Published online Aug 15, 2014. doi: 10.4291/wjgp.v5.i3.252
Revised: February 13, 2014
Accepted: May 15, 2014
Published online: August 15, 2014
Processing time: 302 Days and 21.8 Hours
Pancreatitis is defined as the inflammation of the pancreas and considered the most common pancreatic disease in children and adults. Imaging plays a significant role in the diagnosis, severity assessment, recognition of complications and guiding therapeutic interventions. In the setting of pancreatitis, wider availability and good image quality make multi-detector contrast-enhanced computed tomography (MD-CECT) the most used imaging technique. However, magnetic resonance imaging (MRI) offers diagnostic capabilities similar to those of CT, with additional intrinsic advantages including lack of ionizing radiation and exquisite soft tissue characterization. This article reviews the proposed definitions of revised Atlanta classification for acute pancreatitis, illustrates a wide range of morphologic pancreatic parenchymal and associated peripancreatic changes for different types of acute pancreatitis. It also describes the spectrum of early and late chronic pancreatitis imaging findings and illustrates some of the less common types of chronic pancreatitis, with special emphasis on the role of CT and MRI.
Core tip: Imaging plays an important role in the diagnosis and staging of acute and chronic pancreatitis. Wider availability and good image quality makes computed tomography (CT) the mostly used imaging technique; however, magnetic resonance imaging (MRI) offers diagnostic capabilities similar to those of CT, with additional intrinsic advantages including lack of ionizing radiation and exquisite soft tissue characterization. This article reviews and illustrates the proposed definitions of the revised Atlanta classification for acute pancreatitis. It also describes the spectrum of early and late chronic pancreatitis imaging findings, with special emphasis on the role of CT and MRI.
- Citation: Busireddy KK, AlObaidy M, Ramalho M, Kalubowila J, Baodong L, Santagostino I, Semelka RC. Pancreatitis-imaging approach. World J Gastrointest Pathophysiol 2014; 5(3): 252-270
- URL: https://www.wjgnet.com/2150-5330/full/v5/i3/252.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v5.i3.252
Pancreatitis is defined as the inflammation of the pancreas and considered the most common pancreatic disease in children and adults. It can be acute; representing an acute inflammatory process of the pancreas, or chronic; progressing slowly with continued, permanent inflammatory injury to the pancreas.
The incidence of acute pancreatitis is increasing in the United States and worldwide contributing to be one of the major sources of hospitalization. Acute pancreatitis was the most common gastrointestinal diagnosis for hospitalization (with 274119 discharges) in the United States in 2009[1], usually running a mild clinical course[2]. However, a subset of patients develop severe disease independent of the degree of initial insult or etiology, with high morbidity and mortality up to 45%[3]. Over one-half of cases of acute pancreatitis in adults are related to cholelithiasis or alcohol consumption; whereas trauma, viral infections and systemic diseases account for the majority of cases in children[4].
The incidence of chronic pancreatitis is between five and twelve cases per 100000 persons per year; accounting for more than 120000 outpatient visits and 50000 hospitalizations annually[5]. Alcohol consumption accounts for the majority (80%) of cases of chronic pancreatitis in adults in developed countries; whereas malnutrition is the most common cause worldwide[4].
The purpose of our review is to illustrate the different imaging findings of pancreatitis on computed tomography (CT) and magnetic resonance imaging (MRI); with special emphasis on the revised terminology for acute pancreatitis and substantiate the increasing importance of imaging in the diagnosis, staging and follow-up of acute and chronic pancreatitis[5].
Acute pancreatitis results from the exudation of fluid containing activated proteolytic enzymes into the interstitium of the pancreas and leakage of this fluid into surrounding tissue.
There is general acceptance that a diagnosis of acute pancreatitis requires two of the following three features: (1) Sudden onset abdominal pain suggestive of acute pancreatitis (epigastric pain radiating to the back); (2) Serum amylase and/or lipase levels at least 3 times greater than the upper limit of normal; and (3) Characteristic imaging findings of acute pancreatitis on contrast-enhanced computerized tomography (CECT), MRI, or transabdominal ultrasonography (US) studies.
If abdominal pain is strongly suggestive of acute pancreatitis but the serum amylase and/or lipase activity is less than 3 times the upper limit of normal, characteristic findings on a CECT or MRI are required to confirm the diagnosis[6].
In order to assess and predict local or systemic effects of pancreatic injury, several disease severity-scoring systems were developed (e.g., Ranson score, APACHE-II). In 1992, the Atlanta classification for acute pancreatitis was introduced to establish international standards of definitions of acute pancreatitis and its complications[7]. This system was designed to facilitate understanding and correlation of findings seen by gastroenterologists, pathologists, radiologists and surgeons; aiding improved communication between clinicians.
This initial Atlanta classification system represented major progress; however, advancing knowledge of the disease process, improved imaging and ever-changing treatment options warranted a revision, which was undertaken in 2012.
The revision of the Atlanta classification focuses heavily on morphologic criteria for defining the various manifestations of acute pancreatitis as outlined principally by means of CT and MRI.
Two distinct phases of acute pancreatitis were introduced: a first, or early, phase that occurs within the 1st wk of onset of disease; and a second, or late, phase that takes place after the 1st week of onset[7].
During this phase, pancreatic or peripancreatic ischemia or edema may completely resolve, develop fluid collections or progress to permanent necrosis and liquefaction. Severity of the acute pancreatitis in the early phase is entirely based on clinical parameters; mainly determined by the presence and duration of organ failure, but not the morphologic characteristics and its extent in and around the pancreas[8].
This phase occurs mostly in patients with moderate to severe acute pancreatitis and may extend for weeks to months. It is characterized by the presence of local complications, systemic manifestations (due to ongoing inflammation) and/or by transient or persistent organ failure. In this stage, the need for treatment is determined by presence of symptoms or complications, and the type of management is mainly based on the morphologic characteristics of pancreatic and peripancreatic region seen on cross sectional imaging. The severity of acute pancreatitis in late phase is determined by both morphologic criteria and clinical criteria like persistence of organ failure.
The web based international consensus[7] revised the original Atlanta classification of 1992 and proposed a new classification of acute pancreatitis to avoid the confusion in terminology seen over the last 2 decades. This consensus classification defines criteria for the diagnosis of acute pancreatitis (see above), differentiates the two types of acute pancreatitis (interstitial edematous pancreatitis and necrotizing pancreatitis) classifies the severity of acute pancreatitis into three categories and defines the morphology seen on imaging of pancreatic and peripancreatic collections that arise as complications of acute pancreatitis.
Imaging plays a significant role in the diagnosis of acute pancreatitis in clinically suspected cases or suggesting alternative diagnoses. It helps determine the causes of pancreatitis: gallstones, biliary duct obstruction or structural abnormalities. It also helps in grading the severity of the disease and identifying pancreatic or peripancreatic complications. Additionally, imaging can be utilized to guide therapeutic interventions.
The choice of appropriate imaging modality depends on the reason for investigation, clinical symptoms, time of onset of symptoms and lab findings. However, CECT is the most commonly used modality in the evaluation of acute pancreatitis. In 2010, the ACR committee on appropriateness criteria and its expert panels have developed guidelines for determining the most appropriate imaging examinations for the diagnosis and treatment of acute pancreatitis and have given high score ratings[8,9] to CECT in different clinical scenarios. This is based on it is wide availability and high degree of accuracy. They also stated that MRI appears to offer diagnostic capabilities similar to multi-detector computed tomography (MDCT) with intrinsic advantages including the lack of ionizing radiation and the exquisite soft tissue characterization unmatched by any other imaging modality; allowing better depiction of stones and evaluation of the pancreaticobiliary ductal system.
Ultrasound is frequently the first investigation performed on admission; although it has little value in the diagnosis of pancreatitis or its complications. Ultrasound is usually reserved to confirm or exclude the presence of stones or biliary dilatation. Early identification and treatment of these calculi may have a significant positive impact on outcome. However, body habitus of patient, operator dependence pose a limitation in detection of distal common bile duct stones accurately compared to CECT or MR imaging[9].
Ultrasound is limited in evaluating the entire pancreatic parenchyma; which is often partially or completely obscured by overlying bowel gas. It can however be helpful in monitoring the evolution of fluid collections, which occur as a result of acute pancreatitis, and in guiding diagnostic and therapeutic interventions.
CECT can show morphologic characteristic findings that allow for establishing the diagnosis of acute pancreatitis and determining the extent of disease severity. The best time for performing CECT in acute pancreatitis not well established and if performed immediately after the onset of symptoms, the full extent of pancreatic damage and its severity can be easily underestimated[10,11]. Conversely, a CECT obtained more than 5 d after onset of symptoms that reveals a normal aspect of the pancreas or only mild inflammatory changes (fat stranding) surrounding the pancreas virtually excludes a severe form of acute pancreatitis[12].
Not all patients with acute pancreatitis need to undergo contrast-enhanced CT. In general, CT is not indicated in patients who are clinically classified as having mild pancreatitis (no clinical signs of severe pancreatitis) and show rapid improvement with appropriate medical management. CT should be used in patients who are classified as having severe pancreatitis or are at risk of developing severe pancreatitis; ideally after 72 h, to best assess the full extent of the disease[13].
CT should be repeated when the clinical picture drastically changes, such as with sudden onset of fever, decrease in hematocrit or sepsis. CT can also be useful to guide catheter placement for drainage and to assess success of treatment in patients who underwent percutaneous drainage or other interventions.
Furthermore, in patients with their first episode of pancreatitis who are over 40 years of age and have no identifiable cause for pancreatitis, contrast-enhanced CT should be used to exclude a possible neoplasm[13] (Table 1).
| Types | Indications |
| Initial imaging | 1 When the diagnosis of acute pancreatitis is uncertain 2 Patients with hyperamylasemia, severe clinical pancreatitis, abdominal distention and tenderness, fever > 102°, and leukocytosis for the detection of complications 3 Ranson score > 3 or APACHE score > 8 4 Patients who fail to improve after 72 h of conservative medical therapy 5 Acute change in clinical status, such as new fever, pain, and shock after successful initial medical therapy |
| Followup imaging | 1 Acute change in clinical status suggesting complication 2 7-10 d after presentation if CT severity score is 3-10 at presentation or grade 3 To determine response to treatment after surgery or interventional radiologic procedures to document response to treatment. 4 Before discharge of patients with severe acute pancreatitis |
The main limiting factors for CECT are ionizing radiation, use of iodinated contrast material; especially in patients in with renal failure or contrast allergy and moderate sensitivity in identifying gallstones and biliary stones[14,15]. The above limiting factors can be overcome by using MRI; which does not use ionizing radiation; allowing it to be used during pregnancy, in patients with recurrent pancreatitis and for patients requiring multiple follow-up exams. Non-enhanced MRI seems to be more accurate and reliable for the early assessment of severity and prognosis of acute pancreatitis than is contrast-enhanced CT[16-18]; thus proving beneficial in patients with renal failure and history of contrast allergy.
Recent technological developments have dramatically improved the quality of abdominal MRI. Respiration, bowel peristalsis and vascular pulsations are major sources for artifacts affecting the accuracy and reproducibility of MRI. Breathing-independent sequences and respiratory gating techniques form the foundation of high-quality abdominopelvic MRI. New motion-robust MRI techniques provide promising results even in detection and characterization of pancreatic disease in patients that are not able to cooperate with breath-hold instructions[19].
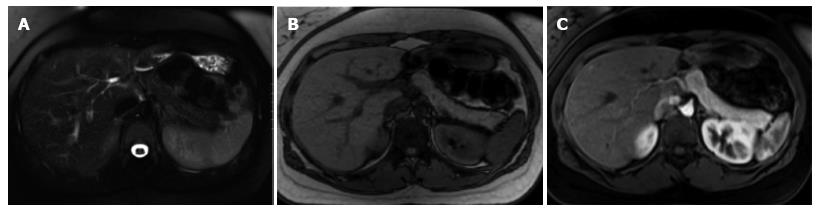
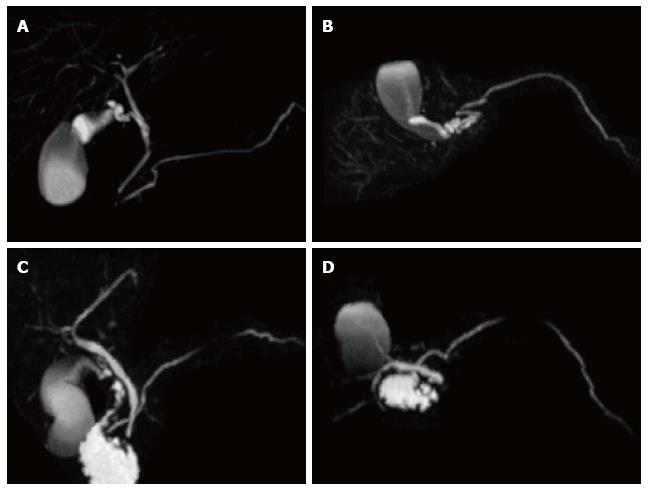
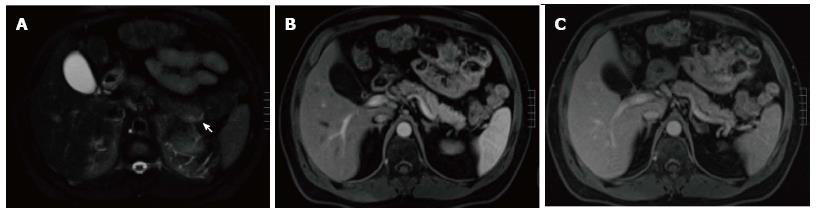
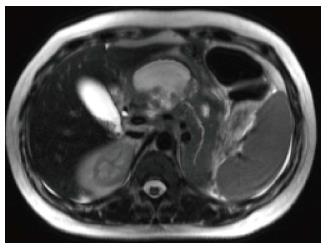
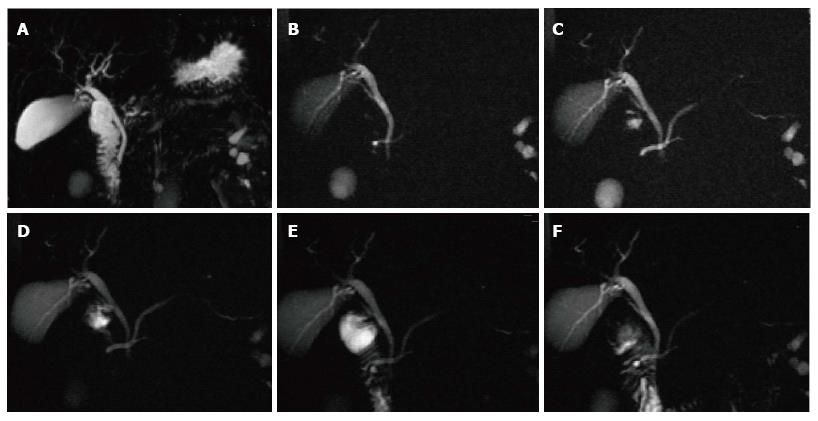
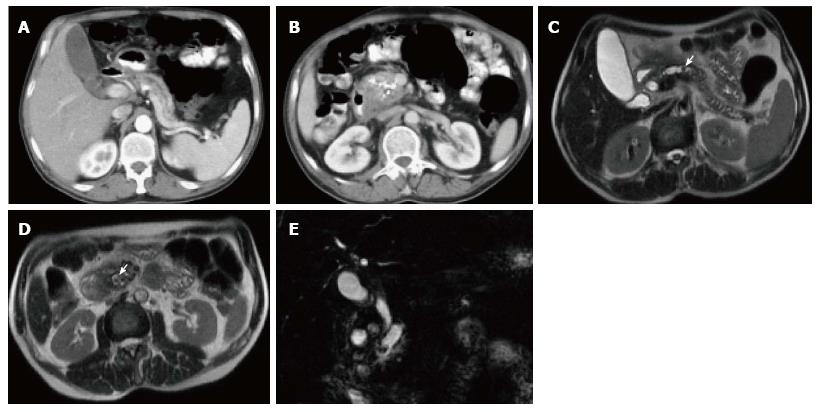
A variety of pulse sequences are currently used for abdominal MRI including T1- and T2-weighted sequences with or without fat-suppression and post-gadolinium T1-weighted sequences (Figure 1). MR Cholangiopancreatography (MRCP) is routinely added to abdominal protocols to assess ductal obstruction, dilatation or course[20-22] (Figure 2); providing comprehensive evaluation of full range of pancreatic diseases. Due to the increasing incidence of acute pancreatitis due to gallstones in the United States, it is more beneficial to consider MRCP as an initial diagnostic study.
MRI is sensitive for detection of subtle changes of acute pancreatitis; particularly minor peripancreatic inflammatory changes; even in the setting of a morphologically normal pancreas on CT imaging; which may appear normal in up to 15%-30% of patients with clinical features of acute pancreatitis[23]. The sensitivity of MRI exceeds that of CT imaging, emphasizing its role in the evaluation of patients with clinically suspected acute pancreatitis and negative CT imaging findings.
It should be emphasized that MRI is a non-ionizing cross sectional imaging method and has a safer intravenous contrast profile in comparison to CT. This is particularly important in radiosensitive populations and those requiring repeated imaging follow up. Additionally, patients who present with acute pancreatitis often have a degree of renal impairment.
The factors that make CECT the most frequently applied imaging approach in pancreatitis are related to its universal availability (especially near the emergency room), faster scanning times, and relatively easier interpretability of CT images by physicians and general radiologists. For early presentation of acute pancreatitis, CT might be the preferred method for the reasons stated above. However, the adequate diagnostic performance of MRI along with the mentioned additive advantages favors MRI as the preferred method.
Endoscopic ultrasound (EUS) has shown great utility in providing high-resolution images of the pancreatic duct and parenchyma as well as extra hepatic biliary system; as the probe can be positioned in close proximity to the pancreas. Furthermore, EUS has become an invaluable technique for its ability to obtain targeted biopsies of lesions in and around the pancreas; thus, playing a prominent role in evaluating patients with atypical findings on other imaging studies.
The disadvantages of EUS are the requirement of monitored anesthesia care, need for expert endo-sonographer, modality operator dependence, and interobserver variability.
According to ACR appropriateness criteria, the role of endoscopic US in the evaluation of acute pancreatitis is primarily reserved for assessing and/or confirming choledocolithiasis and subsequent stone removal, as well as for identifying anatomic abnormalities (e.g., pancreas divisum or malignancy) that can lead to acute pancreatitis. However, it has been recently proposed to use EUS in acute pancreatitis, as it is was found to contribute for the detection of causes like cancer, microlithiasis and chronic pancreatitis[24].
Interstitial edematous pancreatitis (IEP) is a milder form of acute pancreatitis that usually resolves over the first week. IEP is characterized by diffuse or localized enlargement of the pancreas secondary to interstitial or inflammatory edema without necrosis.
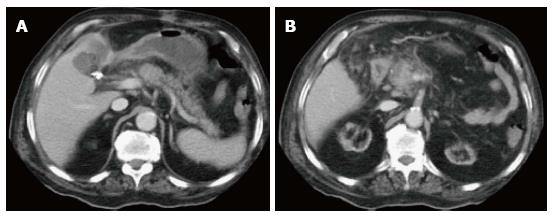
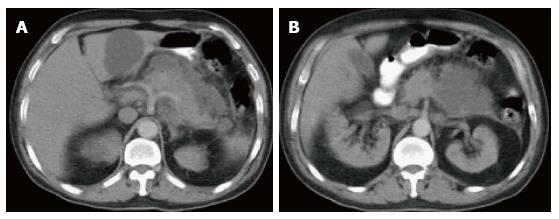
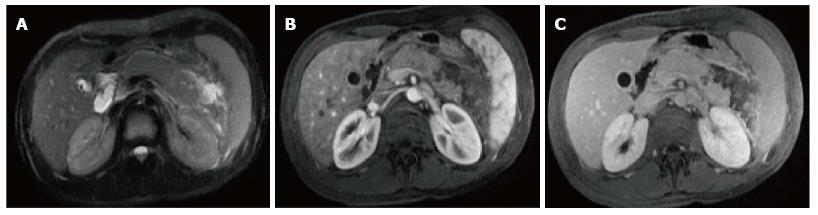
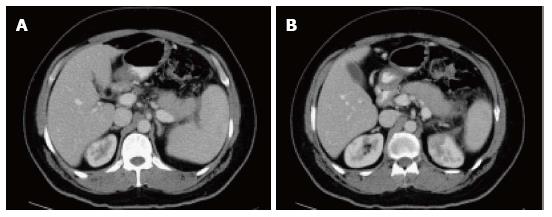
On CECT, findings include enlarged pancreas with relatively normal enhancement. Peripancreatic fat may be normal or show mild stranding and ground glass opacity due to inflammation, with small to varying amounts of non-enhancing peripancreatic fluid (Figure 3). The characteristic CECT finding that distinguishes IEP is absence of pancreatic parenchymal and peripancreatic necrosis.
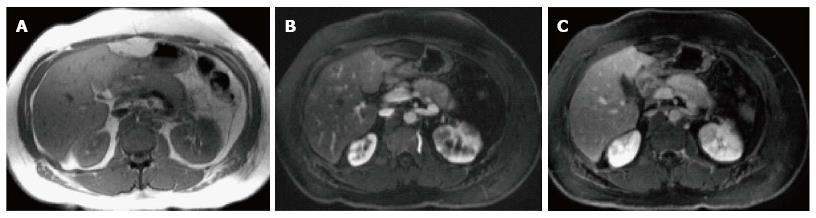
On MRI, the signal intensity characteristics of the pancreas in IEP resemble those of normal pancreatic tissue. Enlargement of the pancreas, parenchymal edema and fat stranding are well demonstrated on T1-weighted images (Figure 4). T1-weighted imaging with fat suppression improves the delineation of the pancreas and pancreatic borders[25]. The pancreas demonstrates high signal intensity on pre-contrast fat suppressed T1-weighted images and enhances uniformly on immediate post-gadolinium images, reflecting a normal capillary blush. Fat suppressed T2-weighted sequences are very sensitive for detecting edema or minimal fluid and therefore have a role in detecting even milder forms of pancreatitis[26](Figure 5).
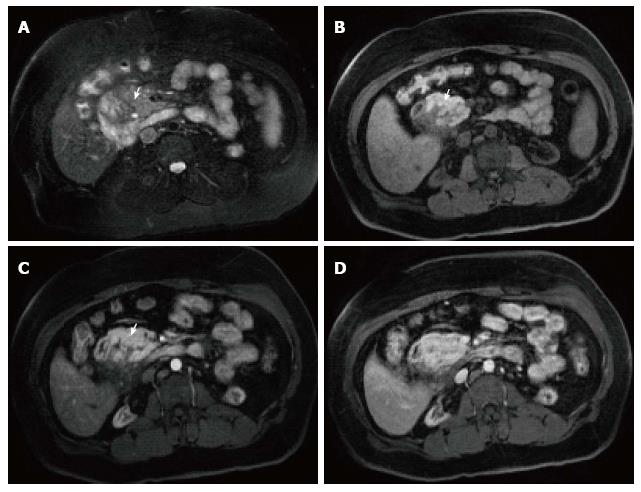
Necrotizing pancreatitis is the inflammation of the pancreas with obvious pancreatic and peripancreatic tissue necrosis. About 5%-10% of patients develop necrosis; affecting the pancreatic parenchyma in 5%, peripancreatic tissue in 20% and both in 70%. Pancreatic parenchymal necrosis carries a worse prognosis than peripancreatic necrosis[27].
Atlanta classification defines necrotizing pancreatitis as being associated with more than 30% parenchymal necrosis. The presence of less than 30% necrosis demands follow-up scanning in 1 wk to confirm true necrosis vs IEP[27].
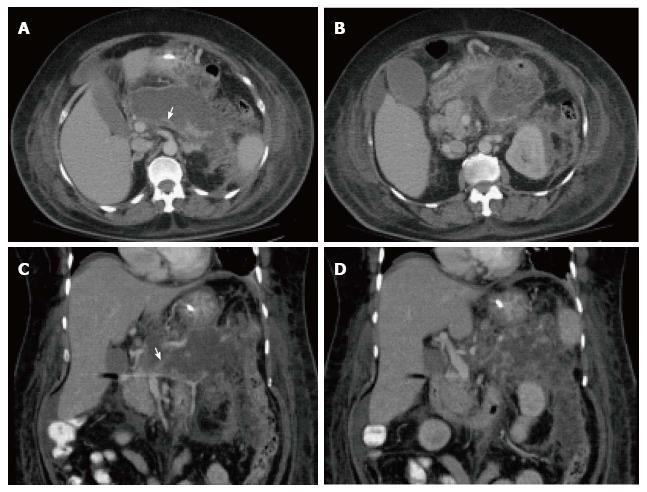
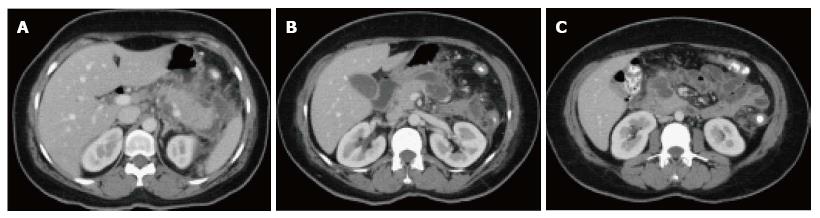
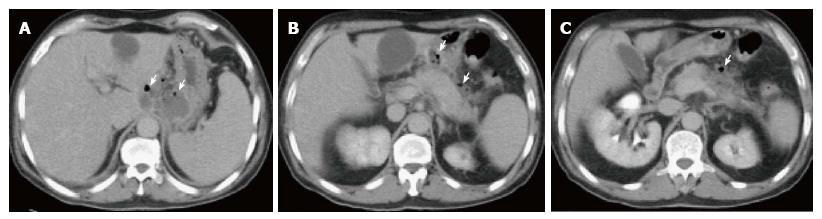
On CECT, findings include areas of compromised pancreatic parenchymal enhancement on the post-Gadolinium images with or without peripancreatic inhomogeneous fluid collections (Figures 6 and 7). The impairment of pancreatic perfusion and signs of peripancreatic necrosis evolve over several days[28], which explains why an early CECT may underestimate the eventual extent of pancreatic and peripancreatic necrosis.
On MRI, necrosis shows appears as hypointense areas on T1-weighted images corresponding to areas of increased signal on fat-suppressed T2 weighted-images, associated with well defined areas of non-enhancing pancreatic parenchyma on post-Gadolinium sequences[29-31] (Figure 8).
For both CT and MRI, acquisition of an adequate arterial phase is of the utmost importance; as the maximum enhancement of pancreas is reached on the late arterial phase, and higher difference in signal between viable and necrotic is achieved on this phase.
Pancreatic duct disruption is an important prognostic factor. It is seen in 30% of the patients of necrotizing pancreatitis[32] when necrosis involves the central gland[33,34]. Drake et al[35] study showed that MRCP, a noninvasive imaging method, achieved 95% accuracy in detecting pancreatic duct disruption; thus helping in identifying patients who might benefit from early treatment.
An important distinction is made between collections that are composed of fluid alone and those that arise from necrosis and contain a solid component (and which may also contain varying amounts of fluid). Below, we define and illustrate the following terms: acute peripancreatic fluid collection; occurring in interstitial edematous pancreatitis, pancreatic pseudocyst as a delayed (usually after 4 wk) complication of interstitial edematous pancreatitis and necrosis; which may be an acute necrotic collection (in the early phase and before demarcation) or walled-off necrosis surrounded by an identifiable capsule on imaging (rarely develops before 4 wk).
Fluid collections less than 4 wk in IEP lacking a discrete wall, with no internal solid components in the peripancreatic region are called acute peripancreatic fluid collections (APFC). Approximately 50% of APFC’s develop within 48 h following the onset of acute pancreatitis[36].
On CT scan, they appear as homogenous collections with low attenuation. They do not have well-defined walls and are confined by normal fascial planes in the retroperitoneum (Figure 9). They can be single or multiple (Figure 10). Most acute fluid collections remain sterile and usually resolve spontaneously without intervention[30].
On MRI, T2-weighted sequences are very sensitive in detecting peripancreatic fluid; which demonstrate high T2 signal intensity. On T1-weighted gradient echo images, APFC’s demonstrate low signal intensity in a background of high signal intensity fat. No perceptible enhancement is depicted on post-gadolinium fat-suppressed T1-weighted images. The majority of fluid collections are typically confined to the lesser sac and anterior pararenal space or may track down to the pelvis and superiorly into mediastinum[29]. These collections are usually sterile and are spontaneously reabsorbed.
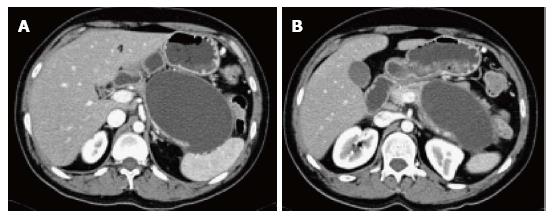
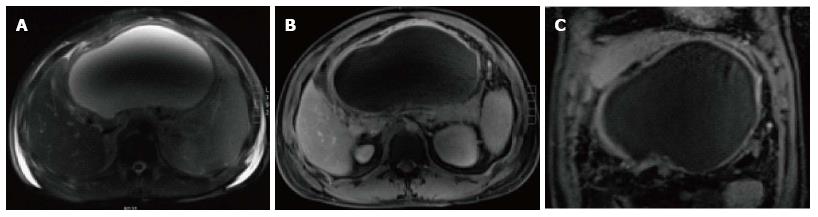
Peripancreatic fluid collections that persist more than 4 wk in IEP, with a well-defined wall and no internal solid components in the peripancreatic region are called pseudocysts.
On CECT, they appear as homogenous collections of low-attenuation surrounded by a uniform enhancing capsule (Figure 11). Typically, an increase of enhancement is observed in the interstitial phase; reflecting the presence of granulation tissue.
On MRI, pseudocysts demonstrate low in signal intensity on and T1-weighted gradient-echo images and relatively homogeneous high signal intensity on T2-weighted images. Pseudocysts walls enhance minimally on early post-gadolinium images and show progressively intense enhancement on 5-min post-gadolinium images; due to the presence of fibrous tissue (Figure 12).
Pseudocysts may sometime have communication with pancreatic duct and detecting this communication is helpful in the further patients’ management. MRCP; a noninvasive imaging modality has an advantage of demonstrating possible communication between pancreatic pseudocyst and pancreatic duct.
The majority of pseudocysts resolve spontaneously. Infection and hemorrhage may complicate simple pseudocysts. Infected pseudocyst may contain gas bubbles on CT. However, absence of these findings on CT may further require confirmation by fine needle aspiration, when there is a strong clinical suspicion.
During the first 4 wk, a collection containing variable amounts of fluid and necrotic tissue is termed an acute necrotic collection (ANC). Unlike APFCs, ANCs are present within the pancreas and peripancreatic regions. ANC’s may often maintain communication with the main pancreatic duct or one of its side-branches; for which, MRI can be useful in delineating this connection.
On CECT, ANC’s demonstrate heterogeneous attenuation variably higher that of thin fluid (Figure 7). Follow-up imaging may be useful to characterize acute collections. CECT often shows ANC’s as a homogenous non-enhancing area during the first week of necrotizing pancreatitis; making it difficult to be differentiated from APFC’s. MRI may be helpful to confirm the presence of solid content in the collection.
On MRI, the necrotic debris may appear as irregularly shaped regions of low signal intensity within the necrotic collections. Breathing-independent T2-weighted sequences such as single-shot echo-train spin echo are useful to evaluate these necrotic collections (Figure 13); not only because of their high sensitivity in demonstrating the complexity of fluid, but also because many of these patients are very debilitated and are unable to cooperate with breath-holding instructions.
An advantage of MRI relative to MDCT in the evaluation of peripancreatic fluid collections is easier appreciation of solid debris with MRI[37]. The sensitivity and specificity of MRI in detecting solid debris of necrosis is 100% when compared to CT; which has a sensitivity of 25% and a specificity of 100%[38]. MRI can help in differentiating fluid collections secondary to pancreatitis from other cystic neoplasms.
After 4 wk, APFC’s mature and develop thick non-epithelialized wall; acquiring the term walled-off necrosis (WON). They commonly occur in the pancreatic body and tail. Management for WON is different from pseudocyst as it contains non-liquefied debris; which needs to be surgically removed. Previously suggested nomenclature for this entity includes: organized pancreatic necrosis, pancreatic sequestration, pseudocyst associated with necrosis and subacute pancreatic necrosis.
On CECT, walled-off necrosis demonstrates a heterogeneous fluid and non-fluid attenuation with varying degree of loculations surrounded by a well-defined and enhancing encapsulating wall; which may involve both the pancreatic and extrapancreatic tissue. CECT, however, may not readily distinguish solid from fluid contents; as a result, pancreatic and peripancreatic necrosis may be misdiagnosed as a pancreatic pseudocyst. For this purpose, MRI may be required for this distinction (Figure 14).
Pancreatic and peripancreatic necrosis can remain sterile or become infected. The development of secondary infection in pancreatic necrosis is associated with increased morbidity and mortality[3]. Most studies suggest that there is no absolute correlation between the extent of necrosis and the risk of infection and duration of symptoms[7]. The early diagnosis of infected pancreatic necrosis is very important in the initiation of antibiotic therapy.
The diagnosis of infected ANC or WON can be suspected in the presence of extraluminal gas on CT or MRI. This extraluminal gas is present in areas of necrosis and may or may not form a gas/fluid level depending on the amount of fluid content present at that stage of the disease (Figure 15). The diagnosis may be confirmed by aspiration and analysis including microscopy and culture.
Several clinical scoring systems like Marshal, SOFA. APACHE or Ranson criteria were designed to accurately correlate the complications like organ failure and mortality in acute pancreatitis. In the last two decades, radiological scoring systems were developed to accurately diagnose and correlate complications in acute pancreatitis.
For the first time in 1990, Balthazar et al[28] introduced the CT severity index for assessment of AP; which correlated well with morbidity, mortality and length of hospital stay. CTSI was widely adopted in clinical and research settings; however, a potential limitation was its inability to detect pancreatic necrosis. MCTSI introduced by Mortele et al[39] in 2004 to account for the limitations of CTSI (Table 2); which showed improved correlation with severity.
| Prognostic Indicators | Characteristics | MCTSI1 |
| Pancreatic inflammation | Normal pancreas | 0 |
| Pancreatic ± peripancreatic inflammatory changes | 2 | |
| One or more collection or peripancreatic fat necrosis | 4 | |
| Pancreatic necrosis | No necrosis | 0 |
| < 30% | 2 | |
| > 30% | 4 | |
| Extrapancreatic complications (pleural effusions, ascites, vascular, gastrointestinal, etc.) | 2 |
MCTSI incorporated extrapancreatic manifestations and simplified the evaluation of extent of parenchymal necrosis by categorizing into none, less than 30% or more than 30%; in addition to evaluating peripancreatic inflammation by detecting the presence or absence of peripancreatic fluid. Predictive accuracy of CT scoring systems for severity of AP and comparisons between CTSI and MCTSI were made[40]. They reported that they could not detect any significant differences between CTSI and MCTSI in evaluating the severity of AP. Their study also demonstrated that compared with APACHE II, both CT indexes more accurately diagnosed clinically severe disease and correlated better with the need for intervention and pancreatic infection.
It has been reported that MR severity index (MRSI) significantly correlated with CTSI (Table 3), Ranson score, C-reactive protein levels, appearance of systemic complications, duration of hospitalization and clinical outcome[17,41].
| Prognostic Indicators | Characteristics | MRSI |
| Pancreatic inflammation | Normal pancreas | 0 |
| Focal or diffuse enlargement of the pancreas | 1 | |
| Intrinsic pancreatic abnormalities with inflammatory changes in the peripancreatic fat | 2 | |
| Single, poorly defined fluid collection | 3 | |
| Two or more poorly defined collection or presence of gas in or adjacent to the pancreas | 4 | |
| Pancreatic necrosis | No necrosis | 0 |
| < 30% | 2 | |
| 30%-50% | 4 | |
| > 50% | 6 |
Chronic pancreatitis is defined pathologically by continuous or relapsing inflammation of the organ leading to irreversible morphologic injury and typically leading to permanent impairment of both exocrine and endocrine functions. The incidence of chronic pancreatitis ranges from 5-12 per 100000 people in industrialized countries[1].
Chronic pancreatitis is a cause of abdominal pain, weight loss, steatorrhea and diabetes mellitus, which may occur as a consequence of multiple factors, including biliary stone disease, alcohol consumption, malignancy, metabolic disorders, and various genetic and environmental insults, including trauma[1].
The histopathological changes in chronic pancreatitis evolve from unevenly distributed fibrosis in early chronic pancreatitis to diffuse fibrosis involving the entire gland in late stages. In advanced disease, large areas of acinar parenchyma are replaced with sclerotic tissue causing atrophy. Ductal irregularities like strictures, dilatation and side branches ectasia occur due to surrounding fibrosis. Other characteristic findings of severe chronic pancreatitis are calcifications and presence of complications like pseudocyst, vascular aneurysms and venous thrombosis.
Imaging plays a significant role in detecting parenchymal and ductal abnormalities in chronic pancreatitis and helps in differentiating early from advanced phases to a certain extent; which further guides the management of these patients.
Most commonly accepted CT- and MRI-based criteria for diagnosis of chronic pancreatitis are shown in Table 4.
| CT criteria | MRI/S-MRCP criteria | |
| Moderate chronic pancreatitis | ≥ 2 of the following: | Moderate pancreatogram changes |
| Main duct enlarged (2-4 mm) | Main duct abnormal and | |
| Slight gland enlargement (up to 2 × normal) | Abnormal side branches, > 3 | |
| Heterogeneous parenchyma | ||
| Small cavities (< 10 mm) | ||
| Irregular ducts | ||
| Focal acute pancreatitis | ||
| Increased Density of the main pancreatic duct wall | ||
| Irregular head/body contour | ||
| Marked chronic pancreatitis | with ≥ 1 of the following | Main duct abnormal and |
| Large cavities (> 10 mm) | Abnormal side branches, > 3 | |
| Gross gland enlargement (2 × normal) | Plus one or more of the following: | |
| Intraductal filling defects or pancreatic calculi | Large cavity | |
| Duct obstruction, stricture, or gross irregularity | Obstruction | |
| Contiguous organ invasion | Filling defects | |
| Severe dilatation or irregularity |
Ultrasound and CT are insensitive in diagnosis of early chronic pancreatitis, as they often show no abnormalities. A recent study showed that parenchymal changes might precede ductal changes in chronic pancreatitis; thus depicting the importance of MRI compared to MRCP in early diagnosis of disease[42].
On MRI, normal pancreas is hyperintense on T1 weighted images and shows uniform enhancement on the late arterial phase (Figure 1). MRI detects not only morphologic characteristics, but also early fibrotic changes. Fibrosis is shown by diminished signal intensity on T1-weighted fat-suppressed images and diminished enhancement on immediate post-Gadolinium gradient-echo images[43]. Low signal intensity on fat-suppressed T1-weighted images reflects loss of the aqueous protein in the acini of the pancreas. Diminished enhancement on capillary phase images reflects disruption of the normal capillary bed and increased chronic inflammation and fibrosis.
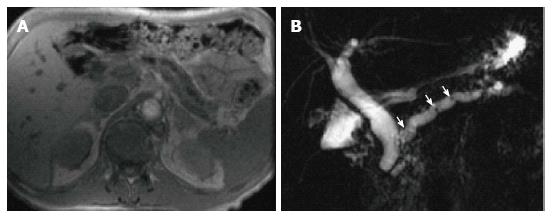
MRCP findings in early chronic pancreatitis often demonstrate normal main pancreatic duct with dilated and irregular side duct branches. The limiting factor is the underestimation of ductal size. Some investigators reported that patients with abnormal MR imaging findings but normal MRCP might benefit from dynamic secretin-MRCP (S-MRCP) (Figure 16); which may reveal ductal abnormalities due to improved visualization otherwise not detected on MRCP[42]. Secretin-MRCP has been reported to show ductal changes, like dilatations and strictures in early chronic pancreatitis.
EUS has a prominent role in chronic pancreatitis for its ability to detect early morphologic changes. Endoscopic retrograde cholangiopancreatography (ERCP) is considered to be gold standard test in detecting early changes, but unlike ERCP, EUS is relatively a non-invasive procedure, and also helps in the evaluation of both pancreatic duct and parenchymal changes compared to ERCP that has limitation in evaluating pancreatic side branches and parenchyma[44]. Chong et al[45] showed sensitivity of 83% and specificity of 80% of EUS for the diagnosis of chronic pancreatitis.
CT is reported to be 60% to 95% sensitive in diagnosing advanced disease as it can readily detect parenchymal changes associated with advanced chronic pancreatitis[46]. Most common findings on CT include dilatation of main pancreatic duct and its side branches; which can be seen in 68% of patients. The ductal contour may be smooth, beaded or irregular[47].
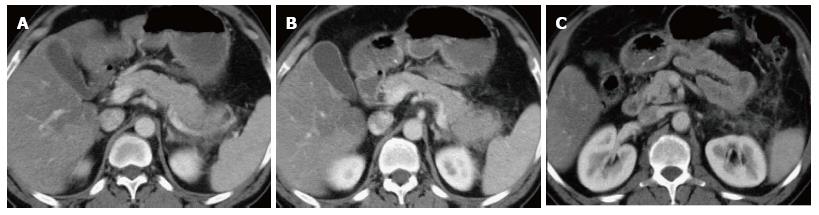
Other findings include intraductal calcifications, which is the most specific finding and is seen in nearly half of the patients with chronic pancreatitis and parenchymal atrophy (Figure 17). However, parenchymal atrophy is neither specific nor sensitive as it seen normally with aging. Intraductal or parenchymal calcifications are usually seen with alcohol related chronic pancreatitis but not on chronic pancreatitis resultant from other causes.
All patients with late or advanced chronic pancreatitis show diminished signal intensity of the pancreas on T1-weighted fat-suppressed images, an abnormally low percentage of contrast enhancement on immediate post-contrast images, and progressive parenchymal enhancement on the 5-min delayed post-contrast images; reflecting the pattern of enhancement of fibrous tissue.
MRCP in advanced phase demonstrates dilatation of the main pancreatic duct with ectasia of the side branches (Figure 18); giving chain of lakes appearance manifested as pancreatic ductal strictures, irregularities and intraductal calculi, appearing as hypointense filling defects.
Chronic pancreatitis may involve only the pancreatic head in 30% of patients, resulting in focally enlarged pancreatic head. In these cases, the focus of chronic pancreatitis can simulate the appearance of pancreatic ductal adenocarcinoma.
Both chronic pancreatitis and adenocarcinoma show similar imaging characteristics on CT and MRI due to abundant fibrosis and ductal obstruction; therefore, making the differentiation between these two entities very difficult. Both are generally seen as hypodense lesions on CT, mildly hypointense on T1-weighted images and heterogeneously mildly hyperintense signal on T2-weighted images. However, certain imaging characteristics are helpful in distinguishing enlarged pancreatic head in chronic pancreatitis from adenocarcinoma (Table 5).
| Chronic pancreatitis | Pancreatic adenocarcinoma |
| Preserved glandular, feathery or marbled texture similar to that of the remaining pancreas | Definable, circumscribed mass lesion is most often diagnostic for tumor, which disrupts the underlying architecture and results in loss of anatomic detail |
| Heterogeneous pancreatic enhancement with presence of signal void (cysts and calcifications) on immediate post-gadolinium images | Irregular, heterogeneous, diminished enhancement on postgadolinium images compared to adjacent pancreatic parenchyma |
| Irregular dilatation of main pancreatic duct with gradual narrowing. Presence of multiple intraductal calcifications (the most specific finding) | Abrupt cut off of the pancreatic duct with significant proximal dilatation +/- presence of double duct sign. Very few ductal calculi compared to chronic pancreatitis |
| Dilatation of main pancreatic duct with and ectasia of the side branches, giving chain of lakes appearance | Minimal dilatation of side branches |
| No vascular encasement, significant lymphadenopathy or distant metastasis. | Vascular encasement, lymphadenopathy or distant metastasis |
Rarely, chronic pancreatitis may involve only the focally enlarged portion of the pancreas, with the reminder of the pancreas having no inflammatory changes. In these cases, the focus of chronic pancreatitis can also simulate the appearance of pancreatic ductal adenocarcinoma. The inflammatory process may also be sufficiently destructive that underlying stromal pattern is lost. In these rare cases, diagnosis can only be established by surgical resection and histopathological examination to confirm the absence of malignancy.
Despite the high-resolution images produced by conventional EUS, there are no specific EUS imaging features that can differentiate pancreatic cancer from other common mimics, including lymphoma, focal pancreatitis, neuroendocrine tumors, metastases, and focal AIP[48]. However, one of the strengths of EUS is its ability to allow guided fine needle aspiration (FNA); which may overcome this problem.
In a retrospective analysis by Agarwal et al[49], 110 patients with abnormal CT or MRI with an enlarged head of the pancreas or dilated pancreatic duct with or without dilation of the common bile duct underwent EUS or EUS-FNA. The study revealed an accuracy of 99.1% for EUS and/or EUS-FNA in diagnosing pancreatic neoplasm with a sensitivity of 88.8% and specificity of 100%[49]. Given the high accuracy in the evaluation of pancreatic tumors, Eloubeidi et al[50] proposed routine EUS-FNA for the differential diagnosis of solid pancreatic masses. Other studies have shown that a negative EUS in ambiguous cases (where a mass is suspected) has a high negative predictive value[51,52].
Positron emission tomography-computed tomography (PET-CT) has an established role in the diagnosis of pancreatic carcinoma, especially when cross sectional imaging or biopsies are equivocal or nondiagnostic. In patients with a suspicion of pancreatic malignancy, a focal increase in 18F-fluorodeoxyglucose (FDG) uptake suggests the diagnosis of malignancy. Nonetheless, the cutoff value of maximum standardized uptake value (SUVmax) is not defined, as it overlaps in benign and malignant pancreatic disease processes[53,54].
Furthermore, FDG-PET’s detectability of pancreatic cancer depends on lesion size and degree of FDG uptake and surrounding background uptake. In the setting of chronic pancreatitis, FDG-PET is shown to detect pancreatic adenocarcinoma with a sensitivity of 92% and with a negative predictive value of 87%. In the setting of acute pancreatitis, the specificity can be as low as 50%, as it is known that inflammatory tissue can also demonstrate FDG activity[47].
The most common non-neoplastic complications of chronic pancreatitis include pseudocysts, pseudoaneurysms (due to erosion of the arterial wall), splenic vein thrombosis with subsequent development of collaterals, biliary obstruction (due to pseudocysts), and gastrointestinal complications like gastric outlet obstruction or bowel ischemia[6,55]. These complications are well depicted with CT and MRI.
MRI with MRCP may be superior to CT in detecting specific complications like pseudocysts, fistula formation, distal common biliary dilatation and vascular complications associated with higher morbidity and mortality[46].
Special types of chronic pancreatitis autoimmune pancreatitis.
Autoimmune pancreatitis is a distinct form of pancreatitis characterized clinically by obstructive jaundice (with or without pancreatic mass), histologically by a lymphoplasmacytic infiltrate and fibrosis and therapeutically by a dramatic response to steroids[56].
Autoimmune pancreatitis accounts for 2%-6% of chronic pancreatitis[57,58]. It is associated with other autoimmune disorders like Sjogren’s syndrome, primary biliary cirrhosis, and primary sclerosing cholangitis[59,60]. Early diagnosis of autoimmune pancreatitis is crucial as it often responds to steroid therapy; thus avoiding complications.
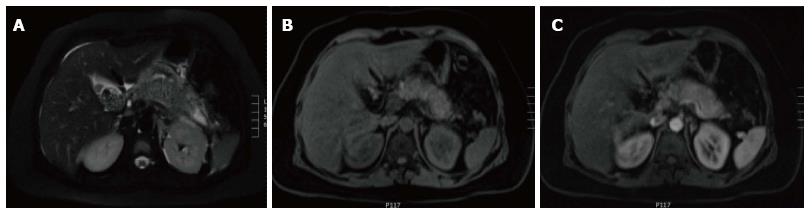
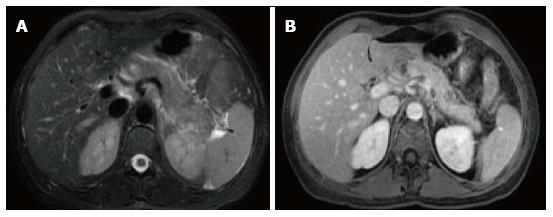
In AIP, affected areas appear enlarged and hypodense on CT. CECT demonstrates diminished enhancement of the involved parenchyma on the late arterial phase and delayed enhancement on the delayed phase (Figure 19). The MR appearance of autoimmune pancreatitis is similar and is characterized by enlarged pancreas with moderately decreased signal intensity on T1-weighted images, mildly high signal intensity on T2-weighted images and delayed post-gadolinium enhancement of the pancreatic parenchyma (Figure 20). Additional findings that may be observed in autoimmune pancreatitis include: (1) capsule like rim surrounding the diseased parenchyma, that is hypointense on T2-weighted images and may show delayed post-gadolinium enhancement[59]; (2) absence of parenchymal atrophy; (3) ductal dilatation proximal to the site of stenosis; (4) absence of peripancreatic fluid; and (5) clear demarcation of the abnormality[60].
MRCP depicts diffuse or segmental narrowing and irregularity of the main pancreatic duct as characteristic findings. The most commonly involved segment is the intrapancreatic common bile duct, and less frequently multifocal intrahepatic biliary strictures are noted.
Autoimmune pancreatitis is has 3 types based on morphologic patterns: diffuse, focal, and multifocal. Diffuse disease is the most common type. CT and MRI commonly show a swollen, sausage-like pancreas with poorly demonstrated borders and a capsule-like rim of low-density/intensity[61].
The diffuse form of AIP may mimic diffuse disorders like lymphoma, metastases or other diffuse infiltrative processes. In most of these disorders, unlike AIP, the parenchyma is heterogeneous and shows irregular contours.
Focal disease is less common and manifests as a well-defined hypodense mass, often involving the head and mimicking pancreatic adenocarcinoma. In patients who underwent pancreatic resection for suspected malignancy, 2.5%-8% were ultimately diagnosed with AIP without malignancy[58,62]. However, the probability of AIP vs pancreatic cancer in patients with obstructive jaundice can be predicted based on CT/MRI findings.
Diffusely enlarged pancreas showing low density mass with enhancement on delayed phases on CT/MRI, especially with a capsule-like rim, and no pancreatic ductal cutoff is highly likely to have AIP. Low-density mass on CECT, pancreatic ductal cutoff in presence or absence of pancreatic atrophy mostly suggests pancreatic cancer.
Groove pancreatitis is a rare form of focal chronic pancreatitis involving the anatomic groove between the pancreatic head, duodenum and common bile duct. Groove pancreatitis is categorized into 2 forms: pure, involving exclusively the groove; and segmental, involving the groove and extending in to the pancreatic head[63] (Figure 21).
Pathogenesis remains controversial but may result from obstruction of the accessory pancreatic duct as it drains into the second portion of the duodenum through the minor ampulla[64]. Presence of cystic changes, frequently located in the expected region of the pancreatic accessory duct, is considered a prominent feature of this process, likely related to accessory duct obstruction[65]. It is commonly seen in patients with history of alcohol abuse[64].
The classic MDCT features in the pure form can range from ill-defined fat stranding to frank soft tissue within the pancreaticoduodenal groove with increased delayed enhancement due to fibrosis. Thickening of medial duodenal wall on coronal images and presence of cysts can be appreciated sometimes[66]. On MRI, groove pancreatitis is characterized by a sheet-like mass in the groove that shows low signal on T1-weighted images, slightly high signal on T2-weighted images relative to the pancreas and may show delayed enhancement. Cystic lesions are well shown on T2-weighted images in the groove or duodenal wall[63].
It may be challenging to differentiate groove pancreatitis from pancreatic head duct adenocarcinoma. Recently, it was shown that by using three strict diagnostic criteria for groove pancreatitis: (1) focal thickening of the second portion of the duodenum; (2) abnormal increased enhancement of the second portion of the duodenum; and (3) cystic changes in the region of the pancreatic accessory duct, distinction from pancreatic duct adenocarcinoma could be achieved with high diagnostic accuracy (87.2% of patients), and a diagnosis of cancer could be excluded with a negative predictive value of 92.9%[67].
Hereditary pancreatitis is an autosomal dominant disease presenting as multiple episodes of pancreatitis in the absence of any predisposing factors. Imaging findings include parenchymal and intraductal calcifications and parenchymal atrophy. However, in hereditary pancreatitis, imaging plays an important role to rule out structural causes of pancreatitis and to closely monitor the development of pancreatic cancer, the risk of which is increased by many folds in these patients.
In summary, imaging plays an important role in the diagnosis and staging of acute and chronic pancreatitis. Both CT and MRI are widely used and represent the best cross sectional techniques in the setting of pancreatitis. Wider availability and good image quality make CT the mostly used imaging technique; however, due to its nonionizing nature, unmatched soft tissue contrast and higher safety profile of intravascular contrast media make MRI particularly valuable in pregnant patients, patients with recurrent pancreatitis and patients requiring multiple follow up examinations. Also, early form of chronic pancreatitis and some specific types of chronic pancreatitis benefit from being imaged with MRI.
P- Reviewer: Clark CJ, Demir IE S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179-1187.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1466] [Article Influence: 112.8] [Reference Citation Analysis (1)] |
| 2. | Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-590. [PubMed] |
| 3. | Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 563] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 4. | Shanbhogue AK, Fasih N, Surabhi VR, Doherty GP, Shanbhogue DK, Sethi SK. A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics. 2009;29:1003-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682-707. [PubMed] |
| 6. | Bollen TL. Imaging of acute pancreatitis: update of the revised Atlanta classification. Radiol Clin North Am. 2012;50:429-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4330] [Article Influence: 360.8] [Reference Citation Analysis (45)] |
| 8. | Perez A, Whang EE, Brooks DC, Moore FD, Hughes MD, Sica GT, Zinner MJ, Ashley SW, Banks PA. Is severity of necrotizing pancreatitis increased in extended necrosis and infected necrosis? Pancreas. 2002;25:229-233. [PubMed] |
| 9. | Megibow AJ, Ralls PW, Balfe DM, Bree RL, DiSantis DJ, Glick SN, Kidd R, Levine MS, Mezwa DG, Saini S. Acute pancreatitis. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000;215 Suppl:203-207. [PubMed] |
| 10. | Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1150] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 11. | Morgan DE. Imaging of acute pancreatitis and its complications. Clin Gastroenterol Hepatol. 2008;6:1077-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Moulton JS. The radiologic assessment of acute pancreatitis and its complications. Pancreas. 1991;6 Suppl 1:S13-S22. [PubMed] |
| 13. | Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262:751-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 14. | Anderson SW, Lucey BC, Varghese JC, Soto JA. Accuracy of MDCT in the diagnosis of choledocholithiasis. AJR Am J Roentgenol. 2006;187:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Anderson SW, Rho E, Soto JA. Detection of biliary duct narrowing and choledocholithiasis: accuracy of portal venous phase multidetector CT. Radiology. 2008;247:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Kim YK, Kim CS, Han YM. Role of fat-suppressed t1-weighted magnetic resonance imaging in predicting severity and prognosis of acute pancreatitis: an intraindividual comparison with multidetector computed tomography. J Comput Assist Tomogr. 2009;33:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Stimac D, Miletić D, Radić M, Krznarić I, Mazur-Grbac M, Perković D, Milić S, Golubović V. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol. 2007;102:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Viremouneix L, Monneuse O, Gautier G, Gruner L, Giorgi R, Allaouchiche B, Pilleul F. Prospective evaluation of nonenhanced MR imaging in acute pancreatitis. J Magn Reson Imaging. 2007;26:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Azevedo RM, de Campos RO, Ramalho M, Herédia V, Dale BM, Semelka RC. Free-breathing 3D T1-weighted gradient-echo sequence with radial data sampling in abdominal MRI: preliminary observations. AJR Am J Roentgenol. 2011;197:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Bret PM, Reinhold C, Taourel P, Guibaud L, Atri M, Barkun AN. Pancreas divisum: evaluation with MR cholangiopancreatography. Radiology. 1996;199:99-103. [PubMed] |
| 21. | Soto JA, Barish MA, Yucel EK, Clarke P, Siegenberg D, Chuttani R, Ferrucci JT. Pancreatic duct: MR cholangiopancreatography with a three-dimensional fast spin-echo technique. Radiology. 1995;196:459-464. [PubMed] |
| 22. | Takehara Y, Ichijo K, Tooyama N, Kodaira N, Yamamoto H, Tatami M, Saito M, Watahiki H, Takahashi M. Breath-hold MR cholangiopancreatography with a long-echo-train fast spin-echo sequence and a surface coil in chronic pancreatitis. Radiology. 1994;192:73-78. [PubMed] |
| 23. | Balthazar EJ. CT diagnosis and staging of acute pancreatitis. Radiol Clin North Am. 1989;27:19-37. [PubMed] |
| 24. | Scaglione M, Casciani E, Pinto A, Andreoli C, De Vargas M, Gualdi GF. Imaging assessment of acute pancreatitis: a review. Semin Ultrasound CT MR. 2008;29:322-340. [PubMed] |
| 25. | Miller FH, Keppke AL, Dalal K, Ly JN, Kamler VA, Sica GT. MRI of pancreatitis and its complications: part 1, acute pancreatitis. AJR Am J Roentgenol. 2004;183:1637-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Kim YK, Ko SW, Kim CS, Hwang SB. Effectiveness of MR imaging for diagnosing the mild forms of acute pancreatitis: comparison with MDCT. J Magn Reson Imaging. 2006;24:1342-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Ashley SW, Perez A, Pierce EA, Brooks DC, Moore FD, Whang EE, Banks PA, Zinner MJ. Necrotizing pancreatitis: contemporary analysis of 99 consecutive cases. Ann Surg. 2001;234:572-579; discussion 572-579. [PubMed] |
| 28. | Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-336. [PubMed] |
| 29. | Balthazar EJ, Freeny PC, vanSonnenberg E. Imaging and intervention in acute pancreatitis. Radiology. 1994;193:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 277] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Lenhart DK, Balthazar EJ. MDCT of acute mild (nonnecrotizing) pancreatitis: abdominal complications and fate of fluid collections. AJR Am J Roentgenol. 2008;190:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Matos C, Cappeliez O, Winant C, Coppens E, Devière J, Metens T. MR imaging of the pancreas: a pictorial tour. Radiographics. 2002;22:e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Lau ST, Simchuk EJ, Kozarek RA, Traverso LW. A pancreatic ductal leak should be sought to direct treatment in patients with acute pancreatitis. Am J Surg. 2001;181:411-415. [PubMed] |
| 33. | Sandrasegaran K, Tann M, Jennings SG, Maglinte DD, Peter SD, Sherman S, Howard TJ. Disconnection of the pancreatic duct: an important but overlooked complication of severe acute pancreatitis. Radiographics. 2007;27:1389-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Tann M, Maglinte D, Howard TJ, Sherman S, Fogel E, Madura JA, Lehman GA. Disconnected pancreatic duct syndrome: imaging findings and therapeutic implications in 26 surgically corrected patients. J Comput Assist Tomogr. 2003;27:577-582. [PubMed] |
| 35. | Drake LM, Anis M, Lawrence C. Accuracy of magnetic resonance cholangiopancreatography in identifying pancreatic duct disruption. J Clin Gastroenterol. 2012;46:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Zaheer A, Singh VK, Qureshi RO, Fishman EK. The revised Atlanta classification for acute pancreatitis: updates in imaging terminology and guidelines. Abdom Imaging. 2013;38:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Macari M, Finn ME, Bennett GL, Cho KC, Newman E, Hajdu CH, Babb JS. Differentiating pancreatic cystic neoplasms from pancreatic pseudocysts at MR imaging: value of perceived internal debris. Radiology. 2009;251:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Morgan DE, Baron TH, Smith JK, Robbin ML, Kenney PJ. Pancreatic fluid collections prior to intervention: evaluation with MR imaging compared with CT and US. Radiology. 1997;203:773-778. [PubMed] |
| 39. | Mortele KJ, Wiesner W, Intriere L, Shankar S, Zou KH, Kalantari BN, Perez A, vanSonnenberg E, Ros PR, Banks PA. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol. 2004;183:1261-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 40. | Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, Mortele KJ. Comparative evaluation of the modified CT severity index and CT severity index in assessing severity of acute pancreatitis. AJR Am J Roentgenol. 2011;197:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Arvanitakis M, Koustiani G, Gantzarou A, Grollios G, Tsitouridis I, Haritandi-Kouridou A, Dimitriadis A, Arvanitakis C. Staging of severity and prognosis of acute pancreatitis by computed tomography and magnetic resonance imaging-a comparative study. Dig Liver Dis. 2007;39:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Balci NC, Alkaade S, Magas L, Momtahen AJ, Burton FR. Suspected chronic pancreatitis with normal MRCP: findings on MRI in correlation with secretin MRCP. J Magn Reson Imaging. 2008;27:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Semelka RC, Shoenut JP, Kroeker MA, Micflikier AB. Chronic pancreatitis: MR imaging features before and after administration of gadopentetate dimeglumine. J Magn Reson Imaging. 1993;3:79-82. [PubMed] |
| 44. | Raman SP, Fishman EK, Lennon AM. Endoscopic ultrasound and pancreatic applications: what the radiologist needs to know. Abdom Imaging. 2013;38:1360-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc. 2007;65:808-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 46. | Choueiri NE, Balci NC, Alkaade S, Burton FR. Advanced imaging of chronic pancreatitis. Curr Gastroenterol Rep. 2010;12:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Perez-Johnston R, Sainani NI, Sahani DV. Imaging of chronic pancreatitis (including groove and autoimmune pancreatitis). Radiol Clin North Am. 2012;50:447-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Papanikolaou IS, Adler A, Neumann U, Neuhaus P, Rösch T. Endoscopic ultrasound in pancreatic disease--its influence on surgical decision-making. An update 2008. Pancreatology. 2009;9:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Agarwal B, Krishna NB, Labundy JL, Safdar R, Akduman EI. EUS and/or EUS-guided FNA in patients with CT and/or magnetic resonance imaging findings of enlarged pancreatic head or dilated pancreatic duct with or without a dilated common bile duct. Gastrointest Endosc. 2008;68:237-42; quiz 334, 335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Eloubeidi MA, Varadarajulu S, Desai S, Shirley R, Heslin MJ, Mehra M, Arnoletti JP, Eltoum I, Wilcox CM, Vickers SM. A prospective evaluation of an algorithm incorporating routine preoperative endoscopic ultrasound-guided fine needle aspiration in suspected pancreatic cancer. J Gastrointest Surg. 2007;11:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 51. | Voss M, Hammel P, Molas G, Palazzo L, Dancour A, O’Toole D, Terris B, Degott C, Bernades P, Ruszniewski P. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244-249. [PubMed] |
| 52. | Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720-726. [PubMed] |
| 53. | Santhosh S, Mittal BR, Bhasin D, Srinivasan R, Rana S, Das A, Nada R, Bhattacharya A, Gupta R, Kapoor R. Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in the characterization of pancreatic masses: experience from tropics. J Gastroenterol Hepatol. 2013;28:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Asagi A, Ohta K, Nasu J, Tanada M, Nadano S, Nishimura R, Teramoto N, Yamamoto K, Inoue T, Iguchi H. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas. 2013;42:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 55. | Remer EM, Baker ME. Imaging of chronic pancreatitis. Radiol Clin North Am. 2002;40:1229-1242, v. [PubMed] |
| 56. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1056] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 57. | Sah RP, Pannala R, Chari ST, Sugumar A, Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD. Prevalence, diagnosis, and profile of autoimmune pancreatitis presenting with features of acute or chronic pancreatitis. Clin Gastroenterol Hepatol. 2010;8:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Nishimori I, Tamakoshi A, Otsuki M. Prevalence of autoimmune pancreatitis in Japan from a nationwide survey in 2002. J Gastroenterol. 2007;42 Suppl 18:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 59. | Irie H, Honda H, Baba S, Kuroiwa T, Yoshimitsu K, Tajima T, Jimi M, Sumii T, Masuda K. Autoimmune pancreatitis: CT and MR characteristics. AJR Am J Roentgenol. 1998;170:1323-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 217] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | Van Hoe L, Gryspeerdt S, Ectors N, Van Steenbergen W, Aerts R, Baert AL, Marchal G. Nonalcoholic duct-destructive chronic pancreatitis: imaging findings. AJR Am J Roentgenol. 1998;170:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Takahashi N, Fletcher JG, Fidler JL, Hough DM, Kawashima A, Chari ST. Dual-phase CT of autoimmune pancreatitis: a multireader study. AJR Am J Roentgenol. 2008;190:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Hardacre JM, Iacobuzio-Donahue CA, Sohn TA, Abraham SC, Yeo CJ, Lillemoe KD, Choti MA, Campbell KA, Schulick RD, Hruban RH. Results of pancreaticoduodenectomy for lymphoplasmacytic sclerosing pancreatitis. Ann Surg. 2003;237:853-858; discussion 853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 63. | Blasbalg R, Baroni RH, Costa DN, Machado MC. MRI features of groove pancreatitis. AJR Am J Roentgenol. 2007;189:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Chatelain D, Vibert E, Yzet T, Geslin G, Bartoli E, Manaouil D, Delcenserie R, Brevet M, Dupas JL, Regimbeau JM. Groove pancreatitis and pancreatic heterotopia in the minor duodenal papilla. Pancreas. 2005;30:e92-e95. [PubMed] |
| 65. | Triantopoulou C, Dervenis C, Giannakou N, Papailiou J, Prassopoulos P. Groove pancreatitis: a diagnostic challenge. Eur Radiol. 2009;19:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 66. | Raman SP, Salaria SN, Hruban RH, Fishman EK. Groove pancreatitis: spectrum of imaging findings and radiology-pathology correlation. AJR Am J Roentgenol. 2013;201:W29-W39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 67. | Kalb B, Martin DR, Sarmiento JM, Erickson SH, Gober D, Tapper EB, Chen Z, Adsay NV. Paraduodenal pancreatitis: clinical performance of MR imaging in distinguishing from carcinoma. Radiology. 2013;269:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | O'Connor OJ, McWilliams S, Maher MM. Imaging of acute pancreatitis. AJR Am J Roentgenol. 2011;197:W221-W225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 69. | Yang R, Jing ZL, Zhang XM, Tang W, Xiao B, Huang XH, Yang L, Feng ZS. MR imaging of acute pancreatitis: correlation of abdominal wall edema with severity scores. Eur J Radiol. 2012;81:3041-3047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Conwell DL, Wu BU. Chronic pancreatitis: making the diagnosis. Clin Gastroenterol Hepatol. 2012;10:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |