, and function. Seitz HK, Kommerell B, editors. Berlin: Springer 1985; 342-360. [DOI] [Full Text] |
| 29. | Wang SL, Xie DP, Liu KJ, Qin JF, Feng M, Kunze W, Liu CY. Nitric oxide mediates the inhibitory effect of ethanol on the motility of isolated longitudinal muscle of proximal colon in rats. Neurogastroenterol Motil. 2007;19:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Addolorato G, Montalto M, Capristo E, Certo M, Fedeli G, Gentiloni N, Stefanini GF, Gasbarrini G. Influence of alcohol on gastrointestinal motility: lactulose breath hydrogen testing in orocecal transit time in chronic alcoholics, social drinkers and teetotaler subjects. Hepatogastroenterology. 1997;44:1076-1081. [PubMed] |
| 31. | Bagyánszki M, Torfs P, Krecsmarik M, Fekete E, Adriaensen D, Van Nassauw L, Timmermans JP, Kroese AB. Chronic alcohol consumption induces an overproduction of NO by nNOS- and iNOS-expressing myenteric neurons in the murine small intestine. Neurogastroenterol Motil. 2011;23:e237-e248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1099] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 33. | Vannucchi MG, Corsani L, Bani D, Faussone-Pellegrini MS. Myenteric neurons and interstitial cells of Cajal of mouse colon express several nitric oxide synthase isoforms. Neurosci Lett. 2002;326:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Talapka P, Bódi N, Battonyai I, Fekete E, Bagyánszki M. Subcellular distribution of nitric oxide synthase isoforms in the rat duodenum. World J Gastroenterol. 2011;17:1026-1029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Krecsmarik M, Izbéki F, Bagyánszki M, Linke N, Bódi N, Kaszaki J, Katarova Z, Szabó A, Fekete E, Wittmann T. Chronic ethanol exposure impairs neuronal nitric oxide synthase in the rat intestine. Alcohol Clin Exp Res. 2006;30:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Bódi N, Jancsó Z, Talapka P, Pál A, Poles MZ, Bagyánszki M, Hermesz E, Fekete É. Gut region-specific rearrangement of the cellular and subcellular compartments of nitric oxide synthase isoforms after chronic ethanol consumption in rats. Histol Histopathol. 2014;29:1547-1555. [PubMed] |
| 37. | Nagano T, Yoshimura T. Bioimaging of nitric oxide. Chem Rev. 2002;102:1235-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 316] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 38. | Itoh Y, Ma FH, Hoshi H, Oka M, Noda K, Ukai Y, Kojima H, Nagano T, Toda N. Determination and bioimaging method for nitric oxide in biological specimens by diaminofluorescein fluorometry. Anal Biochem. 2000;287:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Wang C, Wang S, Qin J, Lv Y, Ma X, Liu C. Ethanol upregulates iNOS expression in colon through activation of nuclear factor-kappa B in rats. Alcohol Clin Exp Res. 2010;34:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol Clin Exp Res. 2009;33:1220-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Giaroni C, De Ponti F, Cosentino M, Lecchini S, Frigo G. Plasticity in the enteric nervous system. Gastroenterology. 1999;117:1438-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Giaroni C, Marchet S, Carpanese E, Prandoni V, Oldrini R, Bartolini B, Moro E, Vigetti D, Crema F, Lecchini S. Role of neuronal and inducible nitric oxide synthases in the guinea pig ileum myenteric plexus during in vitro ischemia and reperfusion. Neurogastroenterol Motil. 2013;25:e114-e126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Qu XW, Wang H, Rozenfeld RA, Huang W, Hsueh W. Type I nitric oxide synthase (NOS) is the predominant NOS in rat small intestine. Regulation by platelet-activating factor. Biochim Biophys Acta. 1999;1451:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Charles F, Evans DF, Castillo FD, Wingate DL. Daytime ingestion of alcohol alters nighttime jejunal motility in man. Dig Dis Sci. 1994;39:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Schmidt T, Eberle R, Pfeiffer A, Kaess H. Effect of ethanol on postprandial duodenojejunal motility in humans. Dig Dis Sci. 1997;42:1628-1633. [PubMed] |
| 46. | Addolorato G, Capristo E, Gasbarrini G, Stefanini GF. Depression, alcohol abuse and orocaecal transit time. Gut. 1997;41:417-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Lu G, Sarr MG, Szurszewski JH. Effects of ethyl alcohol on canine jejunal circular smooth muscle. Dig Dis Sci. 1997;42:2403-2410. [PubMed] |
| 48. | Izbéki F, Wittmann T, Csáti S, Jeszenszky E, Lonovics J. Opposite effects of acute and chronic administration of alcohol on gastric emptying and small bowel transit in rat. Alcohol Alcohol. 2001;36:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Teyssen S, Singer MV. Alcohol-related diseases of the oesophagus and stomach. Best Pract Res Clin Gastroenterol. 2003;17:557-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 50. | De Man JG, De Winter BY, Herman AG, Pelckmans PA. Study on the cyclic GMP-dependency of relaxations to endogenous and exogenous nitric oxide in the mouse gastrointestinal tract. Br J Pharmacol. 2007;150:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Yazir Y, Tugay M, Utkan Z, Utkan T. Effects of chronic ethanol consumption on rat upper gastrointestinal system: functional and histologic findings. Alcohol. 2012;46:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Gao N, White P, Kaestner KH. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell. 2009;16:588-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 299] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 53. | Doodnath R, Wride M, Puri P. The spatio-temporal patterning of Hoxa9 and Hoxa13 in the developing zebrafish enteric nervous system. Pediatr Surg Int. 2012;28:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Zhong W, Zhou Z. Alterations of the gut microbiome and metabolome in alcoholic liver disease. World J Gastrointest Pathophysiol. 2014;5:514-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Vonlaufen A, Spahr L, Apte MV, Frossard JL. Alcoholic pancreatitis: A tale of spirits and bacteria. World J Gastrointest Pathophysiol. 2014;5:82-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Wirth R, Bódi N, Maróti G, Bagyánszki M, Talapka P, Fekete É, Bagi Z, Kovács KL. Regionally distinct alterations in the composition of the gut microbiota in rats with streptozotocin-induced diabetes. PLoS One. 2014;9:e110440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Schoster A, Arroyo LG, Staempfli HR, Weese JS. Comparison of microbial populations in the small intestine, large intestine and feces of healthy horses using terminal restriction fragment length polymorphism. BMC Res Notes. 2013;6:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Guarner F. Enteric flora in health and disease. Digestion. 2006;73 Suppl 1:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 59. | Cummings JH, Beatty ER, Kingman SM, Bingham SA, Englyst HN. Digestion and physiological properties of resistant starch in the human large bowel. Br J Nutr. 1996;75:733-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 249] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 60. | Gordon JI, Hooper LV, McNevin MS, Wong M, Bry L. Epithelial cell growth and differentiation. III. Promoting diversity in the intestine: conversations between the microflora, epithelium, and diffuse GALT. Am J Physiol. 1997;273:G565-G570. [PubMed] |
| 61. | Bode JC, Bode C, Heidelbach R, Dürr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30-34. [PubMed] |
| 62. | Bode C, Kolepke R, Schäfer K, Bode JC. Breath hydrogen excretion in patients with alcoholic liver disease--evidence of small intestinal bacterial overgrowth. Z Gastroenterol. 1993;31:3-7. [PubMed] |
| 63. | Gabbard SL, Lacy BE, Levine GM, Crowell MD. The impact of alcohol consumption and cholecystectomy on small intestinal bacterial overgrowth. Dig Dis Sci. 2014;59:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 64. | Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8:e53028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 427] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 65. | Malaguarnera G, Giordano M, Nunnari G, Bertino G, Malaguarnera M. Gut microbiota in alcoholic liver disease: pathogenetic role and therapeutic perspectives. World J Gastroenterol. 2014;20:16639-16648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 66. | Fleming S, Toratani S, Shea-Donohue T, Kashiwabara Y, Vogel SN, Metcalf ES. Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcohol Clin Exp Res. 2001;25:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605-G611. [PubMed] |
| 68. | Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276:G965-G974. [PubMed] |
| 69. | Gratz SW, Mykkanen H, El-Nezami HS. Probiotics and gut health: a special focus on liver diseases. World J Gastroenterol. 2010;16:403-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881-G884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 71. | Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010;298:G625-G633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 72. | Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 73. | Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 74. | Seitz HK, Oneta CM. Gastrointestinal alcohol dehydrogenase. Nutr Rev. 1998;56:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Visapää JP, Tillonen J, Salaspuro M. Microbes and mucosa in the regulation of intracolonic acetaldehyde concentration during ethanol challenge. Alcohol Alcohol. 2002;37:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Hill MJ, Drasar BS. The normal colonic bacterial flora. Gut. 1975;16:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Jokelainen K, Siitonen A, Jousimies-Somer H, Nosova T, Heine R, Salaspuro M. In vitro alcohol dehydrogenase-mediated acetaldehyde production by aerobic bacteria representing the normal colonic flora in man. Alcohol Clin Exp Res. 1996;20:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Visapää JP, Jokelainen K, Nosova T, Salaspuro M. Inhibition of intracolonic acetaldehyde production and alcoholic fermentation in rats by ciprofloxacin. Alcohol Clin Exp Res. 1998;22:1161-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990;345:346-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 745] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 80. | Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 260] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 81. | Barocelli E, Ballabeni V, Ghizzardi P, Cattaruzza F, Bertoni S, Lagrasta CA, Impicciatore M. The selective inhibition of inducible nitric oxide synthase prevents intestinal ischemia-reperfusion injury in mice. Nitric Oxide. 2006;14:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Takahashi T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J Gastroenterol. 2003;38:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 83. | Cellek S, Qu W, Schmidt AM, Moncada S. Synergistic action of advanced glycation end products and endogenous nitric oxide leads to neuronal apoptosis in vitro: a new insight into selective nitrergic neuropathy in diabetes. Diabetologia. 2004;47:331-339. [PubMed] |
| 84. | Izbéki F, Wittman T, Rosztóczy A, Linke N, Bódi N, Fekete E, Bagyánszki M. Immediate insulin treatment prevents gut motility alterations and loss of nitrergic neurons in the ileum and colon of rats with streptozotocin-induced diabetes. Diabetes Res Clin Pract. 2008;80:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 85. | Talapka P, Nagy LI, Pál A, Poles MZ, Berkó A, Bagyánszki M, Puskás LG, Fekete É, Bódi N. Alleviated mucosal and neuronal damage in a rat model of Crohn’s disease. World J Gastroenterol. 2014;20:16690-16697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 86. | Rivera LR, Poole DP, Thacker M, Furness JB. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol Motil. 2011;23:980-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 87. | Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death. Annu Rev Pathol. 2006;1:405-434. [PubMed] |