Published online Aug 15, 2014. doi: 10.4291/wjgp.v5.i3.188
Revised: May 1, 2014
Accepted: May 28, 2014
Published online: August 15, 2014
Processing time: 219 Days and 1.6 Hours
Intrahepatic cholangiocarcinoma is macroscopically classified into three subtypes, mass-forming-type, periductal infiltrating-type, and intraductal growth-type. Each subtype should be preoperatively differentiated to perform the valid surgical resection. Recent researches have revealed the clinical, radiologic, pathobiological characteristics of each subtype. We reviewed recently published studies covering various aspects of intrahepatic cholangiocarcinoma (ICC), focusing especially on the macroscopic subtypes and stem cell features to better understand the pathophysiology of ICC and to establish the valid therapeutic strategy.
Core tip: We reviewed recently published studies covering various aspects of intrahepatic cholangiocarcinoma (ICC), focusing especially on the macroscopic subtypes and stem cell features to better understand the pathophysiology of ICC and to establish the valid therapeutic strategy.
- Citation: Sanada Y, Kawashita Y, Okada S, Azuma T, Matsuo S. Review to better understand the macroscopic subtypes and histogenesis of intrahepatic cholangiocarcinoma. World J Gastrointest Pathophysiol 2014; 5(3): 188-199
- URL: https://www.wjgnet.com/2150-5330/full/v5/i3/188.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v5.i3.188
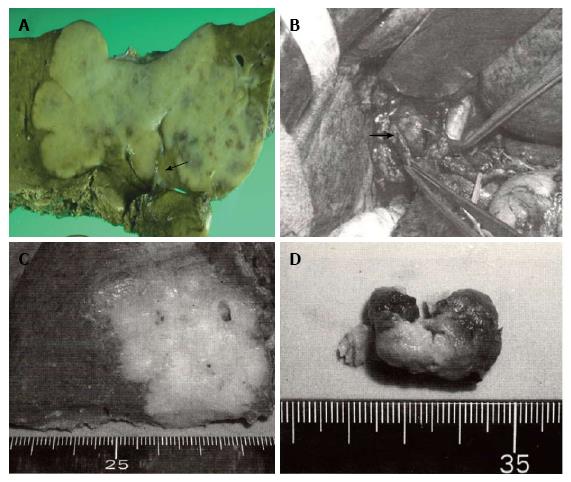
The Liver Cancer Study Group of Japan has applied the same TNM staging system used for hepatocellular carcinoma (HCC) to that for intrahepatic cholangiocarcinoma (ICC)[1]. A recent increase in the number of surgically resected cases of ICC has clarified some characteristics inherent in this disease. The most prominent feature of ICC is that of the macroscopic findings reflecting its growth patterns. ICC is grossly classifiable into mass-forming (MF), periductal infiltrating (PI), and intraductal growth (IG) types[2]. The MF type presents as a gray to gray-white, firm and solid mass in the hepatic parenchyma, and of these three subtypes, MF-type ICC is the most common (59%). The PI type shows spreading of the carcinoma along the portal tracts with stricture of the central bile ducts and dilation of the peripheral bile ducts. The IG type presents as a papillary tumor within the dilated bile duct lumen. Some IG-type ICCs are considered to be an intraductal papillary neoplasm of the bile duct. This classification system provides useful information during surgery (Figure 1). For example, the efficacy of hilar resection is not emphasized except in the case of PI-type ICCs. This macroscopic classification cannot be applied to HCC. Therefore, studies focusing on the association of the macroscopic subtypes with biological behavior, clinical features, and radiologic findings are needed to establish the therapeutic strategy for ICC. Although the macroscopic features are prominent in ICCs, another aspect of ICCs, in which ICCs cannot be discussed independently of other primary liver cancers, exists. Recent histopathologic and immunohistochemical studies have reported that hepatic progenitor cells (HPC) or stem cells play important roles in liver carcinogenesis including both HCCs and ICCs, supporting the hypothesis that HCCs and ICCs share a common evolutionary origin[3,4]. In 2010, the World Health Organization (WHO) established a new classification system of combined hepatocellular-cholangiocarcinoma (cHCC-CC) based on the presence of stem-cell features[5]. According to this new system, cHCC-CCs are classified into two major subtypes, classic type and subtypes with stem-cell features. Subtypes with stem-cell features are further subclassified into three types: typical type, intermediate-cell type, and cholangiocellular type. In addition, recent reports showed that some cases of HCCs and ICCs are associated with hepatic stem cells. However, little is known about the clinical significance of stem cells in ICCs. This review summarizes recently published studies (from 2011 to 2013) covering various aspects of ICC and cHCC-CC, focusing especially on the macroscopic subtypes and stem-cell features.
Recent clinical researches of ICC are summarized in Tables 1 and 2[6-24]. The association between macroscopic subtypes and survival rate and lymph node metastasis has been discussed ever since the macroscopic subtype was established. IG-type ICCs have a favorable outcome because this tumor type shows intraductal growth without invasiveness[2]. Of the three subtypes, MF+PI-type ICCs have the highest incidence of lymph node metastasis (50% to 73%)[15] and are associated with the lowest 5-year survival rate (0% to 19.4%). PI-type and MF-type have relatively favorable outcomes when lymph node metastasis or hilar invasion is absent.
| Ref. | n | Survival rate (%) | MST (mo) | Prognostic factor |
| Marubashi et al[6] | 111 | 59.7 (3 yr) | - | IM, Hilar inv, LN |
| Guglielmi et al[7] | 145 | - | 19 (LN+), 42 (LN-) | LNR > 0.25, LN |
| Zhu et al[8] | 37 | - | - | CA19-9, Low prealbmin |
| Dhanasekaran et al[9] | 105 | - | 16 | V |
| Wang et al[10] | 367 | - | - | CEA, CA19-9, Size, V |
| De Rose et al[11] | 79 (MF) | - | - | Doubling time < 70 d |
| Sulpice et al[12] | 87 | - | - | BT, Maj, Size, V, IM |
| Ribero et al[13] | 434 | 39.8 (5 yr) | - | LN, CA19-9, IM |
| Liu et al[14] | 132 | - | - | Por, CA19-9, Dis(-) |
| Uchiyama et al[15] | 334 | - | - | Shown in Table 2 |
| Chen et al[16] | 64 | 32 (3 yr) | - | LN, PN, Size |
| Uno et al[17] | 273 | - | - | Shown in Table 2 |
| Morine et al[18] | 22 | - | - | Shown in Table 2 |
| Jiang et al[19] | 102 | - | - | CA19-9, IM |
| Murakami et al[20] | 44 | 47 (5 yr) | - | LN |
| Clark et al[21] | 4893 | 8.4 (5 yr, LN+) | - | LN |
| 25 (5 yr, LN-) | ||||
| de Jong et al[22] | 449 | 31 (5 yr) | 27 | IM, V, LN |
| Li et al[23] | 115 | - | - | Cirrhosis |
| Chen et al[24] | 320 | - | - | - |
| Ref. | n | Findings or conclusion |
| Uchiyama et al[15] | 334 | Lymph node metastasis: MF: 16%; IG: 0%; PI and MF + PI: 60% |
| Survival rate (5 yr): MF: 26%; IG: 79.3%; PI and MF + PI: 19.4% | ||
| Uno et al[17] | 273 | Rate of PI-type: 7.9% |
| The PI-type shows significantly better survival than MF- and MF + PI-type. | ||
| Morine et al[18] | 22 | The PI-type shows a lower incidence of intrahepatic metastasis |
| Routine lymph node dissection do not improve survival in MF-type |
Over the most recent 3 years, 19 studies have been published (Tables 1 and 2). Most of these studies describe the poor prognostic factors of resected cases of ICC. The most significant prognostic factor is lymph node metastasis. However, whether routine lymph node dissection improves postoperative survival is still unclear.
The literature on the macroscopic subtypes is very scant. Uchiyama et al[15] and Uno et al[17] reported that the PI type showed significantly better survival than the MF and MF+PI types, supporting the results of previous reports. The difference in malignant potential between each subtype emphasizes the importance of the preoperative identification of each subtype.
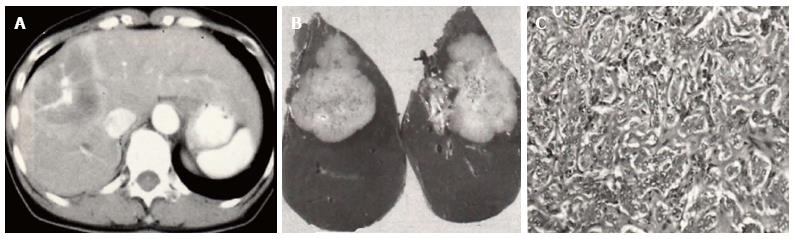
Table 3 summarizes recent radiologic studies of ICC[25-30]. The typical enhancement pattern of ICC on CT and MRI is that of ringed enhancement in the early phase with central delayed enhancement, reflecting the abundant fibrous stroma in ICC. However, Kim et al[26] reported that 6 (30%) of 20 ICCs appeared as hypervascular lesions with washout in the delayed phase, resembling HCCs. In addition, Ariizumi et al[29] pointed out that MF-type ICCs with hypervascular-type pattern had more favorable prognosis than those with the typical enhancement pattern. The histopathological characteristics of hypervascular-type ICCs have not been clarified. Cholangiocellular carcinoma (CoCC), a subtype of ICC, has been reported to originate from the ductules, or canals of Hering, and appears as a hypervascular mass similar to HCC[31]. These results of recent radiologic studies suggest the possibility that some ICCs share the same origin with that of CoCC, i.e., HPCs. Especially in MF-type ICCs, comparative studies between the enhancement patterns and histopathologic findings are needed for further exploration. However, these descriptions can be applied to only MF-type ICCs. Xu et al[28] reported the difference of enhancement patterns on contrast-enhanced ultrasonography between each subtype and demonstrated that most IG-type ICCs appeared as a mass showing homogenous hyperenhancement. This finding provides useful knowledge for preoperative differentiation between IG-type and PI-type ICC.
| Ref. | n | Method | Findings or conclusion |
| Nanashima et al[25] | 42 | CT | Factor for poor prognosis: case showing arterial enhancement with lower attenuation |
| Kim et al[26] | 20 | MRI | 6 (30%) of the 20 cases appeared as hypervascular lesions with washout on delayed phase |
| Kang et al[27] | 50 | MRI | Percentage of relative enhancement on hepatobiliary phase was significantly higher in moderately differentiated tumors than in poorly differentiated tumors and in patients without than in those with lymph node metastasis |
| Xu et al[28] | 40 | Contrast enhanced ultrasono-graphy | MF-type (n = 32): (1) peripheral rim-like hyperenhancement (n = 19); (2) heterogenous enhancement (n = 10); and (3) homogenous hyperenhancement (n = 3) |
| Ariizumi et al[29] | 26 | FDG PET | PI-type (n = 4): heterogenous enhancement (n = 4) IG-type (n = 4): (1) homogenous hyperenhancement (n = 3); and (2) heterogenous enhancement (n = 1) FDG PET was able to predict patient outcome after radioembolization treatment |
During the most recent 3 years, many molecules have been identified as biomarkers for poor prognosis of ICCs (Tables 4-6)[31-71]. Among these, researchers have paid close attention to molecules associated with epithelial-mesenchymal transition (EMT)[32,38,53,55]. The close association between EMT and the progression of ICC was confirmed not only by immunohistochemistry but also by functional and comprehensive analyses. The fact that EMT induces progression of ICC led us to hypothesize that abundant fibrous stroma in ICCs play an important role in the invasive growth and metastasis of this cancer. In addition, Oishi et al[53] reported that activation of miR-200c induced a reduction in EMT and in the expression of neural adhesion molecule (NCAM). Given that NCAM is known to be a hepatic progenitor cell marker, a hypothesis that the hepatic progenitor cell markers and molecules associated with EMT are regulated by common upstream molecules can be proposed. Further functional analyses are needed to confirm this hypothesis.
| Ref. | n | Method | Target | Conclusion |
| Gu et al[32] | 85 | IHC | E-cadherin | (-)por |
| Beta-catenin | (-)por | |||
| Vimentin | (-)por | |||
| Yan et al[33] | 49 | IHC | Smad4 | (-)por, advanced stage, LN |
| Kamphues et al[34] | 65 | DNA-Cyto | DNA-index | (+)poor prognosis |
| Mano et al[35] | 132 | IHC | Roundabout-1 | (-)Size, Ki67index, poor prognosis |
| Slit-1 | (-)PN, LN | |||
| Yin et al[36] | 411 | Serum | γ-glutamyl transferase | (+)V, LN, poor prognosis, |
| incomplete encapsulation | ||||
| Sulpice et al[37] | 40 | mRNA | Osteopontin | (+)poor prognosis |
| (Stroma) | TGFβ2 | (+)poor prognosis | ||
| Laminin | (+)poor prognosis | |||
| Zhou et al[38] | Cell | mRNA | Notch-1 | (+)EMT |
| line | Western | |||
| Li et al[39] | 173 | IHC | CKAP4 | (+)favorable prognosis |
| Nanashima et al[40] | 38 | IHC | CD44 | (+)PI-type, poor prognosis |
| Gli1 | (+)poor prognosis | |||
| Nutthasirikul et al[41] | - | mRNA | Δ133p53/TA | (+)poor prognosis |
| P53 | ||||
| - | IHC | Mutantp53 | (+)poor prognosis | |
| Zhang et al[42] | 33 | mRNA | Capn4 | (+)LN, advanced stage, |
| Western | Poor prognosis | |||
| Ding et al[43] | 20 | IHC | Integrinα6 | (+)IM, Size, V, poor prognosis |
| Cell | Integrinα6 | (-)decrease of metastasis | ||
| Aishima et al[44] | 134 | IHC | Cox-2 | (+)poor prognosis, LN |
| iNOS | (-) LN | |||
| Chen et al[45] | 61 | IHC | IMP3 | (+)Por, advanced stage, V |
| poor prognosis, CA19-9 |
| Ref. | n | Method | Target | Conclusion |
| Shi et al[46] | 138 | IHC | DKK-1 | (+)poor prognosis |
| elevated sMMP9 and VEGF-C | ||||
| Cell | DKK-1 | (-)decrease in cell migration and invasiveness | ||
| (+)LN, Por, advanced stage, V | ||||
| Yao et al[47] | 96 | IHC | Vimentin | poor prognosis |
| and | ||||
| N-cadherin | (+)MF-type | |||
| Zhou et al[48] | 54 | IHC | HBx-protein | well differentiated tumor |
| (+)well differentiated tumor, IG-type | ||||
| Choi et al[49] | 46 | IHC | CK20 | (+)favorable prognosis |
| MUC6 | (+)Size, LN, V, advanced stage | |||
| Jeong et al[50] | 43 | IHC | FABP-5 | (-)decrease in cell proliferation and |
| Cell | FABP-5 | invasion | ||
| (+)elevated serum CEA and CA | ||||
| Tsai et al[51] | 112 | IHC | S100P | 19-9 value, MUC2 positive |
| poor prognosis | ||||
| (+)perineural invasion | ||||
| 86 | Sequencing | K-ras mutation | poor prognosis | |
| miR-200c | (+)reduction of EMT | |||
| Oishi et al[53] | - | Microarray | reduction of NCAM1 expression | |
| HCV core | (+)enhanced NFAT expression | |||
| Liao et al[54] | - | Cell | protein | (+)enhanced Angiotensin II receptor expression and fibrogenesis of |
| Angiotensin | cancerous stroma, metastasis | |||
| Okamoto et al[55] | - | Cell | II and SDF1 |
| Source | n | Method | Target | Conclusion |
| Li et al[56] | - | Tissues | miR-214 | (-)increased expression of Twist(EMT |
| -associated gene) | ||||
| Gu et al[57] | 123 | IHC | IL-17cells | (+)poor prognosis |
| (intratumoral) | ||||
| Higashi et al[58] | 63 | IHC | MUC16 | (+)poor prognosis |
| Gu et al[59] | 83 | IHC | E-cadherin | (-)poor prognosis |
| Beta-catenin | (-)V | |||
| EGFR | (+)Por | |||
| Wang et al[60] | 77 | IHC | P-70S6K | (+)Por |
| 4EBP1 | (+)poor prognosis | |||
| Hirashita et al[61] | 35 | IHC | MMP-7 | (+)poor prognosis |
| Srimunta et al[62] | 55 | IHC | ABCC-1 | (+)poor prognosis |
| Morine et al[63] | 35 | IHC | HDAC | (+)advanced stage, LN |
| poor prognosis | ||||
| Wakai et al[64] | 34 | IHC | RRM1 | (+)gemcitabine resistance |
| Larbcharoensub et al[65] | 60 | IHC | ABCG2 | (-)poor prognosis, LN, Por |
| Lee et al[66] | 101 | IHC | PTEN | (+)favorable prognosis |
| P-AKT1 | (+)favorable prognosis | |||
| P-MTOR | (+)favorable prognosis | |||
| Dong et al[67] | 108 | IHC | Beclin1 | (-)LN, poor prognosis |
| Shinozaki et al[68] | 83 | IHC | Claudin-18 | (+)LN, PI-type, perineural invasion |
| Wakai et al[69] | 34 | IHC | NQO1 | (-)Por, poor prognosis |
| Aishima et al[70] | 110 | IHC | S100P | (+)PI-type |
| S100P(nuc) | (+)LN, V | |||
| Zhou et al[71] | 89 | IHC | MAGE3/4 | (+)larger tumor size, poor prognosis |
The literature on the association between macroscopic subtypes and the expression of genes are very scant[48,49,68,70], similar to that in the clinical study literature. Shinozaki et al[68] reported that claudin-18 (CLDN18), a tight junction protein specific to the stomach and lung, is highly expressed in precancerous lesions of biliary intraepithelial neoplasms and PI components of ICCs. CLDN18 has been reported to be expressed in various gastrointestinal cancer tissues and to be associated with morphogenesis of the histologic subtype and the specific mucin phenotype[72]. In addition, we previously reported the association between the expression of CLDN18 and intestinal-type differentiation in intraductal papillary-mucinous neoplasm of the pancreas[73]. Thus, there is considerable interest in the crucial role of CLDN18 in the development of PI-type morphology in ICCs.
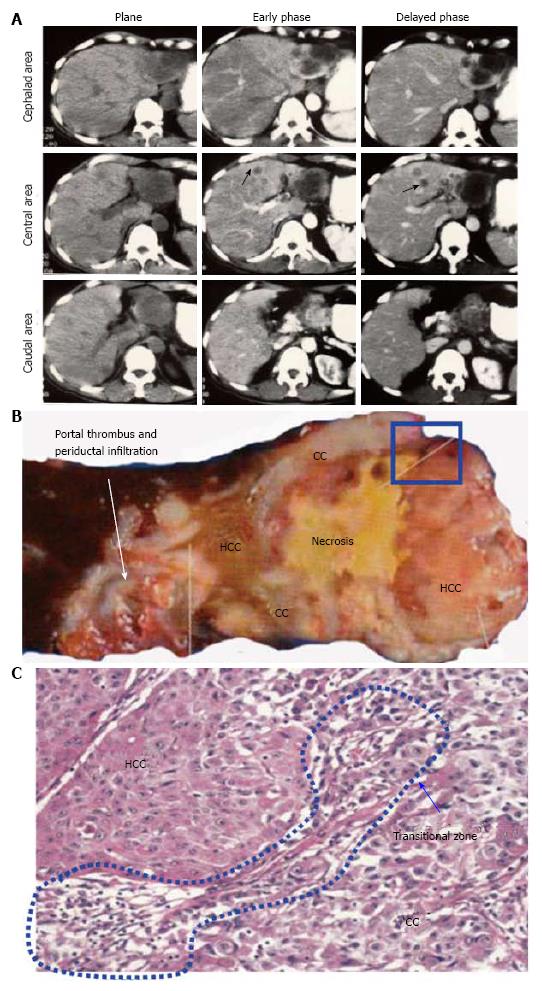
There is a large dissociation in the postoperative survival rates of cHCC-CC reported in the recent researches[74-90] (Tables 7-9), probably because the case numbers are limited. In addition, cHCC-CC is associated with many factors that contribute to poor prognosis including lymph node metastasis, higher levels of serum AFP, and portal vein thrombosis, reflecting intermediate features of cHCC-CC between HCC and ICC (Figure 2). The intermingling of findings of cHCC-CCs are also demonstrated by radiologic studies. Based on the new WHO classification system of cHCC-CC, some immunohistochemical research highlighting the expression of HPC markers has been published in the past 3 years in which YAP1 and EpiCAM, are reported to be markers of poor prognosis. These molecules are mainly distributed across the intermediate- and cholangiocellular-type components. Kim et al[85] reported that YAP1 is localized in the transitional zone between HCC and ICC components. In addition, Akiba et al[87] demonstrated that vimentin is strongly expressed in intermediate-type cHCC-CC. Similar to their role in ICCs, HPC markers may also play a crucial role in the progression of cHCC-CC through EMT. These components may harbor biological instability resembling undifferentiated carcinoma that leads to invasive behavior. However, CoCC, a subtype of ICC, has been known to be a tumor with characteristics resembling those of HCC and to have a relatively favorable prognosis (Figure 3). Given that CoCC is also derived from HPCs[31], a contradictory point exists with regard to the role of HPCs in the progression of ICCs and cHCC-CCs. We speculate that each HPC marker performs various functions involving progression and metastasis of ICCs and cHCC-CCs to a lesser or greater extent.
| Source | n | Conclusion or findings |
| Yap et al[74] | 11 | Survival rate: 69.3% (3 yr) |
| Lee et al[75] | 65 | (1) The clinical characteristics of cHCC-CC are similar to those of HCC |
| (2) Overall survival of cHCC-CC is similar to that of ICC | ||
| Yin et al[76] | 113 | (1) Findings similar to HCC: infection with hepatitis virus; presence of cirrhosis; elevated AFP levels |
| (2) Findings similar to ICC: serum CA19-9 elevation; incomplete capsules; lymph node involvement | ||
| (3) Survival rate: 41.4%(3 yr); 36.4% (5 yr) | ||
| (4) Factors for poor prognosis: radical liver resection | ||
| Ariizumi et al[77] | 44 | (1) Survival rate: 24% |
| (2) Median survival time: 15.4 mo | ||
| Yu et al[78] | 14 | (1) Clinical characteristics: hepatitis B virus infection: 13/14; |
| elevated AFP levels: 11/14 | ||
| (2) Median survival time: 7.9 mo | ||
| (3) Stem cell markers (IHC): c-Kit 71.4%; CD90: 85.7%; CD133: 92.9%; CK19: 78.6% | ||
| Park et al[79] | 21 | Factor for poor prognosis: serum AFP levels |
| Park et al[80] | 43 | (1) median survival time: 34 mo |
| (2) Survival rate: 18.1% (5 yr) | ||
| (3) Factors for poor prognosis: Portal vein thrombosis; distant metastasis | ||
| Zhan et al[81] | 27 | (1) CK-7: 86.4%; CK19: 90.9% |
| (2) Survival rate: 49.4% | ||
| (3) Factors for higher recurrence: lymph node metastasis |
| Ref. | n | Methods | Conclusion or findings |
| Ijichi et al[82] | 3 | FDG | (1) SUVmax value of three cHCC-CC cases: 9.9, 12.0, and 13 |
| -PET | (2) Median SUVmax value of poorly differentiated HCC: 5.7 | ||
| (1) 6/11 showed early ring enhancement with progressive enhancement in central portion. | |||
| (2) 5/11 showed a diffuse heterogenous early enhancement. | |||
| de Campos et al[83] | 11 | MRI | Characteristics findings of cHCC-CC: irregular shape and strong rim enhancement during early phase; absence of target appearance on hepatobiliary-phase |
| Hwang et al[84] | 20 | MRI |
| Ref. | n | Method | Target | Conclusion |
| Kim et al[85] | 58 | IHC | YAP1 | (+): transition zone, poor prognosis |
| EpiCAM | (-)favorable prognosis | |||
| CK19 | (-)favorable prognosis | |||
| Ikeda et al[86] | 36 | IHC | DLK1 | (+)poor prognosis |
| Akiba et al[87] | 54 | IHC | CD56 | (+): components apart from HCC |
| c-Kit | (+): components apart from HCC | |||
| EpiCAM | (+): components apart from HCC | |||
| CD133 | (+): intermediate type or cholangiolocellular type | |||
| Vimentin | (+): intermediate type or cholangiolocellular type | |||
| Coulouarn et al[88] | 152 | Microarray | - | (1) TGFbeta and beta-catenin are identified as the two major signals in the progression of cHCC-CC/ |
| (2) cHCC-CC shares the characteristics of poorly differentiated HCC. | ||||
| (+)poor prognosis | ||||
| Both HCC and CC components of most | ||||
| Of the cHCC-CC express both AFP and | ||||
| Cai et al[89] | 80 | IHC | PCNA | CK19 |
| Itoyama et al[90] | 20 | IHC | AFP and | |
| CK19 |
Recent research in ICC has revealed that each tumor shows different clinical and radiologic characteristics between the macroscopic subtypes. However, there are still many unclear points regarding the molecular mechanisms yielding these subtypes. It is of particular interest to identify the molecular markers inducing invasion, metastasis, and the macroscopic growth patterns of ICC. Many researchers have noted that HPC markers and EMT are involved in the progression of ICCs. Because most cHCC-CCs show MF-type morphology, we infer that HPC markers are closely associated with the morphogenesis and histogenesis of MF-type ICCs. Therefore, studies of ICC, and especially of its molecular pathology, should be designed in conjunction with those of cHCC-CC.
P- Reviewer: Basoli A, Lin ZY, Qin JM, Ramia JM, Xu R S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Igami T, Ebata T, Yokoyama Y, Sugawara G, Takahashi Y, Nagino M. Staging of peripheral-type intrahepatic cholangiocarcinoma: appraisal of the new TNM classification and its modifications. World J Surg. 2011;35:2501-2509. [PubMed] |
| 2. | Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2:419-427. [PubMed] |
| 3. | Lo RC, Ng IO. Hepatic progenitor cells: their role and functional significance in the new classification of primary liver cancers. Liver Cancer. 2013;2:84-92. [PubMed] |
| 4. | Zheng YW, Nie YZ, Taniguchi H. Cellular reprogramming and hepatocellular carcinoma development. World J Gastroenterol. 2013;19:8850-8860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Theise ND, Nakashima O, Park YN. Combined hepatocellular-cholangiocacinoma in WHO classification of tumors of the digestive system; World health organization of tumors. : IARC, Lyon 2010; 225-227. |
| 6. | Marubashi S, Gotoh K, Takahashi H, Ohigashi H, Yano M, Ishikawa O, Sakon M. Prediction of the postoperative prognosis of intrahepatic cholangiocarcinoma (ICC): importance of preoperatively- determined anatomic invasion level and number of tumors. Dig Dis Sci. 2014;59:201-213. [PubMed] |
| 7. | Guglielmi A, Ruzzenente A, Campagnaro T, Valdegamberi A, Bagante F, Bertuzzo F, Conci S, Iacono C. Patterns and prognostic significance of lymph node dissection for surgical treatment of perihilar and intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2013;17:1917-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Zhu HF, Li J, Huang L, Yan YQ. Intrahepatic cholangiocarcinoma: a clinicopathologic study of 37 resected cases. Hepatogastroenterology. 2013;60:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Dhanasekaran R, Hemming AW, Zendejas I, George T, Nelson DR, Soldevila-Pico C, Firpi RJ, Morelli G, Clark V, Cabrera R. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol Rep. 2013;29:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 833] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 11. | De Rose AM, Cucchetti A, Clemente G, Ardito F, Giovannini I, Ercolani G, Giuliante F, Pinna AD, Nuzzo G. Prognostic significance of tumor doubling time in mass-forming type cholangiocarcinoma. J Gastrointest Surg. 2013;17:739-747. [PubMed] |
| 12. | Sulpice L, Rayar M, Boucher E, Pele F, Pracht M, Meunier B, Boudjema K. Intrahepatic cholangiocarcinoma: impact of genetic hemochromatosis on outcome and overall survival after surgical resection. J Surg Res. 2013;180:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Ribero D, Pinna AD, Guglielmi A, Ponti A, Nuzzo G, Giulini SM, Aldrighetti L, Calise F, Gerunda GE, Tomatis M. Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 434 Patients. Arch Surg. 2012;147:1107-1113. [PubMed] |
| 14. | Liu ZH, Chen Z, Ma LL, Li XH, Wang LX. Factors influencing the prognosis of patients with intrahepatic cholangiocarcinoma. Acta Gastroenterol Belg. 2012;75:215-218. [PubMed] |
| 15. | Uchiyama K, Yamamoto M, Yamaue H, Ariizumi S, Aoki T, Kokudo N, Ebata T, Nagino M, Ohtsuka M, Miyazaki M. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2011;18:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Chen LP, Li C, Wang C, Wen TF, Yan LN, Li B. Predictive factors of recurrence for patients with intrahepatic cholangiocarcinoma after hepatectomy. Hepatogastroenterology. 2012;59:1765-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Uno M, Shimada K, Yamamoto Y, Nara S, Esaki M, Sakamoto Y, Kosuge T, Ojima H. Periductal infiltrating type of intrahepatic cholangiocarcinoma: a rare macroscopic type without any apparent mass. Surg Today. 2012;42:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Morine Y, Shimada M, Utsunomiya T, Imura S, Ikemoto T, Mori H, Hanaoka J, Kanamoto M, Miyake H. Clinical impact of lymph node dissection in surgery for peripheral-type intrahepatic cholangiocarcinoma. Surg Today. 2012;42:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Jiang BG, Ge RL, Sun LL, Zong M, Wei GT, Zhang YJ. Clinical parameters predicting survival duration after hepatectomy for intrahepatic cholangiocarcinoma. Can J Gastroenterol. 2011;25:603-608. [PubMed] |
| 20. | Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Sueda T. Intrahepatic cholangiocarcinoma: clinicopathological differences between peripheral type and hilar type. J Gastrointest Surg. 2012;16:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Clark CJ, Wood-Wentz CM, Reid-Lombardo KM, Kendrick ML, Huebner M, Que FG. Lymphadenectomy in the staging and treatment of intrahepatic cholangiocarcinoma: a population-based study using the National cancer institute SEER database. HPB (Oxford). 2011;13:612-620. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 559] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 23. | Li YY, Li H, Lv P, Liu G, Li XR, Tian BN, Chen DJ. Prognostic value of cirrhosis for intrahepatic cholangiocarcinoma after surgical treatment. J Gastrointest Surg. 2011;15:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Chen YX, Zeng ZC, Tang ZY, Fan J, Zhou J, Jiang W, Zeng MS, Tan YS. Prediction of the lymph node status in patients with intrahepatic cholangiocarcinoma: analysis of 320 surgical cases. Front Oncol. 2011;1:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Nanashima A, Abo T, Murakami G, Matsumoto A, Tou K, Takeshita H, Kunizaki M, Hidaka S, Sakamoto I, Hayashi H. Intrahepatic cholangiocarcinoma: relationship between tumor imaging enhancement by measuring attenuation and clinicopathologic characteristics. Abdom Imaging. 2013;38:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Kim SH, Lee CH, Kim BH, Kim WB, Yeom SK, Kim KA, Park CM. Typical and atypical imaging findings of intrahepatic cholangiocarcinoma using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. J Comput Assist Tomogr. 2012;36:704-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Kang Y, Lee JM, Kim SH, Han JK, Choi BI. Intrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR images. Radiology. 2012;264:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Xu HX, Chen LD, Liu LN, Zhang YF, Guo LH, Liu C. Contrast-enhanced ultrasound of intrahepatic cholangiocarcinoma: correlation with pathological examination. Br J Radiol. 2012;85:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Ariizumi S, Kotera Y, Takahashi Y, Katagiri S, Chen IP, Ota T, Yamamoto M. Mass-forming intrahepatic cholangiocarcinoma with marked enhancement on arterial-phase computed tomography reflects favorable surgical outcomes. J Surg Oncol. 2011;104:130-139. [PubMed] |
| 30. | Haug AR, Heinemann V, Bruns CJ, Hoffmann R, Jakobs T, Bartenstein P, Hacker M. 18F-FDG PET independently predicts survival in patients with cholangiocellular carcinoma treated with 90Y microspheres. Eur J Nucl Med Mol Imaging. 2011;38:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 280] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 32. | Gu MJ, Choi JH. Epithelial-mesenchymal transition phenotypes are associated with patient survival in intrahepatic cholangiocarcinoma. J Clin Pathol. 2014;67:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Yan XQ, Zhang W, Zhang BX, Liang HF, Zhang WG, Chen XP. Inactivation of Smad4 is a prognostic factor in intrahepatic cholangiocarcinoma. Chin Med J (Engl). 2013;126:3039-3043. [PubMed] |
| 34. | Kamphues C, Al-Abadi N, Dürr A, Bova R, Klauschen F, Stenzinger A, Bahra M, Al-Abadi H, Neuhaus P, Seehofer D. The DNA index is a strong predictive marker in intrahepatic cholangiocarcinoma: the results of a five-year prospective study. Surg Today. 2014;44:1336-1342. [PubMed] |
| 35. | Mano Y, Aishima S, Fukuhara T, Tanaka Y, Kubo Y, Motomura T, Toshima T, Iguchi T, Shirabe K, Maehara Y. Decreased roundabout 1 expression promotes development of intrahepatic cholangiocarcinoma. Hum Pathol. 2013;44:2419-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Yin X, Zheng SS, Zhang BH, Zhou Y, Chen XH, Ren ZG, Qiu SJ, Fan J. Elevation of serum γ-glutamyltransferase as a predictor of aggressive tumor behaviors and unfavorable prognosis in patients with intrahepatic cholangiocarcinoma: analysis of a large monocenter study. Eur J Gastroenterol Hepatol. 2013;25:1408-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Sulpice L, Rayar M, Desille M, Turlin B, Fautrel A, Boucher E, Llamas-Gutierrez F, Meunier B, Boudjema K, Clément B. Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology. 2013;58:1992-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 38. | Zhou Q, Wang Y, Peng B, Liang L, Li J. The roles of Notch1 expression in the migration of intrahepatic cholangiocarcinoma. BMC Cancer. 2013;13:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Li MH, Dong LW, Li SX, Tang GS, Pan YF, Zhang J, Wang H, Zhou HB, Tan YX, Hu HP. Expression of cytoskeleton-associated protein 4 is related to lymphatic metastasis and indicates prognosis of intrahepatic cholangiocarcinoma patients after surgery resection. Cancer Lett. 2013;337:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Nanashima A, Hatachi G, Tsuchiya T, Matsumoto H, Arai J, Abo T, Murakami G, Tominaga T, Takagi K, Nagayasu T. Clinical significances of cancer stem cells markers in patients with intrahepatic cholangiocarcinoma who underwent hepatectomy. Anticancer Res. 2013;33:2107-2114. [PubMed] |
| 41. | Nutthasirikul N, Limpaiboon T, Leelayuwat C, Patrakitkomjorn S, Jearanaikoon P. Ratio disruption of the ∆133p53 and TAp53 isoform equilibrium correlates with poor clinical outcome in intrahepatic cholangiocarcinoma. Int J Oncol. 2013;42:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Zhang C, Bai DS, Huang XY, Shi GM, Ke AW, Yang LX, Yang XR, Zhou J, Fan J. Prognostic significance of Capn4 overexpression in intrahepatic cholangiocarcinoma. PLoS One. 2013;8:e54619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Ding YB, Deng B, Huang YS, Xiao WM, Wu J, Zhang YQ, Wang YZ, Wu DC, Lu GT, Wu KY. A high level of integrin α6 expression in human intrahepatic cholangiocarcinoma cells is associated with a migratory and invasive phenotype. Dig Dis Sci. 2013;58:1627-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Aishima S, Mano Y, Tanaka Y, Kubo Y, Shirabe K, Maehara Y, Oda Y. Different roles of inducible nitric oxide synthase and cyclooxygenase-2 in carcinogenesis and metastasis of intrahepatic cholangiocarcinoma. Hum Pathol. 2013;44:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Chen YL, Jeng YM, Hsu HC, Lai HS, Lee PH, Lai PL, Yuan RH. Expression of insulin-like growth factor II mRNA-binding protein 3 predicts early recurrence and poor prognosis in intrahepatic cholangiocarcinoma. Int J Surg. 2013;11:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Shi RY, Yang XR, Shen QJ, Yang LX, Xu Y, Qiu SJ, Sun YF, Zhang X, Wang Z, Zhu K. High expression of Dickkopf-related protein 1 is related to lymphatic metastasis and indicates poor prognosis in intrahepatic cholangiocarcinoma patients after surgery. Cancer. 2013;119:993-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Yao X, Wang X, Wang Z, Dai L, Zhang G, Yan Q, Zhou W. Clinicopathological and prognostic significance of epithelial mesenchymal transition-related protein expression in intrahepatic cholangiocarcinoma. Onco Targets Ther. 2012;5:255-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Zhou YM, Cao L, Li B, Zhang XZ, Yin ZF. Expression of HBx protein in hepatitis B virus-infected intrahepatic cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2012;11:532-535. [PubMed] |
| 49. | Choi JE, Noh SJ, Lee JH, Bae JS, Chu HH, Park HS, Jang KY, Chung MJ, Kang MJ, Lee DG. Expression of keratin 20 and its clinicopathological significance in intrahepatic cholangiocarcinoma. Oncol Lett. 2012;4:534-540. [PubMed] |
| 50. | Jeong CY, Hah YS, Cho BI, Lee SM, Joo YT, Jung EJ, Jeong SH, Lee YJ, Choi SK, Ha WS. Fatty acid-binding protein 5 promotes cell proliferation and invasion in human intrahepatic cholangiocarcinoma. Oncol Rep. 2012;28:1283-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Tsai JH, Huang WC, Kuo KT, Yuan RH, Chen YL, Jeng YM. S100P immunostaining identifies a subset of peripheral-type intrahepatic cholangiocarcinomas with morphological and molecular features similar to those of perihilar and extrahepatic cholangiocarcinomas. Histopathology. 2012;61:1106-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Chen TC, Jan YY, Yeh TS. K-ras mutation is strongly associated with perineural invasion and represents an independent prognostic factor of intrahepatic cholangiocarcinoma after hepatectomy. Ann Surg Oncol. 2012;19 Suppl 3:S675-S681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Oishi N, Kumar MR, Roessler S, Ji J, Forgues M, Budhu A, Zhao X, Andersen JB, Ye QH, Jia HL. Transcriptomic profiling reveals hepatic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology. 2012;56:1792-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 54. | Liao Q, Li Z, Chen R, Guo N, Zeng B, Cheng D, Zheng L. [Effect of hepatitis C virus core gene transfection on NFAT1 expression in human intrahepatic cholangiocarcinoma cells]. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:789-793. [PubMed] |
| 55. | Okamoto K, Tajima H, Nakanuma S, Sakai S, Makino I, Kinoshita J, Hayashi H, Nakamura K, Oyama K, Nakagawara H. Angiotensin II enhances epithelial-to-mesenchymal transition through the interaction between activated hepatic stellate cells and the stromal cell-derived factor-1/CXCR4 axis in intrahepatic cholangiocarcinoma. Int J Oncol. 2012;41:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Li B, Han Q, Zhu Y, Yu Y, Wang J, Jiang X. Down-regulation of miR-214 contributes to intrahepatic cholangiocarcinoma metastasis by targeting Twist. FEBS J. 2012;279:2393-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 57. | Gu FM, Gao Q, Shi GM, Zhang X, Wang J, Jiang JH, Wang XY, Shi YH, Ding ZB, Fan J. Intratumoral IL-17⁺ cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2012;19:2506-2514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 58. | Higashi M, Yamada N, Yokoyama S, Kitamoto S, Tabata K, Koriyama C, Batra SK, Yonezawa S. Pathobiological implications of MUC16/CA125 expression in intrahepatic cholangiocarcinoma-mass forming type. Pathobiology. 2012;79:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Gu MJ, Choi JH. Clinicopathological significance of E-cadherin, β-catenin and epidermal growth factor receptor expression in intrahepatic cholangiocarcinoma. Hepatogastroenterology. 2012;59:1241-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Wang Z, Zheng T, Wu Q, Wang J, Wu C, Wang J. Immunohistochemical analysis of the mTOR pathway in intrahepatic cholangiocarcinoma. Neoplasma. 2012;59:137-141. [PubMed] |
| 61. | Hirashita T, Iwashita Y, Ohta M, Komori Y, Eguchi H, Yada K, Kitano S. Expression of matrix metalloproteinase-7 is an unfavorable prognostic factor in intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2012;16:842-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Srimunta U, Sawanyawisuth K, Kraiklang R, Pairojkul C, Puapairoj A, Titipungul T, Hahnvajanawong C, Tassaneeyakul W, Wongkham C, Wongkham S. High expression of ABCC1 indicates poor prognosis in intrahepatic cholangiocarcinoma. Asian Pac J Cancer Prev. 2012;13 Suppl:125-130. [PubMed] |
| 63. | Morine Y, Shimada M, Iwahashi S, Utsunomiya T, Imura S, Ikemoto T, Mori H, Hanaoka J, Miyake H. Role of histone deacetylase expression in intrahepatic cholangiocarcinoma. Surgery. 2012;151:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Wakai T, Shirai Y, Sakata J, Takamura M, Matsuda Y, Korita PV, Muneoka K, Sasaki M, Ajioka Y, Hatakeyama K. Ribonucleotide reductase M1 expression in intrahepatic cholangiocarcinoma. Hepatogastroenterology. 2011;58:1659-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 65. | Larbcharoensub N, Sornmayura P, Sirachainan E, Wilasrusmee C, Wanmoung H, Janvilisri T. Prognostic value of ABCG2 in moderately and poorly differentiated intrahepatic cholangiocarcinoma. Histopathology. 2011;59:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Lee D, Do IG, Choi K, Sung CO, Jang KT, Choi D, Heo JS, Choi SH, Kim J, Park JY. The expression of phospho-AKT1 and phospho-MTOR is associated with a favorable prognosis independent of PTEN expression in intrahepatic cholangiocarcinomas. Mod Pathol. 2012;25:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 67. | Dong LW, Hou YJ, Tan YX, Tang L, Pan YF, Wang M, Wang HY. Prognostic significance of Beclin 1 in intrahepatic cholangiocellular carcinoma. Autophagy. 2011;7:1222-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 68. | Shinozaki A, Shibahara J, Noda N, Tanaka M, Aoki T, Kokudo N, Fukayama M. Claudin-18 in biliary neoplasms. Its significance in the classification of intrahepatic cholangiocarcinoma. Virchows Arch. 2011;459:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Wakai T, Shirai Y, Sakata J, Matsuda Y, Korita PV, Takamura M, Ajioka Y, Hatakeyama K. Prognostic significance of NQO1 expression in intrahepatic cholangiocarcinoma. Int J Clin Exp Pathol. 2011;4:363-370. [PubMed] |
| 70. | Aishima S, Fujita N, Mano Y, Kubo Y, Tanaka Y, Taketomi A, Shirabe K, Maehara Y, Oda Y. Different roles of S100P overexpression in intrahepatic cholangiocarcinoma: carcinogenesis of perihilar type and aggressive behavior of peripheral type. Am J Surg Pathol. 2011;35:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 71. | Zhou JX, Li Y, Chen SX, Deng AM. Expression and prognostic significance of cancer-testis antigens (CTA) in intrahepatic cholagiocarcinoma. J Exp Clin Cancer Res. 2011;30:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Sanada Y, Oue N, Mitani Y, Yoshida K, Nakayama H, Yasui W. Down-regulation of the claudin-18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J Pathol. 2006;208:633-642. [PubMed] |
| 73. | Sanada Y, Hirose Y, Osada S, Tanaka Y, Takahashi T, Yamaguchi K, Yoshida K. Immunohistochemical study of claudin 18 involvement in intestinal differentiation during the progression of intraductal papillary mucinous neoplasm. Anticancer Res. 2010;30:2995-3003. [PubMed] |
| 74. | Yap AQ, Chen CL, Yong CC, Kuo FY, Wang SH, Lin CC, Liu YW, Lin TL, Li WF, Millan CA. Clinicopathological factors impact the survival outcome following the resection of combined hepatocellular carcinoma and cholangiocarcinoma. Surg Oncol. 2013;22:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 75. | Lee CH, Hsieh SY, Chang CJ, Lin YJ. Comparison of clinical characteristics of combined hepatocellular-cholangiocarcinoma and other primary liver cancers. J Gastroenterol Hepatol. 2013;28:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Yin X, Zhang BH, Qiu SJ, Ren ZG, Zhou J, Chen XH, Zhou Y, Fan J. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol. 2012;19:2869-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 77. | Ariizumi S, Kotera Y, Katagiri S, Nakano M, Yamamoto M. Combined hepatocellular-cholangiocarcinoma had poor outcomes after hepatectomy regardless of Allen and Lisa class or the predominance of intrahepatic cholangiocarcinoma cells within the tumor. Ann Surg Oncol. 2012;19:1628-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Yu XH, Xu LB, Zeng H, Zhang R, Wang J, Liu C. Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2011;10:620-625. [PubMed] |
| 79. | Park HS, Bae JS, Jang KY, Lee JH, Yu HC, Jung JH, Cho BH, Chung MJ, Moon WS. Clinicopathologic study on combined hepatocellular carcinoma and cholangiocarcinoma: with emphasis on the intermediate cell morphology. J Korean Med Sci. 2011;26:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 80. | Park H, Choi KH, Choi SB, Choi JW, Kim do Y, Ahn SH, Kim KS, Choi JS, Han KH, Chon CY. Clinicopathological characteristics in combined hepatocellular-cholangiocarcinoma: a single center study in Korea. Yonsei Med J. 2011;52:753-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Zhan Q, Shen BY, Deng XX, Zhu ZC, Chen H, Peng CH, Li HW. Clinical and pathological analysis of 27 patients with combined hepatocellular-cholangiocarcinoma in an Asian center. J Hepatobiliary Pancreat Sci. 2012;19:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Ijichi H, Shirabe K, Taketomi A, Yoshizumi T, Ikegami T, Mano Y, Aishima S, Abe K, Honda H, Maehara Y. Clinical usefulness of (18) F-fluorodeoxyglucose positron emission tomography/computed tomography for patients with primary liver cancer with special reference to rare histological types, hepatocellular carcinoma with sarcomatous change and combined hepatocellular and cholangiocarcinoma. Hepatol Res. 2013;43:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | de Campos RO, Semelka RC, Azevedo RM, Ramalho M, Heredia V, Armao DM, Woosley JT. Combined hepatocellular carcinoma-cholangiocarcinoma: report of MR appearance in eleven patients. J Magn Reson Imaging. 2012;36:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Hwang J, Kim YK, Park MJ, Lee MH, Kim SH, Lee WJ, Rhim HC. Differentiating combined hepatocellular and cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma using gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2012;36:881-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 85. | Kim GJ, Kim H, Park YN. Increased expression of Yes-associated protein 1 in hepatocellular carcinoma with stemness and combined hepatocellular-cholangiocarcinoma. PLoS One. 2013;8:e75449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Ikeda H, Harada K, Sato Y, Sasaki M, Yoneda N, Kitamura S, Sudo Y, Ooi A, Nakanuma Y. Clinicopathologic significance of combined hepatocellular-cholangiocarcinoma with stem cell subtype components with reference to the expression of putative stem cell markers. Am J Clin Pathol. 2013;140:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Akiba J, Nakashima O, Hattori S, Tanikawa K, Takenaka M, Nakayama M, Kondo R, Nomura Y, Koura K, Ueda K. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol. 2013;37:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 88. | Coulouarn C, Cavard C, Rubbia-Brandt L, Audebourg A, Dumont F, Jacques S, Just PA, Clément B, Gilgenkrantz H, Perret C. Combined hepatocellular-cholangiocarcinomas exhibit progenitor features and activation of Wnt and TGFβ signaling pathways. Carcinogenesis. 2012;33:1791-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 89. | Cai X, Zhai J, Kaplan DE, Zhang Y, Zhou L, Chen X, Qian G, Zhao Q, Li Y, Gao L. Background progenitor activation is associated with recurrence after hepatectomy of combined hepatocellular-cholangiocarcinoma. Hepatology. 2012;56:1804-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 90. | Itoyama M, Hata M, Yamanegi K, Yamada N, Ohyama H, Hirano H, Terada N, Nakasho K. Expression of both hepatocellular carcinoma and cholangiocarcinoma phenotypes in hepatocellular carcinoma and cholangiocarcinoma components in combined hepatocellular and cholangiocarcinoma. Med Mol Morphol. 2012;45:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |