INTRODUCTION
Studies have demonstrated that reactive oxygen species (ROS) are involved in the pathogenesis of pancreatitis[1]. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), a transmembrane flavoprotein enzyme, uses NADPH as an electron donor to catalyze the univalent reduction of oxygen, resulting in the production of superoxide free radical, which might be a source of oxidants in injured pancreas[1]. NOX is mainly distributed in the phagocytic cell membrane with cytochrome C and flavin adenine dinucleotide groups, which can produce ROS, scavenging pathogenic microorganisms such as bacteria[2]. ROS, being generated by NOX, also participate in intracellular signaling processes in the pancreas. Recently, research on NOX is no longer limited to inflammatory cells, but extends to the aspect of pancreatic acinar cells and pancreatic stellate cells (PSCs) in pancreatitis patients[2]. The function of NOX, which is involved in the pathogenesis of inflammation in pancreatic acinar cells and PSCs, has become the hotspot of research. Non-phagocytic NOX derived ROS function as a messenger molecule to participate in the modulation of cell differentiation, proliferation and apoptosis in the pancreas. In this review, we summarize the literature on NOX protein structure, activation, function and its role in the pathogenesis of pancreatitis.
STRUCTURE, LOCATION AND FUNCTION OF NOX IN THE PANCREAS
NOX is a multicomponent enzyme consisting of five different subunits, including the subunits p22phox and gp91phox (also known as NOX2) located in the membrane, together with the cytosolic subunits p40phox, p47phox and p67phox. The participation of Rac would elicit full oxidase activity[3-5]. Relative to gp91phox (the catalytic subunit of NOX), p22phox, p47phox, p40phox and p67phox are regulatory subunits. Gp91phox in different types of cells has other six homologues, termed NOX1, NOX3, NOX4, NOX5, DUOX1 and DUOX2, which constitute the NOX family proteins[6-8]. NOX is an enzyme which was initially discovered in phagocytes[4,5]. NOX in neutrophils is composed of constitutive subunits (p22phox and gp91phox) positioned in membrane and regulatory subunits (p47phox and p67phox, and possibly p40phox) stationed in the cytosol[9]. In recent years, NOX has been discovered in several nonphagocytic cells such as fibroblasts[10], vascular smooth muscle cells[11] and hepatic stellate cells[12]. More recently, it has been found that NOX was present in pancreatic β cells[13,14], pancreatic acinar cells[15-18] and PSCs[19,20]. The main intrinsic components of NOX comprising the NOX2 isoform are present in human pancreatic islets[14]. Cytosolic subunits p47phox and p67phox as well as membrane-bound subunits p22phox and NOX1 are constitutively expressed in pancreatic acinar AR42J cells[16,21,22]. The key subunits of NOX including p22phox, p47phox, NOX activator 1 (a homologue of p67phox), NOX1, NOX4, and NOX2 (gp91phox) are expressed in PSCs[19,20]. The activation of non-phagocytic NOX is similar to that in neutrophils[23]. Upon activation of NOX, p47 translocates to the membrane and then recruits p67 to interact with the p22 subunit, thus facilitating NADPH-dependent formation of superoxide (O2-), which increases the production of secondary ROS such as hydrogen peroxide (H2O2)[21]. Non-phagocytic NOX derived ROS function as a messenger molecule to participate in the modulation of cell differentiation, proliferation and apoptosis[6-8]. NOX protein family can be activated quickly under pathophysiological conditions, leading to high production of ROS, which contributes to oxidative stress and a wide range of diseases.
ACTIVATION AND INHIBITION FACTORS OF NOX IN THE PATHOGENESIS OF PANCREATITIS
Cholecystokinin analogues
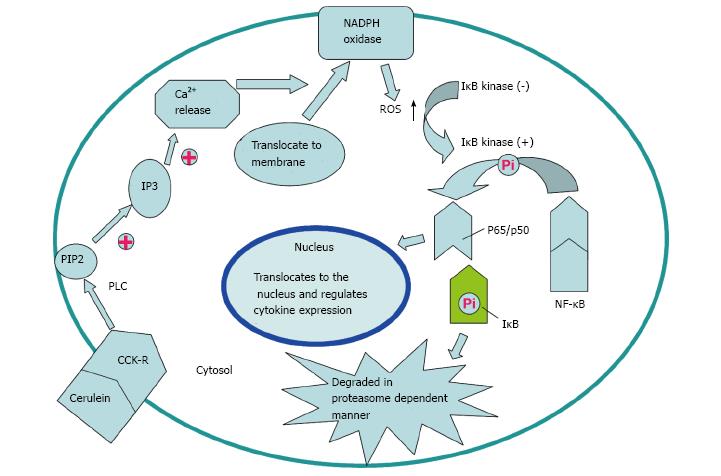
Cerulein, an analogue of cholecystokinin (CCK), can stimulate the pancreatic exocrine secretion by binding CCK receptors, causing the autolysis of pancreatic acinar cell[24]. There are two kinds of CCK receptor subtypes, CCK1 and CCK2 receptors. CCK1 receptors regulate pancreatic digestive enzymes, satiety and feeding behavior, while CCK2 receptors enhance the level of gastric acid, as well as gastrin which has anti-apoptotic effects on pancreatic cells[25]. Experimental pancreatitis induced with high dosages of cerulean, similar to human edematous pancreatitis, is characterized by cytoplasmic vacuolization, formation of edema and acinar cell death as well as elevation in serum levels of digestive enzymes caused by unconventional secretion of digestive enzymes[26]. ROS are involved in the activation of oxidant-sensitive nuclear transcription factor (NF-κB), expression of cytokine, apoptosis and further occurrence of pancreatitis[27]. P47phox, p67phox, NOX1 and p22phox in pancreatic AR42J cells could produce ROS after cerulein stimulation[21]. Intrapancreatic trypsin is not only activated by high-dose cerulein, but also regulated by neutrophils via NADPH oxidase[28]. The mechanism for the activation of NF-κB and expression of cytokines in pancreatic acinar cells stimulated by cerulein may be summarized as the following steps. Cerulein binds to the CCK receptor, a G-protein-coupled receptor, to activate phospholipase C (PLC) and inositol 1,4,5-trisphosphate (IP3), triggering transient Ca2+ release from the endoplasmic reticulum in pancreatic acinar cells. NOX activated by Ca2+ produces ROS to activate IκB kinase and then to phosphorylate IκB. Phosphorylated IκB can be ubiquitinated and degraded in a proteasome dependent manner to eliminate the inhibition of NF-κB, a p65/p50 heterodimer in the cytosol. NF-κB then translocates to the nucleus to mediate the expression of cytokines which are involved in the pathogenesis of pancreatitis (Figures 1 and 2)[27].
Figure 1 Potential mechanism of nicotinamide adenine dinucleotide phosphate oxidase activation via cholecystokinin receptor.
Cerulein and cholecystokinin (CCK) receptor binding triggers transient Ca2+ release from the endoplasmic reticulum to activate nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which is mediated by PLC and IP3. Reactive oxygen species (ROS) generated by NADPH oxidase activate IκB kinase to phosphorylate IκB in the cytosol. Phosphorylated IκB is ubiquitinated and degraded in a proteasome-dependent manner. NF-κB translocates to the nucleus and regulates expression of cytokines to participate in the pathogenesis of pancreatitis.
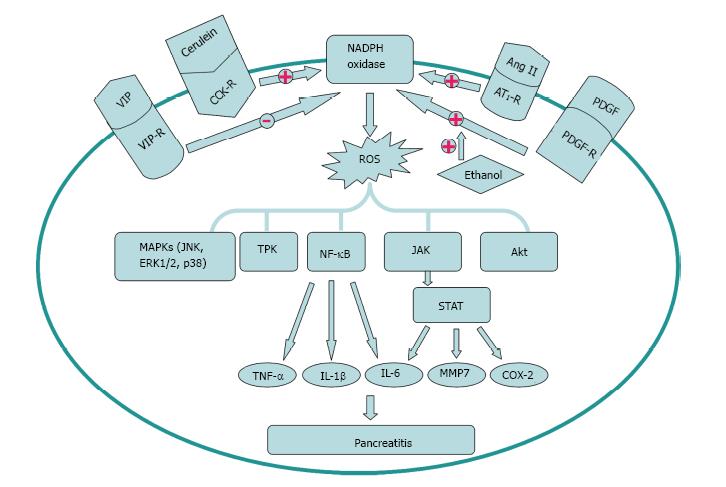
Figure 2 Activation and inhibition factors of nicotinamide adenine dinucleotide phosphate oxidase signal transduction in the pathogenesis of pancreatitis.
Cerulein, Ang II and platelet derived growth factor (PDGF) can enhance, while VIP can decrease the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Ethanol can augment the activation of the cell’s NADPH oxidase system stimulated by PDGF. The downstream signal molecules including MAPKs, TPK, NF-κB, JAK/STAT and Akt participate in the pathogenesis of pancreatitis. TNF: Tumor necrosis factor; IL: Interleukin; TPK: Tyrosine protein kinase; MAPK: Mitogen activated protein kinase.
Renin-angiotensin system
The Renin-angiotensin system (RAS) is generally considered to regulate blood pressure and body fluid homeostasis[29]. The pancreatic RAS activation that is related to the production of ROS might contribute to oxidative stress and tissue injury[30,31]. Angiotensin II, an active mediator of RAS, is transformed from angiotensin I by the angiotensin-converting enzyme (ACE)[32]. The effect of angiotensin II is regulated by its receptors, including angiotensin II type 1 receptor (AT1R) and angiotensin II type 2 receptor (AT2R)[32]. Many reports indicate that interaction of angiotensin II with AT1R promotes superoxide anion production through NOX system[30,31,33,34]. Inhibition of the AT1R, but not AT2R, may play a significant role in decreasing the severity of acute pancreatitis. Mechanism of NOX activation by AT1R and AT2R might contribute to different effects of AT1R and AT2R inhibitors on pancreatic injury induced by cerulein. Activation of pancreatic NOX was associated with oxidative stress which can be indicated by the level of protein oxidation in rats stimulated with cerulein[30,35]. However, further investigations about the potential application of RAS inhibitors including AT1R in treating acute pancreatitis are needed in the future (Figure 2).
Ethanol and platelet derived growth factor
Alcohol abuse has long been recognized as the most common factor leading to chronic pancreatitis[36]. Activated stellate cells are viewed as vital regulators of chronic alcoholic pancreatitis or fibrosis. Hu et al[20] investigated the mechanisms of action of alcohol on PSCs to determine the correlation of NOX system and alcohol with the proliferation of PSCs. The results demonstrated that NOX activity was predominantly located in the cell membrane fraction (95%) compared to the cytosolic fraction (5%) of the stellate cells. platelet derived growth factor (PDGF) could increase NOX activity in a dose- and time-dependent manner. PSC proliferation caused by alcohol is mediated by the activation of PDGF induced NADPH oxidase system. However, ethanol did not show a significant effect on stellate cell DNA synthesis, which provides a new perspective for the mechanism of fibrosis stimulated with alcohol (Figure 2)[20].
Vasoactive intestinal peptide
Previous reports found that vasoactive intestinal peptide (VIP) could decrease the production of cytokines to alleviate experimental acute pancreatitis[37]. VIP could decrease the level of ROS significantly and increase cell viability in acini cells in a dose dependent manner. NOX1 and NOX2 markedly increased following treatment with H2O2 in pancreatic acini. Besides, H2O2 can stimulate the activation of NOX. The production of ROS was affected by VIP via NADPH oxidase and the cAMP/PKA pathway because decreased NOX activity by administration of VIP could be abolished by PKA inhibitor H89. Oxidative stress and tissue injury in acini can be decreased by VIP through NOX inhibition (Figure 2)[38].
NOX SIGNAL TRANSDUCTION IN THE PATHOGENESIS OF PANCREATITIS
NOX protein family can be activated quickly under pathophysiological conditions, leading to high production of ROS, which contributes to oxidative stress and a wide range of diseases. Furthermore, ROS can act as an intracellular second messenger or chemoattractant to enhance the level of cytokines, resulting in the aggravation of pancreatitis[38]. Studies indicate that pro-inflammation cytokines such as IL-1β, IL-6 and TNF-α mediate the local or systemic manifestations of acute pancreatitis. IL-1β and TNF-α released from activated pancreatic macrophages respond to local tissue damage. Locally, these cytokines may aggravate the severity of acute pancreatitis. Systemically, IL-6 can increase the capillary permeability and accelerate the leukocyte adherence, leading to multiple organ failure (Figure 2)[27].
NF-κB and Janus kinase/signal transducers and activators of transcription
NF-κB, a member of the Rel family of transcription factors, can regulate the activation of cellular stress-related genes or early response genes such as growth factors, cytokines, adhesion molecules, and acute-phase proteins[39,40]. The Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway was relevant to the immune response mediated by numerous cytokines and non-immune response mediated by hormones and growth factors. The JAK/STAT pathway activated by the family of cytokine receptors regulate a variety of biological processes, such as immune response, cell survival, differentiation, proliferation and oncogenesis[41]. Recently, reports indicated that cerulein could activate the JAK2/STAT3 pathway through NOX in pancreatic acinar cells[27].
NOX may be the source of ROS in pancreatic acinar cells during pancreatitis. ROS can induce expression of cytokines, apoptosis, NF-κB and JAK/STAT pathway activation, thus regulating the inflammation and apoptosis in pancreatic acinar cells. Consequently, NOX, NF-κB and JAK2/STAT3 may be involved in the pathogenesis of acute pancreatitis[27]. Inflammation and apoptosis in pancreatic acinar cells during pancreatitis may be alleviated by inhibition of NOX, NF-κB and JAK/STAT through suppression of inflammatory cytokines, apoptosis and caspase-3 activity. Ju et al[23] found that NOX inhibition suppresses STAT3-DNA binding, JAK2/STAT3 activation and TGF-β1 level in AR42J cells stimulated by cerulein. Therefore, ROS may activate NF-κB to induce cytokine production in pancreatic acinar cells through activation of NOX during pancreatitis[21]. NOX, NF-κB and JAK/STAT may be potential targets for treatment of acute pancreatitis.
Mitogen activated protein kinase and tyrosine protein kinase
Recently, studies found that mitogen activated protein kinase (MAPK) and tyrosine protein kinase (TPK) might be involved in NOX signal transduction pathway. ROS induced by the family of NOX can cause protein phosphorylation and cell apoptosis directly or indirectly.
In the direct way, ROS mediate the activation of the MAPK pathway and TPK pathway to promote protein phosphorylation in pancreatic acinar cells. ROS activate the signal transduction pathway which consists of different MAPK family members probably owing to the activation of the upstream ERK1/2 kinase pathway. ROS stimulate TPK signaling pathway through increasing the TPK activity, thereby promoting protein tyrosine phosphorylation and affecting signal transduction to regulate cell proliferation, differentiation, metabolism and apoptosis. Inhibition of NOX or ROS significantly reduced the p38MAPK signaling cascade[42]. Activation of the MAPK signaling pathway including SAPK/JNK, ERK1/2 and p38 by ROS induce cell apoptosis. The activation of the MAPK pathway is mainly dependent on the inhibition of tyrosine phosphatase by ROS[43].
In the indirect way, ROS reduce phosphatase activity, decrease protein dephosphorylation, and thus indirectly increase protein phosphorylation. ROS injure DNA, lipid and protein, thus indirectly inducing apoptosis. In some cases, NOX family can also inhibit cell apoptosis through ROS, which activate the pathway of NF-κB and Akt/ASK1, thereby reducing cell apoptosis[44].
NOX ACTIVATION IN DIFFERENT PANCREATIC CELLS INVOLVED IN THE PATHOGENESIS OF PANCREATITIS
Phagocytes
In support of the involvement of oxygen free radicals in acute pancreatitis, studies have addressed the possibility that the severity of pancreatitis can be reduced by inhibiting the activity of oxygen-derived free radicals[45]. ROS could have different origins, and the role of the NOX system in neutrophils but not pancreatic acinar tissue is originally considered essential. The phagocytic NOX is a multicomponent enzyme complex that is composed of membranous and cytosolic proteins in the resting cell. During activation, approximately 10% of cytosolic proteins including p47phox and p67phox are phosphorylated and translocate to the cell membrane to form active catalytic complexes with p22phox and gp91phox, resulting in the generation of ROS[4]. Intrapancreatic trypsin activation and acinar cell trypsin-activation peptide (TAP) labeling induced by high dose cerulein were significantly decreased in neutrophil depleted rats. NOX deficient mice displayed attenuation of the cerulean-induced trypsin activation, while myeloperoxidase (MPO) deficient mice did not. Neutrophils have been considered to be implicated in pathologic activation of digestive enzymes by infiltrating the pancreas in acute pancreatitis, which is mediated by products of NOX[28].
Evidence suggests that inflammatory cell infiltration is an early and vital event in acute pancreatitis, which will lead to local and systemic complications[46]. Many of the pathological failures of acute pancreatitis may be a consequence of the overstimulation of leukocytes[47]. The argument put forward was that once pancreatitis has been initiated, chemoattractants for polymorphonuclear leukocytes, macrophages and platelets are released, possibly via the action of oxygen derived free radicals. The chemoattractants induce leukocytes and macrophages to adhere to the endothelium of the postcapillary venule and to migrate into the interstitial spaces. Stimulus-secretion coupling causes synthesis of a range of enzymes including elastase, cathepsins, phospholipase A2, phospholipase C, platelet-activating factor (PAF) and MPO. When the quantity of material to be digested is excessive, phagocytosis may become so vigorous that the contents of leukocyte and macrophage granules are spilled outside the cell where they increase the severity of inflammation. As a result, large amounts of oxygen-derived free radicals are produced and may exceed the capacity of superoxide dismutase (SOD) and catalase to inactivate them[48].
Pancreatic acinar cells
ROS and apoptosis can be observed in pancreatic acinar cells in cerulein induced pancreatitis[49,50]. NADPH has been considered to be the major source of ROS in pancreatitis[18,21,22]. Oxidative stress induced inflammation and apoptosis have been implicated in pancreatitis[51,52]. Cerulein induced the expression of apoptosis-inducing factor (AIF). AIF is located in the mitochondrial membrane of pancreatic acinar cells. During apoptosis, AIF translocates from mitochondria to the cytoplasm and then enters into the nucleus, resulting in nuclear DNA aggregation and breakage to induce apoptosis of pancreatic acinar cells[53,54]. Antisense oligonucleotides (AS ODN) transfection or Ca2+ chelator treatment decreased the expression of AIF induced by cerulein in AR42J cells. These results suggested that intracellular Ca2+ increase and NOX activation might be the upstream events of AIF expression, which result in cerulein induced apoptosis of AR42J cells[18,55].
The activation of NOX was inhibited and the production of ROS was decreased when cerulein-stimulated pancreatic acinar cells were treated with Ca2+ chelator, which indicates that Ca2+ activate NOX and ROS. Transfection with AS ODN for NOX subunits p22phox and p47phox can inhibit the ROS generation, illustrating that NOX mediates the production of ROS. The apoptotic indices including apoptotic genes bax and p53, DNA fragmentation, caspase 3 activity, TUNEL staining and cell viability were inhibited by treatment with Ca2+ chelator or AS ODN transfection, indicating that NOX regulates ROS-induced apoptosis in a Ca2+ dependent manner in pancreatic acinar cells[22]. Diphenyleneiodonium (DPI), an inhibitor of NOX, reduces the AIF expression and caspase-3 activation, and thus inhibits apoptosis of AR42J cells[16]. During the stimulation with cerulein, the increase of NOX accelerates the formation of ROS in cells and mitochondria, thus further inducing the apoptosis of acinar cells[56,57]. ROS generated by pancreatic acinar cells stimulated with bile acids or cerulein can induce apoptosis and, at the same time, induce pancreatitis[58-60].
Research indicates that JAK2/STAT3 activation and increases of MAPKs and TGF-β1 induced by administration of cerulein were inhibited by AS ODN transfection in AR42J cells, which shows that NOX can activate JAK2/STAT3, MAPKs and TGF-β1[23]. NOX may regulate the production of cytokines by activating NF-κB in AR42J cells stimulated with cerulein. Rebamipide, an antiulcer agent, can scavenge ROS and decrease the level of superoxide[61,62]. Transfection with AS ODN for NOX subunits or administration of DPI or rebamipide inhibited cerulein induced NF-κB activation and IL-6 expression[21]. Cerulein also could produce large amounts of ROS to activate NF-κB and thus stimulate the expression of cytokines in freshly isolated pancreatic acinar cells without inflammation[63].
Numerous studies have shown that increases of ROS and peroxidation products are accompanied with endogenous antioxidant depletion in the early stage of pancreatitis. Many preclinical antioxidant treatments, including genetic manipulation, significantly reduce pancreatic injury and inflammation[1,64-66]. However, randomized clinical trials of antioxidants have produced conflicting results[67], and treatment of pancreatitis with antioxidants has even been discontinued because of adverse events[68]. Moreover, several studies indicated that NOX was only present in neutrophils but not in pancreatic acinar cells[28,69].
PSCs
PSCs are the major fibrogenic cells in chronic injury of the pancreas, which encircle the acinus[70,71]. PSCs account for approximately 4% of the total pancreatic cells[72]. PSCs are quiescent in normal pancreas and can be identified by the character of vitamin A containing lipid droplets in the cytoplasm. When chronic pancreatitis happens, PSCs are activated and transformed into myofibroblast-like cells. As a result, intracellular lipid droplets disappear and α-smooth muscle actin (α-SMA) and extracellular components such as fibronectin and collagen arise[19,73]. Besides, PSCs may be involved in the pathogenesis of acute pancreatitis[72]. Therefore, suppression of PSC activation is a potential target to treat pancreatic inflammation and fibrosis.
Studies showed that p22phox, p47phox, NOX1, gp91phox (NOX2), and NOX4 were expressed in rat quiescent and culture-activated PSCs as well as human activated PSCs, while p67phox and NOX3 were not detected. NOX activator 1 was present in human PSCs, while NOX organizer 1 was not detected. NOX can activate PSCs, which can be verified by DPI inhibition experiments. Studies showed that DPI could inhibit the activation of PSCs, that is, to inhibit proliferation, chemokine production, α-SMA and collagen expression. Platelet-derived growth factor BB (PDGF-BB) promoted proliferation of rat PSCs, which was inhibited by DPI in a dose-dependent manner, showing that NOX underlies the PDGF induced PSC proliferation. DPI decreased the chemokine production, which indicates that NOX also regulates the production of chemokines. DPI decreased the levels of α-SMA and collagen, once again, proving that NOX activate PSCs. DPI also inhibited interleukin 1β (IL-1β) and PDGF induced activation of MAPKs in PSCs, and this evidence indicates that NOX mediates the activation of MAPKs induced by IL-1β and PDGF in PSCs[19].
FUTURE RESEARCH ON THE PATHOGENESIS OF PANCREATITIS IN NOX
Accumulated evidence suggested that ROS induced by NOX play a significant role in pancreatitis. The activation of ROS mediates the activation of many cytokines[56,57]. ROS can induce cell apoptosis through direct and indirect pathways[43,44]. ROS induced by bile acids and cerulein can promote apoptosis of pancreatic acinar cells[18,69]. NOX is usually induced by cerulein, inflammatory factors, cytokines and growth factors as well as other stimuli in pancreatic acinar cells and PSCs. NOX can generate ROS, which in turn increase cytokines levels downstream to initiate the next activation cycle. The positive feedback of activation process might be one of the causes of pancreatitis. Although many scholars have made a great deal of research about the pathogenic mechanisms of NOX in the inflammation of pancreatic acinar cells and stellate cells, the relative importance of different pathogenic mechanisms of NOX in the pathogenesis of pancreatitis, the relationship between various pathogenic mechanisms of NOX, the specific pathways involved in each mechanism of NOX in pancreatitis, and the feasibility of NOX targeted therapy applied to pancreatitis are all needed to be studied in the future.
P- Reviewer: Coelho AMM S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Lu YJ