INTRODUCTION
Inflammatory bowel disease (IBD) is a phrase widely used to describe a diverse group of chronic inflammatory conditions of the gastrointestinal tract, including of the colon and small intestine. The major types of IBD can be characterized as Crohn’s disease (CD) and ulcerative colitis (UC), and about 10%-15% of the patients are diagnosed as having indeterminate colitis (IC). IC is typically exemplified by clinical, endoscopic and histological findings comprising elements which classically characterize those of both CD and UC. However, the differentiating characteristics between CD and those of UC are usually obvious, the main difference between these two conditions being the location and type of inflammatory changes. Thus, although CD and UC are both the result of an inflammatory condition of the gut they are clearly distinguished by the location of the disease and by disease conduct. CD normally affects any part of the gastrointestinal tract (GI), from mouth to anus, with any number of “skip areas” representing macroscopically and microscopically normal mucosa. Furthermore, the inflammation in CD is typically transmural, extending from the mucosa to the serosa, occasionally associated with granulomas, and may affect all of the layers of the bowel wall. The transmural inflammation may lead to perforating, fistulazing complications and to a stricturing disease. In sharp contrast, UC is restricted to the colon and rectum areas while the inflammation in UC is normally only restricted to the mucosal layer of the colon[1].
The etiologies of IBD remain unclear and appear to involve interplay between genetic susceptibility, environmental factors and the immune system. It should be noted that the intestinal epithelium and the enteric immune system act as the main “defense” barriers between the gastrointestinal tract, enteric bacteria, food antigens and noxious compounds that pass through it. Therefore, the gut mechanism of “defense” comprises the epithelial cell layer, mucus-secreting goblet cells, lymphoid tissue such as Peyer’s patches, nonimmune system mediators and cells of the immune system including the innate and the adaptive cells and their secreted mediators. The chronic stimulation by the intestinal microbiota and food antigens requires tight control mechanisms. An activation of the innate immune response and excessive inflammatory reaction or impaired innate immune response (i.e, NOD2, ATG16L1) can lead to the development of IBD. It is thought that alterations in the gut microbial environment also contribute to inflammatory bowel disease either by causing inflammation or by altered the immune system[1-5].
In 20%-25% of IBD patients, the disease has developed early in life, i.e., in childhood and adolescence, about 80% of the pediatric patients are diagnosed in adolescence (i.e., 10-18 years old children). The incidence of CD continues to increase worldwide. CD has a higher incidence in industrialized countries and higher rates have been noted in countries residing in the north and west as compared to countries residing in the south and east.
CD in children and adolescents can exemplify many similarities to the disease in adults, however, there may be clinical and pathological findings that are specific to children/adolescents as compared to adults. This can lead to different and specific treatment options. For instance, there is a difference between young children, aged less than 10 years, and adolescents in the location of the disease. In younger children the disease is more prevalent as isolated colonic inflammation while in adolescents the disease is more commonly detected in the terminal ileum and ileocecal regions. Young children and young adolescents have the potential for growth impairment due to the chronic inflammation and the low food intake. Furthermore, children and adolescents with CD experience greater levels of distress, emotional stress and the disease impacts their psychological well-being and reduces their quality of life. Quality of life is typically reduced due to pain, vomiting, diarrhea, and other socially unacceptable symptoms.
In pediatric CD patients, the main objective of therapy has been to induce and maintain clinical remission while optimizing the patient’s growth and well-being. Every effort needs to be made to choose a therapy that is effective with minimal adverse effect and easiest to use. The appropriate treatment plan should be based on disease activity and severity, disease location (i.e., in the gastrointestinal tract), phenotypic behavior, the patient’s linear growth and the patient’s psychosocial condition. After remission is achieved, the patient is usually switched to a lighter therapy with fewer potential side effects. However, disease flare-ups may occur any time between several weeks to several years resulting in an acute reappearance of disease symptoms. Depending on the situation, the flare-up may disappear on their own or may require a more intense therapy.
The treatment options summarized herein are used by pediatric gastroenterologists and are extrapolated from adult trials. As such, most doses and dosing interval are based on pharmacokinetic in adults and may be slightly different in children and adolescents. Furthermore, the therapeutic recommendations depend on the disease conduct, location, severity and related complications and further should take into consideration the type of treatment required, e.g., treatment of an acute disease, induction of a clinical remission or maintenance of remission.
5-AMINOSALICYLATE AGENTS
5-aminosalicylic acid (5-ASA) agents are used often to manage mild to moderate cases of CD. They are currently considered by many physicians as first line therapy in cases of mild to moderate CD and in maintenance of disease remission. Conversely, 5-ASA agents are less effective in induction of remission as illustrated in randomized double blind, placebo controlled studies which did not illustrate any advantage of using 5-ASA over placebo[6]. Moreover a Cochrane review published in 2011 illustrated the modest efficacy of sulfasalazine as compared to placebo in treatment of mild to moderate CD[7]. Furthermore, only two clinical trials have been carried out with 5-ASA in children[8,9] and the dosing in pediatric patients is thus extrapolated from adult treatment. The most convincing evidence for a beneficial treatment with 5-ASA has been the use of sulfazaline in the treatment of active CD involving the colon[10]. It should be noted, however, that 5-ASA are frequently being used as they are considered to have less adverse events and have some protective effect against cancer.
5-ASA exploits their therapeutic effect within the lumen of the gastrointestinal tract and is believed to have multiple anti-inflammatory effects. As unprotected 5-ASA is rapidly absorbed in the upper gastrointestinal tract, several slow release preparations have been developed to permit entrance of 5-ASA to the terminal ileum and to the colon. Currently there are several oral preparations of 5-ASA agents which differ from each other in the location of their therapeutic efficacy. These agents include pH-dependent delayed release formulations (e.g., Mesalamine), slow release formulations and azo-bound pro-drugs (e.g., Sulfasalazine), the later needing colonic bacteria to break the azo-bound drug and “activate” the drug. Azo-bound pro-drugs should mainly be used in patients with predominantly colonic disease. Furthermore, topical formulations (e.g., suppository and enema) can be used to treat or control mucosal inflammation in proctitis[11].
ANTIBIOTICS
Alterations in the gut microbial environment are believed to be one of the contributing factors to CD and to flare-ups. This has lead to the use of antibiotic therapy in mild to moderate disease[12]. Antibiotic therapy is frequently used for the treatment of perianal disease, e.g., fistulae, and abscess. The two main antibiotics currently being used for treatment of acute CD and flare-ups of CD comprise Metronidazole (10-20 mg/kg per day) and Ciprofloxacin (20 mg/kg per day), either as monotherapy or as combination therapy. In 2011 Kahan et al[13] published a systemic review and meta-analysis wherein they determined that treatment with antibiotics is superior to placebo in patients with active CD. Metronidazole may be more effective in patients with active colonic involvement[14] while Ciprofloxacin may be effective in the treatment of ileitis. Furthermore, a combination of the two antibiotics may be preferentially used for a better efficacy.
In the long run, however, the clinical efficacy of antibiotics appears to be inadequate, and the majority of patients will experience flares in their disease after discontinuing antibiotic therapy[15]. Furthermore, it is not expected that antibiotic therapy will result in a successful remission in patients with moderate to severe CD.
SYSTEMIC CORTICOSTEROIDS AND BUDESONIDE
Corticosteroids comprise a general potent anti-inflammatory effect for the treatment of CD. The mechanism of action of corticosteroids remains to be determined, however, it is generally accepted that corticosteroids attach to a cell’s cytoplasmic receptor, as a complex they enter the nuclease to interact with glucocorticoid response components on the chromosomal DNA. Corticosteroids comprise a wide variety of anti-inflammatory influences including inhibition of the proliferation and recruitment of monocytes, macrophages and lymphocytes; inhibition of migration of neutrophils to sites of inflammation; and decreased production of inflammatory mediators including prostaglandins, cytokines and leukotrienes[16].
In CD patients, systemic corticosteroids are considered to be very effective in the treatment of active disease and in achieving clinical remission. Corticosteroids continue to be the basic treatment to control acute disease that has not responded to first line therapy (e.g., antibiotics and 5-ASA)[17]. As previously reported by Bousvaros et al[18], only a minority of patients responding clinically to corticosteroid therapy exhibit endoscopic mucosal healing. A paper published by Canani in 2006 teaches endoscopic improvement in 4 of 10 children treated with steroids however, none of the treated children showed mucosal healing[19]. Oral therapy (with prednisone) is typically initiated at a dose of 1-2 mg/kg per day with a maximal daily dose of 40-60 mg/d for 2 to 4 wk. Conventional corticosteroid therapy is commonly used for short term treatment of moderate to severe symptoms and is typically used for achieving a quick relief of symptoms. It is important to minimize the use of corticosteroids as long term use results in adverse effects including hypertension, glucose intolerance, bone osteopenia, cataracts, decreased linear growth and increased risk of infections. Therefore, once the patient has improved clinically the daily corticosteroid dose should be reduced slowly over an 8 to 10 wk period until complete discontinuation. The induction and the tapering time of steroids relies on the physician’s experience and the patient’s respond (as illustrated clinically and in laboratory tests)[20,21].
About 10%-20% of moderate to severe CD patients will not respond to oral corticosteroids and will need parenteral administration. In severe cases of CD, parenteral administration should be immediately recommended. Furthermore, approximately 30% of CD patients become dependent on corticosteroid treatment and dose reduction results in clinical flares[21,22]. Thus, alternative therapies should be considered to replace corticosteroid use.
Budesonide is a glucocorticoid steroid that demonstrates a high affinity for the intestinal glucocorticoid receptor, enhances hepatic first-pass metabolism and lowers the systemic corticosteroid absorption[23]. Budesonide is known for its lower risk of systemic corticosteroid related complications due to its high topical activity and low systemic bioavailability (10%)[24]. Furthermore, budesonide is efficient in the treatment of mild to moderate CD locating to the ileum and/or ascending colon[10]. The starting dose of oral budesonide is 9 mg/d and it is typically reduced by 3 mg increments (i.e., to 6 mg/d followed by 3 mg/d) over several weeks. A Cochrane systemic review evaluating the efficacy of budesonide illustrated budesonide to be superior to placebo and mesalamine in induction of remission in CD patients after 8 wk of treatment[25]. In two pediatric trials the efficacy of budesonide in induced remission was 42%-55%[26,27]. Previous work illustrated that administration of a daily dose of 6 mg budesonide was not efficient in preventing relapse and was only efficient in maintaining remission for a period of several months[28]. Moreover, data from several small studies comparing treatment with prednisone to budesonide illustrated no superiority of budesonide over traditional corticosteroids[26,29,30]. In fact, prednisone may be more effective for inducing remission in patients who failed budesonide therapy. Therefore, the use of budesonide should be recommended in specific CD cases.
NUTRITION
Gut rest and total parenteral nutrition (TPN) were discovered to be efficient for improved nutritional status and induced remission in children with CD[31]. Children with CD are at high risk of nutrition malabsorbtion and weight loss, therefore, special nutrition has two goals: first, to improve malabsorption and gain weight, and second, exclusive enteral nutrition (EEN) can induce remission in active disease. EEN is a special liquid formula which is administered as a sole nutrition without the addition of a regular diet. The EEN formula may contain whole proteins (polymeric) or modified proteins (e.g., elemental). The advantage of the polymeric formula over the elemental formula is the better compliance and less need for using nasogastric tube.
EEN can be administered at first diagnosis of CD[32], but may also be considered as a mode of treating CD relapse patients, however, its efficiency tends to decrease. In many pediatric European centers, children with CD are treated with EEN as the first line treatment, however, the use of EEN in North America is less common[33]. There are a few trials comparing EEN to steroids in induction of remission in CD. Their results illustrate that the rate of remission and mucosal healing with EEN is about 75%[20,34]. Some of these trials also demonstrate significantly higher mucosal healing with EEN as compared to steroids[19,35]. Wilschanski et al published in 2006 that nutritional therapy has a role in maintaining disease remission in pediatric patients with CD. The duration of remission is controversial, some trials show relapse after three months and others demonstrate remission for 2 years. It is advisable to initiate an immunomodulatory treatment early or shortly after EEN to maintain remission[36]. The duration of exclusive feeding as treatment for induction of remission is typically 6 to 8 wk[37]. Towards the end of the exclusive feeding period, reintroduction of regular diet should be started gradually over a period of several weeks. However, if exclusive nutrition does not induce clinical improvement after 2 wk or if disease activity is aggravated it is advisable to initiate other treatment options.
Monitoring nutrition is an important part of handling children with CD. As mentioned, children with CD, are at high risk of nutrient malabsorbtion and growth impairment. Deficiencies of iron, vitamin B12, folic acid, vitamin D, zinc, impaired calcium absorption all need to be monitored. Many children with CD need enhanced caloric intake due to disease activity, and therefore may need about 120% reference nutrient intake to overcome nutrient deficiencies and for normal growth[38]. Furthermore, administration of any deficient micronutrient and/or daily multivitamin supplements is advisable in all pediatric CD patients.
IMMUNOMUDOLATOR AGENTS
Following an induction of remission with EEN or with corticosteroids, as mentioned above, maintaining remission may be carried out by administration of immunomodulatory drugs.
Azathioprine and 6-mercaptopurine
The immunomodulatory drugs azathioprine (AZA) and 6-mercaptopurine (6-MP) are closely related chemical compounds classified as thiopurine anti-metabolites. AZA is a prodrug that is quickly converted to 6-MP via a nonenzymatic reaction following administration. 6-MP is subsequently metabolized to its active metabolite 6-thioguanine (6-TG) through a series of reactions.
The clinical effects of AZA and 6-MP are probably indistinguishable, although their exact form of action is still unclear. Both AZA and 6-MP inhibit inflammatory response in several ways including inhibition of pathways in the nucleic acid biosynthesis and by causing damage to DNA through the integration of thiopurine analogues. AZA and 6-MP are further known to alter lymphocyte function, interfere with clonal expansion, reduce the number of lamina propria plasma cells and affect natural killer cell function[39].
AZA and 6-MP are both slow acting drugs, which is why clinical efficacy cannot be expected until 8 to 12 wk or even months after initiation of treatment. After induction of remission with prednisone, the use of AZA and 6-MP demonstrated maintained remission in a higher rate compared to placebo in CD patients. In the known trial of Markowitz et al[40], adolescents who were randomized to 6-MP after induction of remission by steroids, were more likely to remain in steroid free remission with 6-MP maintenance treatment over an eighteen months follow-up than the placebo group. Also the relapse rate was significantly lower with 6-MP as compared to placebo treatment. A Cochrane review assessing seven trials of AZA illustrate that the use of AZA is associated with decreased rates of hospitalization and surgery and with decreased use of corticosteroids. Moreover, higher doses of AZA (2.5 mg/kg per day) were more effective than lower dose of AZA (1 mg/kg per day or 2 mg/kg per day) for decreased flare-ups of the disease[41]. Today, AZA and 6-MP are the most commonly used immunomodulatory drugs in the treatment of CD due to their steroid sparing effect and in maintained remission.
The general guidelines for weight-based dosing of thiopurines, based on clinical trials, demonstrated efficacy for AZA at 2-2.5 mg/kg per day and 6-MP at 1-1.5 mg/kg per day. It is recommended to initiate therapy at low doses and escalate dosing within several weeks.
Severe adverse effects of AZA and 6-MP include bone marrow suppression, leukopenia, acute pancreatitis and hepatitis. In addition, other adverse effects include dizziness, diarrhea, fatigue, skin rashes and increased risk of infections. Therefore, regular blood count checkups are advisable during treatment. If leukopenia occurs or if there is an increase in liver function test, discontinuation or tapering AZA/6-MP treatment is recommended until white blood cell count and liver enzymes normalize. Then, treatment should commence with lower dose. In cases of pancreatitis, it is recommended to discontinue treatment and alternate to other treatment options. Other serious but rare side effects of long term AZA and 6-MP treatment include the development of malignancies including the uncommon Hepatosplenic T cell lymphoma (HSTCL) and non Hodgkin lymphoma[42]. In a single center long-term follow-up, patients with CD who responded to AZA had an increased risk of malignancies twice that of patients who did not receive AZA[43].
AZA and 6-MP are prodrugs that metabolize to the active drug, while thiopurine S-methyltransferase (TPMT) is a rate limiting enzyme involved in AZA/6-MP metabolism. Genetic polymorphisms of TPMT lead to decreased methylation and decreased inactivation of 6-MP which can result in life threatening bone marrow suppression. An assay of TPMT in red blood cells or a TPMT gene test can avoid this complication. Therefore, it is most advisable to check the TPMT enzyme levels or activity prior to initiation of treatment with AZA or 6-MP. If assessing TPMT enzyme levels or activity is not possible, one should start with low dose AZA/6-MP and check complete blood count within seven days of treatment and increase the dose gradually every 1 to 2 wk[44,45].
Combination therapy comprising AZA/6-MP with 5-ASA can be used to increase treatment efficacy. Specifically, 5-ASA therapy can inhibit TPMT activity and thereby increase the potential for 6-TG[46].
AZA and 6-MP are among a small number of treatments of CD that have been demonstrated to clinical remission as well as induce mucosal healing[47].
Methotrexate
The immunomodulatory drug methotrexate (MTX) is an antimetabolite and antifolate drug. MTX is a folic acid antagonist that inhibits purine synthesis, DNA and RNA formation, and eventually inhibits the S phase of the cell cycle. MTX also has multiple anti-inflammatory effects including decreased pro-inflammatory cytokine production and lymphocyte apoptosis.
MTX has been used as an immunomodulatory drug in the treatment of several other autoimmune disorders including lupus and rheumatoid arthritis. It is still not widely used for the treatment of CD mostly due to the need for parenteral administration (intramuscular or subcutaneous) and due to concerns about it’s possible toxicity (e.g., liver fibrosis, hypersensitivity pneumonitis, and teratogenicity). However, while thiopurines remain the most widely used immunomodulatory drugs, there is a need for alternative drugs such as MTX, especially for the 10%-20% of patients who cannot tolerate these drugs.
In placebo-controlled studies in adult CD patients, remission and maintenance was achieved after MTX administration as a dose of 25 mg intramuscularly per week (for remission) and 15 mg intramuscularly per week for maintenance. Significantly more remained in remission on MTX (65%) as compared to placebo (39%)[48-50]. Deducing these adult studies to pediatric patients, MTX should be administered at a dose of 15 mg/m2 per week with a maximum of 25 mg per week. A few pediatric trials demonstrated induction and maintenance of remission of 40%-60% at six months and at a lower rate at twelve months[51-53]. In a retrospective analysis, Turner et al[54] demonstrated that 42% of 60 patients that did not respond to AZA/6-MP achieved and maintain remission when administered MTX therapy.
MTX can be administered by an intramuscular injection or subcutaneously, the effective dose of MTX is 15 mg/m2 weekly with a maximal dose of 25 mg. Supplement of 5 mg folate 2 d after administration of MTX is advisable to reduce the adverse events[50].
Typically patients respond to parenteral MTX treatment within 3 to 4 wk and treatment should continue for at least four to six months. Responders to treatment may then alternate to oral dosing[52], and the lowest effective dose should be maintained. However, if patients relapse on oral treatment, it is advisable to switch from oral treatment back to parenteral administration or alternatively to increase dosing[53].
In low doses, MTX is generally a safe and well tolerated drug. Some side effects such as fatigue, hair loss, diarrhea, nausea, anorexia, headaches, and skin pigmentation are common. However adverse effects, which should be monitored more closely, include bone marrow toxicity, hepatitis and pulmonary disease. Supplemental folate can diminish the severity and the incidence of these adverse effects[54].
BIOLOGIC AGENTS (ANTI-TUMOR NECROSIS FACTOR THERAPY)
Tumor necrosis factor (TNF), also known as TNF-α, is a monocyte-derived pro-inflammatory cytokine that has diverse pro-inflammatory effects within the intestinal mucosa and is a pivotal cytokine in the inflammatory cascade. TNF-α been implicated in various autoimmune diseases including rheumatoid arthritis and psoriasis and has emerged as a key cytokine involved in pathogenesis of CD. Consequently, constant efforts have been made to control TNF-α’s harmful effects in CD. Anti-TNF antibodies neutralize this pro-inflammatory cytokine and thus interrupt the inflammatory cascade.
So far two anti-TNF agents have been approved by the FDA and EMA for the induction of remission and maintenance of remission in CD: infliximab (IFX) and adalimumab (ADA). These monoclonal antibodies differ in their structure and in their mode of administration.
IFX (trade name Remicade) is a chimeric IgG1 monoclonal antibody against TNF-α comprising 75% human and 25% murine sequences. IFX is typically used for the treatment of patients with moderately to severely acute CD and patients with fistulizing CD, who have had an inadequate response to conventional therapy. IFX is also recommended as primary induction therapy for patients with penetrating disease.
ADA (trade name Humira) is a complete human recombinant human IgG1 monoclonal antibody directed against TNF-α. ADA is typically used to decrease signs and symptoms of CD, and to achieve and maintain clinical remission in patients with moderate to severe acute luminal CD who have not responded well to conventional treatments. ADA is also used to treat patients who have lost response to IFX, are unable to tolerate or have an allergic to IFX.
ACCENT I, a large multicenter randomized, double blind trial, clearly demonstrated a statistically significant improvement in the response and remission rates of adult patients who were treated with a scheduled IFX maintenance therapy. These patients further showed a higher rate of free steroid remission at 54 wk and fewer hospitalizations and surgeries[55,56]. In adults with moderate to severe CD it was demonstrated that IFX is effective in induction and maintenance of remission[57]. In pediatric patients, a multi-center international study (REACH) demonstrated that 88% of children with acute CD who were treated with IFX achieved response and 60% were in clinical remission at 54 wk. REACH study also demonstrated the efficacy of IFX in maintaining remission in patients receiving IFX every 8 wk[58].
IFX is administered via an intravenous infusion at a dose of 5 mg/kg at zero, 2 and 6 wk followed by maintenance infusions every 8 wk. Dose of IFX may be increased to 10 mg/kg as needed. Dose escalation (to 10 mg/kg) and/or interval shortening (from 8 wk to 6 wk or 4 wk) may be implemented as needed[59].
In adults, the efficacy of ADA was first evaluated in the CLASSIC I study. This study demonstrated that ADA can be used for the induction of remission in moderate to severe CD[59]. The CLASSIC II study demonstrated clinical remission at 56 wk in 88% of patients who maintained ADA treatment every other week[60], similar results have been demonstrated in the CHARM study[61,62]. In pediatrics patients, a few studies demonstrate the efficacy of ADA in inducing and maintaining remission in acute CD. The efficacy of ADA treatment was also illustrated after treatment failure with IFX[57,63-65].
ADA is administered via subcutaneous injection at an initial injection of 160 mg followed by a 80 mg dose given 2 wk later, initiation of maintenance is administered 2 wk later at a dose of 40 mg followed by maintenance treatment every 2 wk at a dose of 40 mg.
Typically patients respond to anti-TNF therapy within a few days to 3 wk. It should be noted that therapy with anti-TNF antibodies, IFX and ADA, has been proven effective in achieving mucosal healing with a more rapid effect compared to immumomodulants.
Adverse effects of anti-TNF therapy, including IFX and ADA, include increased risk for serious infections including hepatitis, tuberculosis (TB) and unusual infections caused by viruses, fungi, bacteria, and other opportunistic infections. Anti-TNF therapy is also associated with allergic reactions and new or worsening psoriasis. Anti-TNF therapy also increases the risk of lymphoma, such as the extremely rare HSTCL, in adolescents and young adults primarily in male patients. The risk of HSTCL increases in patients being treated with anti-TNF therapy in combination with thiopurines (e.g., azathioprine and/or mercaptopurine). Therefore, the benefits of such a combination therapy must be weight against the risk of HSTCL in young patients, especially in males[65-67].
Due to these adverse effects, every patient must be tested before initiation of anti-TNF therapy for hepatitis B, tuberculosis (TB) and varicella. It is advisable to begin anti-TNF therapy only after proper immunization of the patients.
It is advisable that patients with sustained remission will continue with their scheduled anti-TNF therapy. In case the patients were previously immunemodulator naïve they may be switched to an immunomodulatory drug. Moreover, discontinuing anti-TNF therapy may be considered in cases of longstanding sustained remission.
As mentioned, ADA and IFX are the only two biological drugs which were approved for the treatment of Crohn’s disease in pediatric patients. Currently there are two additional biological drugs that have been used off-label for the treatment of Crohn’s disease in patients who do not respond to ADA and IFX.
The first of these off-label drugs is Certolizumab pegol (CZP) (trade name Cimzia), a monoclonal antibody to TNF-α, which comprises the Fab portion of the antibody conjugated to a polyethylene glycol (PEG) moiety, which prolongs the drug’s half-life. The fact that Certolizumab pegol lacks an Fc region minimizes potential Fc-mediated effects such as complement-dependent cytotoxicity (CDC) or antibody dependent cell-mediated cytotoxicity (ADCC). Four different studies (PRECiSE 1-4) were performed to evaluate the effectiveness of Certolizumab in CD patients. The PRECiSE studies illustrate that Certolizumab pegol administered subcutaneously at a dosage of 400 mg at weeks 0, 2 and 4 and every 4 wk afterwards (at the same dosage) significantly improved the patient’s response rates in comparison with placebo, and to a lesser extent improved remission rates. However, from these studies it is evident that administration of Certolizumab is much more efficient in achieving remission in patients receiving treatment within one year of diagnosis and the efficacy of Certolizumab is more pronounced in patients receiving it as a first anti-TNF-α treatment as compared to a prior infliximab therapy. Furthermore, remission rates decrease as administration of Certolizumab is postponed[68-71].
The second drug, Vedolizumab (VDZ) (trade name Entyvio), is a humanized monoclonal antibody to integrin α4β7. VDZ attaches to the integrin α4β7, a mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1). MAdCAM-1 is typically expressed on the endothelium of venules in the lamina propria of the small intestine and colon, as well as in Peyer’s patches[72]. The attachment of VDZ to integrin α4β7 inhibits adhesion and migration of leukocytes into the gastrointestinal tract. The GEMINI 2 study illustrates that administration of vedolizumab (VDZ) at a dose of 300 mg at weeks 0 and 2 and afterwards every 8 wk demonstrates remission of Crohn’s disease. In the GEMINI 3 study, patients with previous failure to TNF-α antagonist treatment were recruited to assess the efficacy of VDZ treatment. The primary endpoint of clinical remission at week 6 in the population tested was not met. However, secondary endpoints, including clinical remission at week 10 and clinical response at week 6 and week 10, were partially met. This finding implies a modest effect and slow onset of action of VDZ in active CD. VDZ treatment has a better remission rate in Ulcerative colitis as compared to Crohn’s disease[73,74].
CONCLUSION
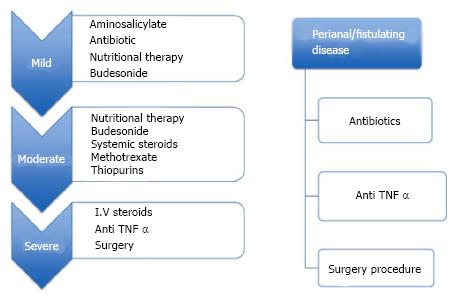
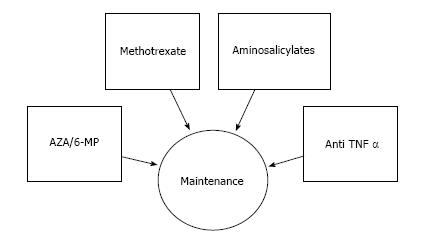
In conclusion, we summarize herein the current treatments for pediatric Crohn’s disease and describe the risk and benefits of each (Figures 1 and 2). While 30 years ago only 2 medications were commonly utilized for the treatment of Crohn’s disease, currently there are more than 15 different therapeutic options of various efficacies and potencies. It should be appreciated that in children as well as in adults CD encompasses a varied range of disease phenotypes and severities and therefore optimal patient selection, timing and therapy requires clinical judgment.
Figure 1 Medication used in the treatment of pediatric Crohn’s disease.
I.V: Intravenous; TNF: Tumor necrosis factor.
Figure 2 Medication for maintenance of remission.
TNF: Tumor necrosis factor; AZA: Azathiopurine; 6-MP: 6-mercaptopurine.
Our knowledge and understanding of CD, its causes and pathogenesis is improving significantly alongside improvement in treatment options. In the near future, we may recognize more specific causes of the disease which will ultimately result in more specific therapeutics.
Although CD is incurable its adverse effects on health and quality of life can be considerably improved by appropriate treatment. Thus, pediatric patients who would otherwise experience chronic illness, symptoms, poor growth and reduced quality of life can benefit from early introduction of proper therapy and may become healthy and thriving. Clinical response is anticipated in pediatric patients but attention must be paid to maintaining it to decreasing adverse events.