INTRODUCTION
Microscopic colitis is regarded as one of the common causes of chronic watery diarrhea. The incidence rate for collagenous colitis is 0.8/100000-6.2/100000. Many cases have been reported in western countries and in Asian countries like India. Lindstrom and Freeman described the term collagenous colitis concurrently in 1976. Microscopic colitis (MC) refers to two medical conditions which cause diarrhea: collagenous colitis and lymphocytic colitis. The following triad of clinicopathological features characterizes both conditions: (1) chronic watery nonbloody diarrhea; (2) normal mucosal appearance on colonoscopy; and (3) characteristic histopathology.
Patients are characteristically, although not exclusively, middle-aged females. They present with a long history of watery nonbloody diarrhea which may be profuse. There is a strong association between autoimmune diseases, for example arthritis, Sjorgen’s syndrome and celiac disease, and microscopic colitis. There are reports of associations with multiple drugs, especially non-steroidal anti-inflammatory drugs. Colonoscopy is normal or near normal. The changes are often patchy, so multiple colonic biopsies must be taken in order to make a correct histological diagnosis[1-3]. A full colonoscopy is required as an examination limited to the rectum will miss cases of MC.
EPIDEMIOLOGY
The true incidence of MC is not known. The disease has been increasingly diagnosed over the past 20 years but is still uncommon. A recently published population-based study found the incidence of microscopic colitis to increase significantly from 1.1 per 100000 persons in the late 1980s to 19.6 per 100000 persons by the end of 2001. More recent epidemiological studies done in this century confirmed these high incidence numbers, showing that actual incidence and prevalence numbers are higher than initially thought and are still able to show rising incidences, although the rise is far less pronounced than before. Most recent north American studies show incidence rates of 7.1 per 100000 person-years for collagenous colitis and 12.6 per 100000 person-years for lymphocytic colitis[4].
Morbidity is limited to the consequences of diarrhea, including metabolic abnormalities such as hypokalemia and dehydration, weight loss and fatigue. This is not considered a life-threatening condition; however, profuse watery diarrhea may lead to severe dehydration and electrolyte abnormalities requiring intensive resuscitation.
Lymphocytic colitis affects similar numbers of men and women, while collagenous colitis is up to 20 times more frequent in women than in men[5].
Both conditions are observed most commonly in people over the age of 40 years, with peak incidence in the sixth and seventh decades of life, and the incidence of both conditions increases with age. Isolated cases have been reported in younger populations, including children[5-8].
ETIOPATHOGENESIS
The etiology of MC is most likely multifactorial with a mucosal inflammatory response to yet not specified noxious luminal agent occurring in a predisposed host. The noxious luminal agent may be a single one or multiple ones summing up to an individual threshold. Technically, MC is an inflammatory bowel disease (IBD) and the disease shares a number of etiological aspects with the so-called classical inflammatory bowel diseases like Crohn’s disease and ulcerative colitis. Among the possible predisposing and/or contributing factors for microscopic colitis, genetic factors and intraluminal noxious factors are best studied. Although a limited number of familial clusters of microscopic colitis have been reported, there is only minimal evidence of a genetic component within the etiology of MC. All reported so-called family clusters are very small and comprise of a maximum of two reported family members. In contrast, there is evidence of a predisposition of sensitivity to gastrointestinal inflammatory insults in patients with microscopic colitis since up to 12% of patients with MC have a family history of celiac disease or even inflammatory bowel disease[8]. The meaning of the association between Human Leukocyte antigen (HLA-DQ2, DQ1, DQ3) and microscopic colitis and the high prevalence of a tumor necrosis factor (TNFα) gene polymorphisms in patients with microscopic colitis deserves further attention as it may lead to a discovery of a hereditary component of microscopic colitis of presently unknown penetration[9]. Furthermore, metalloproteinase-9 gene variations have been reported to be associated with collagenous colitis[10]. However, the meaning of all the presently reported genetic associations is poorly understood and the respective research is presently not driven by hypotheses, rather than by incidental observations or genetic screening. Very strong evidence exists for an autoimmune basis to the development of both collagenous and lymphocytic colitis. The association of MC with autoimmune-based disorders such as celiac, thyroid disease and rheumatoid arthritis, as well as the female preponderance, supports the notion that both forms of MC have a strong association with autoimmune diseases and may well be an autoimmune disorder themselves. However, to date, no specific autoantibody has been identified as being diagnostic for or being associated with collagenous or lymphocytic colitis[11]. It is known that MC can be found together with various autoantibodies and phenotypes, like human leukocyte antigen (HLA)-DR3 phenotype, although these associations are not strong enough to be regarded as being diagnostically relevant or useful, nor do we know what these associations mean. A case series of 4 patients is described in which subjects developed classic symptoms of lymphocytic-collagenous colitis with typical mucosal histopathology during treatment with omeprazole/esomeprazole (proton pump inhibitors)[12]. Symptoms promptly stopped and mucosal biopsies returned to normal with drug withdrawal. Disease quickly recurred in 2 patients who were re-exposed to the drugs, one with biopsy documented recurrent collagenous colitis. Luminal factors of whatever kind seem to play an important role in the pathogenesis of microscopic colitis. Numerous drugs were reported to have a high or at least intermediate probability of causality in microscopic colitis[13]. Other luminal factors like infectious or even toxic agents are supported by studies that found either onset of MC following a gastrointestinal infection or improvement of symptoms with the initiation of antibiotics in the context of a proven or suspected gastrointestinal infection Yesinia species[14], Clostridium difficile[15] and Campylobacter species[16] were suggested in published case reports to cause MC; although, interpreting these observations in the context of current knowledge, it is most likely that these cases are of sporadic nature. In some small retrospective case series, bile acid malabsorption was found in up to 60% of patients with lymphocytic and up to 44% of patients with collagenous colitis, supporting the notion that MC may at least in some patients be caused by bile acid malabsorption. Whether bile acid malabsorption is causative or not remains questionable, as later studies were unable to confirm these observations[17]. Still, this may direct therapeutic decisions and especially in patients with a cholecystectomy, a bile acid directed treatment should be considered. Basic science is still in its infancy when it comes to studying microscopic colitis and possible causes, drivers, mechanisms or even pathophysiological models. A recent bench side study employing sigmoid tissues from patients with collagenous colitis and lymphocytic colitis was able to identify that sodium transport and epithelial barrier function are disturbed in patients with microscopic colitis[18]. Unfortunately, it remains unclear whether these reported changes are of causal nature, of transient nature, or a consequence of the underlying microscopic colitis. Even although these descriptive studies at least initiate a scientific discussion on what may be mechanisms underlying or involved in the development and the resolution of microscopic colitis, these small mechanistic studies have to be carefully taken since such studies are highly artificial in the techniques they use and therefore the results are most likely influenced not only by numerous circumstances like laboratory procedures and protocols but also by patient drug use, age and even nutritional status. Thus, such information has to be considered as hypotheses generating information that hopefully guides future prospective studies that help us to understand the mechanisms involved in the pathogenesis, maintenance and resolution of microscopic colitis and symptoms associated with the histological changes.
Using molecular techniques, it was reported that in patients with MC, increased interferon-γ, TNF-α and IL-1β levels suggest a Th1 cytokine profile being involved in the inflammatory process[18]. The differences in mucosal lymphocyte subsets seen in patients with collagenous and lymphocytic colitis[19] is not fully understood presently. This information may help us to understand the inflammatory mechanisms involved and may be useful for future therapeutic approaches. Environmental factors may play a crucial role in the etiology of MC, although other than cigarette smoking, presently no other factors are confirmed. For both collagenous and lymphocytic colitis, cigarette smoking is more prevalent compared to subjects without MC and first reports suggest that lung cancer is associated with MC[20-22]. The odds ratio (OR) for lymphocytic colitis and smoking (OR = 3.8) is higher than for collagenous colitis and smoking (OR = 2.4), although this difference was calculated on a small cohort of 120 patients with collagenous colitis, 70 patients with lymphocytic colitis, and 128 controls, and thus has to be verified in larger groups of patients[23]. Interestingly, it was additionally shown that MC occurs roughly 10 years earlier when the respective person is an active smoker, stressing the relevance of cigarette smoking to the pathophysiology of microscopic colitis. Beyond the strong results from association studies, it would be of great impact to learn whether cessation of smoking would cure MC or at least be beneficial to the patient’s symptoms, and a prospective clinical trial answering this seems worthwhile. In addition to the inflammatory component in the pathophysiology of MC, there may be an additional neuronal component to pathophysiology. A recent study identified increased chromogranin A, chromogranin B and secretoneurin levels in feces of patients with collagenous colitis compared to relevant control groups. These observations may point to a neurogenic involvement in MC and, additionally, these stool markers are suggested to be helpful in discriminating MC from irritable bowel syndrome or classical inflammatory bowel disease[24].
The precise mechanism of diarrhea in these patients is not well understood. Factors that may play a role include bile salt induced injury, active chloride excretion, decrease in net sodium absorption, creation of a diffusion barrier by collagen band and increased local inflammatory mediators such as nitric oxide and prostaglandins. It remains unclear which of these results in the symptoms reported by patients. Two studies have looked at inflammatory cytokines in MC. Patients with MC seem to have a predominantly TH1 type cytokine profile with significant increases in interferon gamma, TNF-α and interleukin 15, as well as an increased inducible nitric oxide synthetase. Others have found increased levels of Transforming growth factor-β in patients with collagenous colitis[25].
CLINICAL PRESENTATION
Collagenous and lymphocytic colitis present with very similar symptoms and from a clinical perspective, there is no specific symptom or clinical feature that allows discriminating one from the other. Thus, the differentiation between the two entities is made by histology only. The typical clinical presentation involves chronic (either recurrent or intermittent) relapsing watery, nonbloody diarrhea. Symptoms may have been present for several months to 2-3 years before medical attention is sought and a diagnosis is made. Less frequent complaints include abdominal cramping, fecal incontinence and weight loss, although weight loss may be seen in 40% or more of patients with collagenous colitis. Incontinence is probably more a reflection of the advanced age of those individuals who are affected and patients with this problem may do well if treated with antidiarrheal agents[26,27]. The natural history of MC is variable. Many cases are self-limiting, with symptoms lasting a few weeks or months. Others may be symptomatic for years in a relapsing or continuous pattern. Although a small number of case reports have suggested that MC may lead to development of ulcerative colitis, a small case series of patients with MC showed that none developed ulcerative colitis or Cohn’s disease after a follow-up of at least 6 years[28]. There are case reports on spontaneous and colonoscopy induced colonic perforations in patients with MC[29-30].
DIAGNOSIS/HISTOPATHOLOGY
The diagnosis of MC is dependent on (1) a convincing clinical history with other etiologies ruled out; (2) normal or near normal endoscopic and/or radiographic findings; and (3) endoscopic biopsies with histopathological findings consistent with MC.
The first step in the diagnostic process is a thorough history with particular attention paid to risk factors and the disease associated with MC. A complete history helps one to rule out other etiologies that may cause a similar clinical picture, such as IBD, celiac disease, diarrhea-predominant irritable bowel syndrome or infectious colitis.
Laboratory and radiographic investigations can be employed to rule out other entities on the differential diagnosis list but they are typically unremarkable.
Endoscopy with biopsy is necessary to arrive at the diagnosis. Colonoscopy generally reveals normal mucosal appearance. However, non-specific changes such as erythema, edema, abnormal vascular markings or even tears associated with perforation have been described. The hallmark of microscopic colitis is an increase in inflammatory cells (i.e., lymphocytes) in colonic biopsies with an otherwise normal appearance and architecture of the colon. Inflammatory cells are increased both in the surface epithelium (“intraepithelial lymphocytes”) and in the lamina propria. In lymphocytic colitis, these are the only abnormal features.
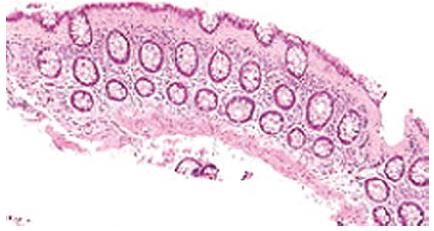
In collagenous colitis, the features of lymphocytic colitis are present, with the additional presence of a characteristic thickened sub epithelial collagen band which may be up to 30 μm thick (Figure 1)[31].
Figure 1 Micrograph of collagenous colitis, a type of microscopic colitis showing sub epithelial band of collagen H and E stain.
As the mucosa is not ulcerated or otherwise disrupted, the diarrhea generally does not contain blood or pus[32]. The diarrhea in collagenous colitis is likely due to inflammatory process and sub epithelial collagen serves as a cofactor in the role of a diffusion barrier and increased levels of immunoreactive prostaglandins E2 in stool water may lead to secretary diarrhea. Some cases may have fibrosis due to increased mucosal secretion of vascular endothelial growth factor[32,33]. One important question is how many biopsies need to be taken and how many biopsies are needed to confirm or rule out microscopic colitis. Numerous studies showed that the microscopic lesions can be skipped and therefore random multiple colonic biopsies should be taken[34].
Treatment
Treatment recommendations for MC are largely based on case reports and uncontrolled studies. Specific agents evaluated include 5-aminosalicylic acid (5-ASA), prednisone, immunomodulators, bismuth, probiotics and Boswellia extract. Small randomized controlled trials have shown that agents such as budesonide offer promise as an effective form of symptomatic therapy for both collagenous and lymphocytic colitis. As a first step in managing MC, an in depth medication history should be taken with potentially precipitating medications stopped where possible. Associated conditions such as celiac disease should be appropriately managed. In patients with mild symptoms, dietary restrictions like avoiding caffeine and lactose might be helpful.
Anti-diarrheal therapies
Non-specific anti-diarrheal therapies such as loperamide are commonly used in the management of MC. Retrospective studies have suggested benefit with doses ranging from 2 to 16 mg/d[34]. Due to the safety of this agent and the possibility of spontaneous remission, loperamide is the first-line therapy for MC.
Aminosalicylates
Uncontrolled retrospective series have suggested symptomatic improvement in up to 50% of patients with MC treated with mesalamine (5-ASA). A recent randomized trial of 64 MC patients compared mesalamine (800 mg tid) to mesalamine (800 mg tid) and cholestyramine (4 g/d). Treatment resulted in resolution of diarrhea in 84% overall after 2 wk. If treatment was continued over 6 mo, clinical and histological remission was achieved in 85% of those with lymphocytic and 91% of those with collagenous colitis. The number of relapsing patients after 6 mo of treatment was low and symptomatic relapses could be successfully retreated. Overall, the combination of mesalamine with cholestyramine was slightly superior[35,36].
Budesonide
Budesonide is currently the most promising treatment for collagenous colitis. Three trials involving 94 patients have shown that budesonide therapy (9 mg/d for 6-8 wk) compared to placebo resulted in statistically significant improvements in clinical symptoms and quality of life. A recent Cochrane database meta-analysis reported pooled OR of 12.3 for clinical response with budesonide with a number needed to treat of two. Although effective in the short-term, all trials showed a high rate (61%-80%) of relapse within 2 wk of budesonide cessation. Age < 60 years was a significant risk factor for relapse. Although there are no studies to support a tapering course of budesonide, many clinicians employ this in an effort to minimize the likelihood of relapse.
One randomized controlled trial of budesonide for the treatment of lymphocytic colitis has been conducted. When compared to the placebo arm, the patients randomized to budesonide (9 mg/d × 6 wk) had a statistically significantly higher rate of remission (< 3 bowel movements per day) at 3 and 6 wk[37-41].
Prednisolone
A double-blind, placebo-controlled randomized trial of oral prednisolone 50 mg/d for 2 wk for collagenous colitis was inconclusive because of the low number of patients enrolled[42]. Studies examining the effect of prednisone in the treatment of lymphocytic colitis have not been performed.
Immunosuppressive therapy
Immunosuppressive therapy with azathioprine or methotrexate has been utilized in patients either refractory to corticosteroid therapy or corticosteroid dependent, but there are no randomized controlled trials to guide therapy with these medications.
Other therapies
Small clinical trials studying bismuth subsalicylate, Boswellia serrata extract, probiotics and empirical antibiotic treatment for collagenous and lymphocytic colitis look promising but cannot be suggested outside of such trials. Finally, case reports suggest that pentoxifylline, verapamil and subcutaneous octreotide might be treatment options, but their use cannot be recommended at this time. When medical therapy was unsuccessful and symptoms were very severe, surgical interventions, such as a temporary or permanent loop ileostomy or even a proctocolectomy, have been employed in smaller case series.
CONCLUSION
To conclude, the term microscopic colitis is now used to describe both lymphocytic and collagenous colitis and the condition should be kept in mind in any patient with unexplained watery nonbloody diarrhea with normal endoscopic findings. Biopsy is a must to rule out either form of microscopic colitis. Based on symptom severity and disease duration, a stepwise approach to treatment is suggested.
P- Reviewers: Jayalakshmi J, Mahajan H, Saran RK S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Wu HL