INTRODUCTION
Gastric ulcers affect many people worldwide and are characterized by the presence of a deep necrotic lesion involving the entire mucosal thickness and the muscularis mucosae[1,2]. Gastric ulcer is a chronic disease featured with repeated healing and recurrence in the original location or elsewhere throughout the patient’s lifetime. Basically, its development is a result from an imbalance between mucosal defensive mechanisms, including mucus, bicarbonate, prostaglandins (PGs) and mucosal blood flow, and damaging factors, including acid and pepsin, in the luminal surface of the gastric mucosa[2]. Among these factors, the two major damaging causes implicated in gastric ulcer and ulcer recurrence are infection with Helicobacter pylori (H. pylori) and long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs)[3,4]. Toxins of H. pylori and NSAIDs disturb the ulcer healing mechanism by inhibiting epithelial cell proliferation, migration and angiogenesis, and by blocking growth factor triggered signaling pathways[5-7]. Gastric ulcers respond to conventional treatment, including H2-RA, acid suppressant drugs and antibiotic drugs for eradication of H. pylori, as well as the withdrawal of NSAIDs. However, several studies have shown that, in the ulcer healing process, acid inhibition or H. pylori eradication is insufficient for complete ulcer healing since the decrease of PGs and the increase of oxygen free radical leads to a varying quality of ulcer healing and is intimately associated with ulcer recurrence[8,9].
In 1993, Arakawa et al[10] proposed the concept and definition of the quality of ulcer healing (QOUH) based on histological maturity of regenerated tissue in the ulcer area, for which the additional intervention might be a prerequisite, including PGs, radical scavengers and the way of spurting regeneration, as well as the removal of inflammation. Since several studies have reported that a low recurrence rate is intimately associated with the achievement of the QOUH, efforts to achieve the QOUH might impose the ideal healing of an ulcer as well as functional restoration. The QOUH is achieved by several kinds of gastroprotective drugs, including rebamipide, sucralfate, misoprostol, ecabet sodium, sofalcone and antacids[6,11-15]. Rebamipide is one of the gastroprotective drugs able to intervene effectively in the process of ulcer healing and effectively improve the QOUH in the chronic acetic acid ulcer model. The combination of omeprazole and rebamipide accelerated the quality of ulcer healing through an increasing level of prostaglandin E2 (PGE2) and a decreasing level of Interleukin-8 (IL-8) and malondialdehyde (MDA) in the gastric mucosa, but not omeprazole alone[6]. In addition, rebamipide inhibited H. pylori CagA-induced effects on gastric epithelial cells, including morphological changes associated with ZO-1, IL-8 production and nuclear factor kappa B (NF-κB) activation in gastric epithelial cells[16].
The antacid talcid activates epidermal growth factor (EGF) and EGF-R expression in normal and ulcerated gastric mucosa. EGF and EGF-R are crucial for cell proliferation, migration, re-epithelialization and gland reconstruction within the scar. In addition, antacids, especially hydrotalcite, cause activation of prostaglandin synthesis; binding to and inactivation of pepsin, lysolecithin, bile acids and H. pylori toxins; activation of heat shock proteins; and activation of the genes encoding EGF, bFGF and their receptors[17]. Several diverse studies revealed the significance of the gastric defense system to overcome these limitations of acid suppressant treatment with ecabet sodium, Artemisia extracts and sucralfate.
Recently, we identified that natural product substances have an increasing interest because of fewer side effects, low cost and a potential source for anti-ulcer treatment through improving the QOUH and preventing ulcer recurrence. In this review, we discuss numerous natural products, including isopropanol, ethanol extracts of Artemisia, extracts from garlic and a special licorice extract, and their secondary metabolites with potential gastroprotection to achieve the QOUH and resistance to ulcer recurrence.
MECHANISMS OF GASTRIC ULCEROGENESIS AND QOUH
Mechanism of gastric ulcerogenesis
Gastric ulcers are a deep defect in the gastric wall involving the mucosal thickness and the muscularis mucosae. A gastric ulcer results from tissue necrosis induced by various conditions, such as aspirin, indomethacin, bile acids, alcohol intake, ischemia, stress, aging and H. pylori infection, the so called excess increment of damaging factors far exceeding the defense capacity. These conditions are known to disturb the mechanisms of gastric mucosal defense and develop characteristic morphological, ultrastructural and functional changes. When gastric mucosa is exposed to damaging agents, it encompasses the disruption of the unstirred mucus/bicarbonate/phospholipid layer, exfoliation of the surface epithelium with loss of its barrier and the deeper gastric mucosal layers, including microvascular endothelial cells, progenitor, parietal and chief cells. When the capillary endothelium was damaged, it led to microvascular stasis with cessation of oxygen and nutrient delivery and hypoxia. Microvascular damage occurs early during mucosal injury, leading to hypoxia and necrosis of glandular cells and thus adding an ischemic component to the direct toxic injury of cells. Vasoconstriction events produced by release of vasoactive and proinflammatory mediators from damaged mast cells, macrophages and endothelial cells further impair the mucosal microcirculation and ultimately result in mucosal necrosis in the form of ulcers[2,17]
Gastric ulcer healing
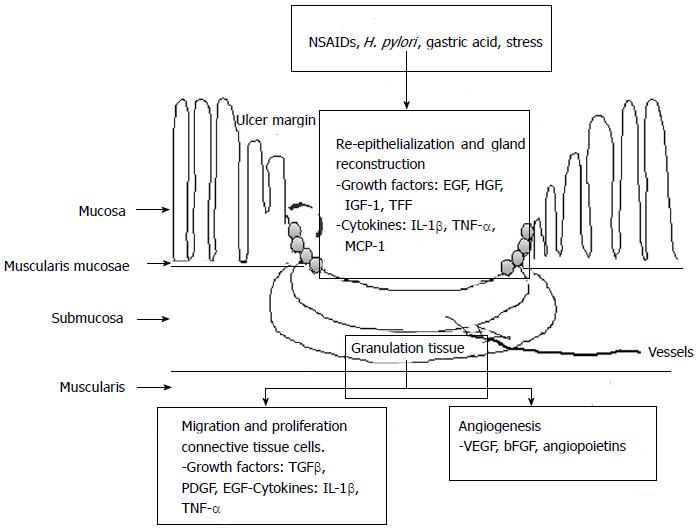
Ulcer healing is an orchestrated process of filling the mucosal defect with epithelial and connective tissue cells, including cell proliferation, migration, differentiation, regeneration, active angiogenesis and extracellular matrix deposition, leading to scar formation. The structure of a gastric ulcer consists of an ulcer margin, which is epithelial tissue formed by adjacent non-necrotic mucosa, and the granulation tissue which is connective tissue[2], as shown in Figure 1. At the ulcer margin, epithelial cells proliferate and migrate onto the granulation tissue to cover (re-epithelialize) the ulcer and initiate reconstruction of the glands within the ulcer scar.
Figure 1 Schematic representation of intracellular pathway on ulcer healing mechanism.
SAC: Artemisia or S-allyl cysteine; PPI: Proton pump inhibitor; NSAIDs: Nonsteroidal anti-inflammatory drugs; TNF-α: Tumor necrosis factor-α; MCP-1: Monocyte chemotactic protein-1; EGF: Epidermal growth factor; HGF: Hepatocyte growth factor; VEGF: Vascular endothelial growth factor; IL-1β: Interleukin-1β; IGF-1: Insulin like growth factor-1.
The processes of re-epithelialization and gland reconstruction are controlled by growth factors, including EGF, hepatocyte growth factor and insulin like growth factor-1 (IGF-1) , as well as trefoil factors, including pS2, Trefoil factor family II (TFF II) and Intestinal trefoil factor, prostaglandins generated through activated cyclooxygenase-2 (COX-2), and other cytokines produced locally by regenerating cells in an orderly and integrated manner. These factors, mainly EGF and prostaglandins, trigger cell proliferation via signal transduction pathways involving both direct activation and transactivation of the EGF receptor. Granulation tissue develops at the ulcer base. It consists of fibroblasts, macrophages and proliferating endothelial cells forming microvessels under the control of angiogenic growth factors, including vascular endothelial growth factor (VEGF), bFGF and angiopoietins. The angiogenesis is essential for the restoration of the blood microcirculation in the mucosa and is thus crucial for oxygen and nutrient supply.
The major mechanism underlying the activation of angiogenic growth factors and expression of their receptors is hypoxia, which activates the transcription factor, hypoxia-inducible factor 1 (HIF-1α). HIF-1α, in turn, up-regulates VEGF transcriptional expression and thus increases the local production of VEGF essential for angiogenesis. The final outcome of the healing process reflects a dynamic interaction between the epithelial component from the “healing” zone at the ulcer margin and the connective tissue component (including microvessels) originating from the granulation tissue and from bone marrow derived stem cells attracted to the site of injury[2,17].
Gastric ulcer recurrence
Numerous neutrophils and macrophages persist in and beneath the regenerated epithelium, even after ulcer healing, the basis for ulcer recurrence. This persistent chronic inflammation may have a key role in causing future ulcer recurrence. Watanabe et al[18] demonstrated that inflammatory cytokines, IL-1β and tumor necrosis factor (TNF)-α administered systemically in rats with a macroscopically healed gastric ulcer cause ulcer recurrence at the site of the previous ulcer. This model of gastric ulcer recurrence has found increased expression of adhesion molecules, intercellular adhesion molecule (ICAM-1) in endothelial cells and leukocytic β2 integrins, lymphocyte function-associated antigen and Mac-1 in leukocytes and cytokines, IL-1β and TNF-α, and chemokine, monocyte chemotactic protein (MCP)-1. This increase occurred in the regenerated tissue of the healed ulcer site, the ulcer scar, 12 h after injection of an inflammatory cytokine and was followed by massive infiltration of macrophages and neutrophils, ultimately resulting in ulcer recurrence. Anti-neutrophil antiserum prevents ulcer recurrence in this model, suggesting that neutrophils producing noxious protease and active oxygen species are the final mediator of tissue injury. These molecules regulate migration of neutrophils from arterioles into the interstitial space. An antibody against MCP-1 prevents gastric ulcer recurrence in this model, suggesting that the overexpression of MCP-1 in resident macrophages accumulated in the interstitial space of the ulcer scar is a first step in the mechanism of ulcer recurrence because neutrophils and macrophages infiltrate the interstitial space of the ulcer scar only after overexpression of MCP-1[19].
FACTORS AFFECTING THE “QOUH”
Tarnawski et al[1] and Arakawa et al[10] first proposed the concept of the QOUH. Since the QOUH is defined as histological maturity of regenerated tissue replaced at an ulcer site, evaluation of the QOUH should be done to assess functional and endoscopic maturity in addition to histological maturity[10]. High QOUH is ideal ulcer healing featuring a fine granular ulcer scar, high functional restoration of mucosa and granulation tissue, and the resistance to recurrence[10]. In a clinical study, QOUH was assessed by chromoendoscopy. The recurrence is minimal from a high quality ulcer scar (flat scar), while it frequently occurs from a poor one (nodular scar)[20]. Accumulation of macrophages and expression of cytokines are much more prominent in a poor quality scar than a high quality scar. The acetic acid ulcer model in rat has been developed[21] and is used as a standard model for screening new anti-ulcer drugs. This model closely resembles human ulcers in terms of both pathological features and healing mechanisms and the ulcer responds well to various anti-ulcer drugs[22].
NSAIDs are a major cause of ulcer complications and deaths worldwide. Arakawa et al[23] demonstrated that indomethacin administered during the initial period of acetic acid-induced gastric ulcer healing affects future ulcer recurrence. Cumulative ulcer recurrence rate was significantly higher in rats initially treated with indomethacin than in controls. Increased polymorphonuclear neutrophil (PMN) infiltration was the major histological abnormality persisting after cessation of indomethacin. Therefore, the administration of indomethacin during the initial period of ulcer healing promoted persistent PMN infiltration and increased ulcer recurrence rates, possibly via a prostaglandin-dependent mechanism[23]. On the other hand, Wang et al[4] demonstrated the effects of aspirin on ulcer recurrence and healing quality. Aspirin was administrated by gavage from day 25 to 54 after ulcer induction by an acetic acid-induced ulcer in rat. The gastric juice volume was significantly increased in the aspirin group compared with those of fasting or saline control groups, while the pH, mucus, mucosal blood flow and PGE2 were significantly decreased in the aspirin treated rats compared with those of the other two groups. COX-2 was significantly augmented in the aspirin group compared with the others. The QOUH in the aspirin group was poorer than that of the fasting or saline control groups. The imbalance between protective and aggressive factors resulted in poor QOUH and ulcer recurrence. Therefore, improvement of the QOUH may contribute to decrease the incidence of ulcer recurrence and the development of a new anti-ulcer treatment.
Prostaglandins
PGs play a critical role in the regulation of gastric acid secretion and maintaining gastric mucosal integrity. The achievement of QOUH is thought to be PG-dependent. Exogenous PGs could reverse events involved in ulcer recurrence, inflammatory response, retarded ulcer healing and defective angiogenesis[4,13,16].
Vascular endothelial growth factor and
angiopoietin 1
Angiogenesis is crucial for gastric ulcer healing which is stimulated by VEGF and angiopoietin-1. Joes et al[24] found that local gene therapy with VEGF and Ang1 into the ulcer base, with limited duration of target gene expression, significantly increased neovascularization and accelerated ulcer healing in rats. Furthermore, co-injection of both plasmids encoding rhVEGF 165 and rhAng1 resulted in formation of more mature vessels and a more complete restoration of gastric glandular structures within the ulcer scar, reflecting better QOUH.
Heat shock protein
Heat shock protein (HSP) acts as a molecular chaperone and exhibits various functions, including protection against apoptosis, protease inhibition and cross-linkage to other proteins. The induction of HSPs is important for protection against apoptosis, protease inhibition, refolding and activity of partially denatured proteins; other reports indicate that their beneficial effect may be especially important for the later phase of regeneration when oxidative stress caused by infiltrating inflammatory cells may oppose tissue repair, signifying that HSP27 induction might be one of the mechanisms that lower recurrence of gastric ulcer after IL-1β in gastroprotective administration[25].
Inflammatory cells and cytokines
The number of macrophages infiltrating scar tissue is five times higher than neutrophils in a non-flat scar, suggesting that these macrophages may play a key role in the pathophysiology of the QOUH and thus future ulcer recurrence. These macrophages produce increased amounts of IL-1β, TNF-α and MCP-1. The proinflammatory cytokines, IL-1β and TNF-α, further activate and stimulate macrophages, thus constituting a self-perpetuating circuit. The increased stimulation of production of these cytokines induced by NSAIDs, stress or H. pylori may cause these macrophages to increase cytokine production and/or release, leading in turn to attraction and accumulation of neutrophils. Neutrophils release proteases and active oxygen species, damage the scar tissue and induce ulcer recurrence[19].
ANTI-ULCER TREAMENT TO ACHIEVE THE QOUH
Clinical treatments for gastric ulcer have allowed rapid development of anti-ulcer drugs and gastric ulcer healing is successful with conventional treatments, including H2-RA, PPI and the eradication of H. pylori infection. However, numerous studies have shown that during the ulcer healing process, either acid inhibition or H. pylori eradication is not enough to prevent relapse as well as complications. These treatments were found to be insufficient to remove or clear accumulated inflammation beneath the ulcer scar, decreased PGs and excessive oxidative stress, all of which affected the QOUH and ulcer recurrence. Therefore, a new concept of the QOUH was initiated to consider the reconstruction of the mucosal structure and ideal functional restoration, as well as clearing gastric inflammation to prevent ulcer recurrence.
More gastric ulcer recurrence develops after completion of conventional treatment in patients whose ulcers heal with an H2-RA than in patients treated with other drugs. Arakawa et al[26] found that inhibition of acid secretion significantly accelerates ulcer healing; however, acid suppressors used alone cannot improve the quality of healing. Combination treatment with both sucralfate (sucrose aluminum sulfate) and omeprazole treatment can improve the QOUH. The stimulating actions of sucralfate on EGF and angiogenesis may be the basis for improving the QOUH[27,28]. Several kinds of gastroprotective drugs are available in clinics, including rebamipide, ecabat sodium and Artemisia extracts, that achieves QOUH and prevents ulcer recurrence. For instance, rebamipide is a gastroprotective drug that induces mucosal prostaglandin generation, accelerates ulcer healing and reduces relapse of the acetic acid ulcer model in rats. Arakawa et al[29] reported that administration of rebamipide during the initial period of acetic acid-induced gastric ulcer affected healing and future ulcer relapse. The ulcer healing rate was higher in rats given rebamipide alone than in those given rebamipide and cimetidine during and after administration, but not in rats given cimetidine alone. The relapse rate was significantly lower in rats initially given rebamipide alone or those given rebamipide and cimetidine than in rats initially given cimetidine alone. Therefore, rebamipide is beneficial for improving the QOUH and reduction of future ulcer relapse[29]. Additionally, rebamipide as well as omeprazole and the combination regimen may improve the QOUH through increasing the level of PGE2 and decreasing the levels of IL-8 and MDA in gastric mucosa. This may potentially result in reduced recurrence of the ulcer. Moreover, the combination regimen was identified as having more anti-ulcer effects than monotherapy[6].
Eradication of H. pylori leads to healing of acute inflammation of the gastric mucosa, followed by changes in the gastric environment such as gastric acid secretion. Therefore, gastroprotective drugs such as repamipide should be given for post-eradication physiological changes in the gastric mucosa to promote ulcer healing and prevent ulcer recurrence[30]. Kato et al[31] showed that the administration of rebamipide with teprenone is a combination for dual therapy for H. pylori eradication. A total of 102 H. pylori-positive gastric ulcer patients were assigned at random to two groups. In addition to dual therapy (amoxicillin 500 mg thrice daily and lansoprazole 30 mg every morning for two weeks), one group received rebamipide 100 mg thrice daily for eight weeks, while the other group received teprenone 50 mg thrice daily for eight weeks. The ulcer healing rate was 85.7% in the rebamipide group and 79.5% in the teprenone group. The eradication rate was 68.4% in the rebamipide group and 47.7% in the teprenone groups. These findings suggest that the efficacy of dual therapy may be increased by the administration of rebamipide. Lafutidine is one of anti-ulcer drugs with antisecretory and gastroprotective activities[32], mediated by capsaicin-sensitive sensory neurons (CSSN). Lafutidine (30 mg/kg) significantly accelerated the ulcer healing and the recurrence rate was much lower than that for the vehicular control. In CSSN-desensitized rats, lafutidine also accelerated the ulcer healing significantly, but the low recurrence rate shown in normal rats was counteracted. The recurrence rate of the combination of famotidine and teprenone (100 mg/kg) was lower than that of famotidine alone. Therefore, the low recurrence rate of ulcers after lafutidine treatment in rats seems to depend on the gastroprotective mechanisms involving CSSN[33].
GASTROPROTECTIVE EFFECT OF NATURAL PRODUCTS TO ACHIEVE QOUH
Natural products and their compounds are highly effective in anti-ulcer treatment, possessing a gastroprotective action and being gentle on the body’s systems without any side effects. While many synthetic and prescription drugs provide symptomatic relief, they also have harsh side effects. Our previous studies have shown that gastroprotective actions from Artemisia ethanol extract treatment was quite efficient in accelerating the ulcer healing at the early phase of ulcer healing and hindering the recurrence of gastric ulcer after complete ulcer healing, whereas an acid suppressant was somewhat inferior in these aspects of ulcer healing. The achievement of ideal QOUH can only be accomplished with intervention of the enhancement of gastric defense systems. The molecular basis for the QOUH achievement with Artemisia treatment were efficient remodeling of regenerated gastric mucosa, intervention of several growth factors, abundant gastric mucins, including trefoil proteins like trefoil peptide (pS2/TFF1), and significant suppression of inflammatory cytokines like IL-2, TNF-α, COX-2 and nitrosative stress. Also, mechanisms of Artemisia extract showing resistance to ulcer recurrence were excellent remodeling activity, enrichments of molecular chaperones like HSP27, as well as significant decreases in inflammatory cytokines like COX-2, IL-2 and TNF-α, and quenching nitrosative stress.
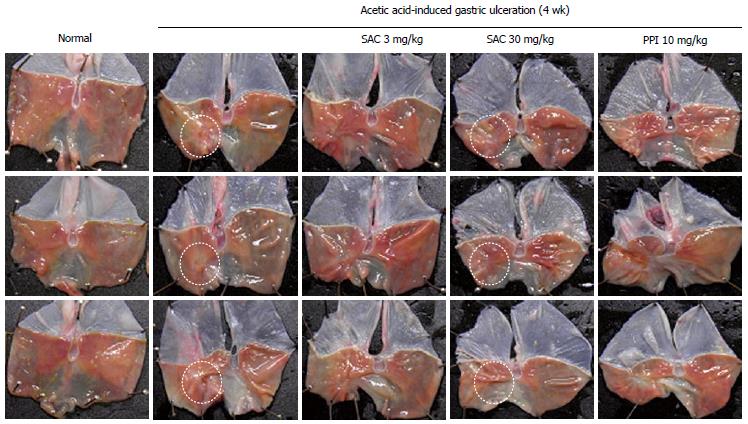
In our recent study, S-allyl cysteine (SAC), an organic compound that is a natural constituent of fresh garlic, has been shown to exert anti-inflammatory and anti-oxidative effects by numerous studies. SAC significantly increased the total antioxidant concentration and decreased levels of MDA, a lipid peroxidation marker in water immersion restraint stress-induced gastritis in vivo, suggesting that SAC prevents stress-induced gastritis through enhancing antioxidative activity. SAC significantly inhibited TNF-α-induced pro-inflammatory signaling, including COX-2, inducible nitric oxide synthase and cytosolic phospholipase A. Moreover, SAC suppressed TNF-α-induced phosphorylation and subsequent degradation of IκBα by preventing IKKβ activation, thereby inhibiting TNF-α-induced nuclear translocation of NF-κB p65. Our results suggest that SAC can be a gastroprotective agent against stress-induced gastric mucosal damage by potentiating antioxidative activity and suppressing NF-κB activation and subsequent proinflammatory cascades. Also, in the acetic acid ulcer model, the healing rate was higher in rats treated with SAC 30 mg/kg than with PPI administration, as shown in Figure 2. Additionally, we have added more evidence that isopropanol extracts of Artemisia possessed higher antioxidative and anti-inflammatory actions (data not shown), inferring a higher achievement of QOUH in gastric ulcer models. Therefore, although acid suppressant is the gold standard treatment for a gastric ulcer, the antisecretory agents alone were not sufficient for reaching the QOUH, necessitating the combined use of antisecretory and gastroprotective agents for anti-ulcer treatment.
Figure 2 Gross features of gastric ulcer stage assessed at 4 wk after acetic acid serosa injection according to group.
SAC: Artemisia or S-allyl cysteine; PPI: Proton pump inhibitor.
Other studies have shown the gastroprotective effect of Pongamia pinnata root flavonoids (PRF) that significantly inhibited ulcerative formation and increased the EGF and TGF-α expression of para-ulcer mucosa tissue and improved the EGF contents in blood serum, which might be one of possible mechanisms by which PRF improves the QOUH[34]. The stem bark of Lafoensia pacari is a traditional medicine in Brazil widely used for the treatment of gastroduodenal ulcers. The gastroprotective and ulcer healing of methanol extract of Lafoensia pacari (MELP) was evaluated using ethanol, indomethacin, cold-restraint stress-induced and acetic acid ulcer models in experimental animals. This study has shown that MELP possesses preventive and curative effects against gastric ulcers. These effects are partly dependent on its antioxidant, antisecretory properties and inhibition of pro-inflammatory cytokines and independent of gastric motility and mucus secretion[35]. The ethanolic extract (EET) of roots from Arctium lappa (bardana) has shown that it accelerates the healing of acetic acid-induced gastric ulcers in rat. Oral administration of EET reduced the gastric lesion area and promoted regeneration of the gastric mucosa. EET also restored the superoxide dismutase activity, prevented the reduction of glutathione levels, reduced lipid hydroperoxide levels, inhibited the myeloperoxidase activity and reduced the microvascular permeability. In addition, EET reduced the free radical generation and increased scavenging of 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals in vitro. Furthermore, intraduodenal EET decreased the volume and acidity of gastric secretion. Therefore, the gastroprotective effect of EET was mediated by the decrease in the volume and total acidity of gastric secretion, cell proliferation, reduction of the inflammatory process and antioxidant-dependent mechanisms[36].
Pimple et al[37] investigated the gastroprotective effect of Luffa acutangula methanolic extract (LAM) and aqueous extract (LAW) on type II diabetes rats. LAM significantly increased mucosal glycoprotein and antioxidant enzyme levels in the gastric mucosa of diabetic rats, more than LAW. LAM was efficient in reversing the delayed healing of gastric ulcers in diabetic rats close to the normal level. LAM exhibited a better ulcer healing effect than glibenclamide and LAW, because of its antihyperglycemic and mucosal defensive actions. Morus alba is a well-known Chinese herb traditionally used for the prevention and treatment of several diseases as this plant possesses antidiabetic, hypolipidemic, antimicrobial, antioxidant and anti-ulcer activities. Five new compounds were isolated, one a new steroid named albo steroid. This new compound exhibits significant anti-ulcer activity in pylorus-ligation and ethanol-induced ulcer models. Furthermore, this compound showed significant dose-dependent reversal of ethanol-diminished activity in antioxidant enzymes, such as superoxide dismutase, catalase, glutathione peroxidase and glutathione, and reduced the ethanol-elevated levels of glutathione reductase and lipid peroxidation[38]. Finally, VSL#3 is a probiotic preparation containing a mixture of eight bacterial species, including Lactobacilli, Bifidobacteria and Streptococcus species which has been shown to be highly effective in accelerated gastric ulcer healing by stimulating the expression and secretion of angiogenesis promoting growth factors, primarily VEGF. The expression and protein production of VEGF was significantly increased on day 7 in the ulcerated tissues of VSL#3 treated animals. In addition, animals treated with VEGF neutralizing antibody significantly delayed gastric ulcer healing in VSL#3 treated animals[39].
CONCLUSION
We cannot ignore the principal role of acid suppressant in the treatment of gastric ulcers. Based on the experimental documentation, we suggest that the combined use of acid suppressants and gastroprotection should be considered to improve the quality of ulcer healing, facilitating rapid symptom relief, accelerated healing and resistance to ulcer recurrence, as well as complete functional restoration. In conclusion, our novel finding is that gastroprotective treatments are not merely supplementary in the treatment of gastric diseases, otherwise signifying that gastroprotection might be essential and a prerequisite for better healing, for which phytoceuticals or phytochemicals can be ideal candidates, supported with safety and effectiveness.
P- Reviewers: Kim BW, Leung FW, Rocha JBT S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Wu HL