Published online Feb 15, 2014. doi: 10.4291/wjgp.v5.i1.33
Revised: October 14, 2013
Accepted: November 15, 2013
Published online: February 15, 2014
Processing time: 175 Days and 20.9 Hours
Intense abdominal pain is the most common symptom in chronic pancreatitis, but the underlying mechanisms are not completely understood and pain management remains a significant clinical challenge. The focus of pain origin in chronic pancreatitis traditionally has been on the pancreatic gland, assuming pain to originate in the pancreas or its surrounding organs. However, research in the last decade points to abnormal central nervous system pain processing. For this reason, electroencephalography has been receiving increasing attention. In contrast to imaging methods such as functional magnetic resonance imaging and positron emission tomography, electroencephalogram has excellent temporal resolution making it possible to investigate central processing of pain on a millisecond time scale. Moreover, continuously advancing methodology made it possible to explore brain sources responsible for generation of evoked potentials and hence to study brain reorganization due to pain in chronic pancreatitis. The aim of this review is to give an overview of the current methods and findings in electroencephalography as a tool to unravel the origin of pancreatic pain.
Core tip: Chronic pancreatitis (CP) is a disease with progressive destruction of the pancreatic gland and intense abdominal pain is one of its main characteristics. The understanding of pain in CP has conventionally focused on the diseased pancreas itself, assuming pain to be due to increased parenchymal or ductal pressure. However, recent research points to possible involvement of abnormal central nervous system pain processing. This review gives an insight into electrophysiology as a tool to unravel brain abnormalities underlying pancreatic pain and provides up to date electrophysiological results in this patient group.
- Citation: Lelic D, Olesen SS, Graversen C, Brock C, Valeriani M, Drewes AM. Electrophysiology as a tool to unravel the origin of pancreatic pain. World J Gastrointest Pathophysiol 2014; 5(1): 33-39
- URL: https://www.wjgnet.com/2150-5330/full/v5/i1/33.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v5.i1.33
Pain is a prominent symptom in chronic pancreatitis (CP), but its underlying mechanisms are incompletely understood and probably multifactorial[1]. Thus, no single remedy for pain relief exists and an optimal pain treatment can only be achieved on the basis of a better understanding of the pain mechanisms underlying pain in the individual patient[2]. While, the focus of pain origin in CP historically has been on the pancreatic gland, assuming pain to originate in the pancreas or its surrounding organs, recent findings indicate that both peripheral and central pain processing are abnormal in CP patients[1,3]. Various mechanisms responsible for the altered pain processing have been proposed, including pancreatic neuropathy and neural remodeling[4,5], sensitization of neurons in the spinal cord and the brain[6,7], reorganization of the brain areas involved in visceral pain processing[8] and alterations in descending pain control from the brainstem and other supraspinal structures[9]. The diagnostic work up of patients with painful CP should therefore not only focus on pancreatic and extra pancreatic causes of pain (e.g., pseudocysts, duct dilation and strictures, pancreatic head mass, etc.), but also include an assessment of central pain processing.
Central pain processing can be studied by various methods, but none of the current available techniques have gained wide clinical use. Neuroimaging methods based on indirect measures of neuronal activity, such as functional magnetic resonance imaging (f-MRI) (changes in haemodynamic responses) or positron emission tomography (changes in metabolic responses), have been used extensively to study pain processing[10,11]. Although these methods possess excellent spatial resolution and have greatly contributed to our knowledge of the structural basis of the pain system, their temporal resolution is relatively poor (order of several seconds). Consequently, in order to address the dynamics of pain processing (i.e., the pain-specific sequential brain activation underlying pain perception), a method with high temporal resolution is needed, such as electroencephalography (EEG) which measures neuronal activity directly[12]. An additional rationale for using electrophysiological methods for objective characterization of pain processing is the relatively low cost and ease of use compared to other neuroimaging methods. This allows evaluating pain processing in the clinical setting and may in the future guide clinicians in tailoring individualized therapies.
The aim of this review is to give an overview of electrophysiology as a tool to unravel the origin of pain in patients with CP. This will be done by giving a summary of up-to-date methods and findings of research done in CP patients by means of: (1) spontaneous EEG and (2) EEG evoked potentials (EPs). The review will be concluded by giving some future perspectives for EEG research in CP patients.
To explore the specific brain circuitry involved in chronic pain conditions such as neuropathic pain and central sensitization[13], spontaneous EEG recorded while the patient is at rest may provide a clinical useful tool to identify pain mechanisms in individual patients.
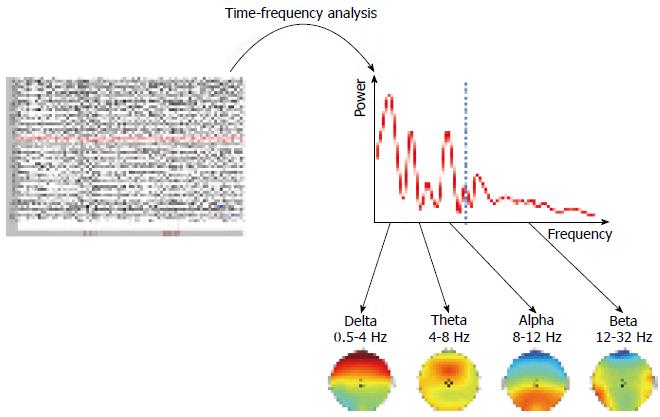
Spontaneous EEG captures the excitatory and inhibitory postsynaptic potentials, which are regulated by various homeostatic processes[14,15]. The potentials oscillate at various frequencies, which have traditionally been split into standard frequency bands such as delta (0.5-4 Hz), theta (4-8 Hz), alpha (8-12 Hz), beta (12-32 Hz), and gamma (32-80 Hz). As the oscillations are regulated by homeostatic processes, quantification of the EEG reveals important information regarding the neurotransmitters in the brain in each patient. As some of these neurotransmitters are involved in pain processing, EEG analysis can be used to study the central nervous system (CNS) state in the patient[16]. Furthermore, spontaneous EEG can be used to identify the pain mechanisms and the neuroplasticity in the CNS caused by many years of pain[17]. For an example of spontaneous EEG analysis, please see Figure 1.
Although spontaneous EEG has been applied to many patient groups[18-20], there seems to be a lack of studies in CP patients. We have compared the resting state EEG of thirty-one patients diagnosed according to the Mayo Clinic diagnostic criteria to that of fifteen healthy volunteers[21]. Delta, theta and alpha activities were increased in the patients as compared to controls. The increase in theta band activity could indicate disturbed thalamocortical interplay[22], while the increased alpha activity may reflect inhibition of sensory stimuli[23]. Delta band activity increase was lost in a sub-analysis adjusting for opioid treatment, diabetes mellitus and alcohol aetiology, indicating a less pain specific role of the slower rhythms in pain processing. A significant interaction between electrode and participant group were evident for all frequency bands and it was plausible that differences in amplitude strengths were confined to specific cortical areas. Differences were seen for most central electrodes in the theta band and this supported the hypothesis that thalamocortical dysrhythmia may play a key role in the altered pain processing in the chronic pancreatitis patients.
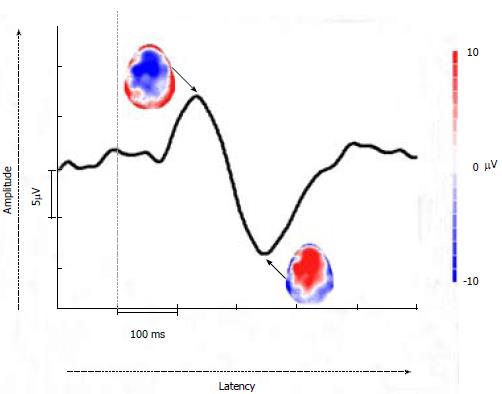
To explore how the pain relevant brain networks are modified due to CP, EEG pain EPs can be utilized. EEG EPs are electrical potentials recorded from the nervous system following presentation of a stimulus, such as pain on the skin or viscera. Since EP amplitudes tend to be much lower than the spontaneous EEG amplitudes, multiple stimulations are required and the corresponding EPs are averaged. During this process, amplitudes of EPs are increased, while the random background activity (spontaneous EEG) cancels out. Then, the amplitudes and latencies of EP peaks can be analysed and compared between healthy controls and patients in order to observe whether there are any alterations in the patient group. Time-frequency analysis of EPs can also be done as discussed in the spontaneous EEG section. Moreover, when enough of recording scalp electrodes are used to provide the full scalp coverage (typically > 30 electrodes), topographical distributions can be analysed in order to investigate at which topographical location the activity is maximal for each peak. Please see Figure 2. Recent advancements in EP analysis methods allow for looking at brain source generators of these topographies and hence it is possible to investigate where in the brain the changes due to CP are occurring on a millisecond time scale[24].
Research with EPs in CP to date is limited. Previous research mainly made use of visceral EPs, but recently somatic contact heat evoked potentials (CHEPs) have also been used. The findings of these studies are summarized in Table 1 and presented below in their respective subsections.
| Ref. | Methods | Results |
| Dimcevski et al[8], | 64-channel EPs; 12 HV and 10 CPP | Decreased latencies of the early EP components |
| Gastroenterology, 2007 | Electrical stimulation of the oesophagus, stomach, and duodenum | The bilateral insular sources localized more medial after stimulation of all 3 gut segments |
| Amplitudes, latencies, and brain sources of the EPs were analysed | The cingulate source localized more posterior after stimulation of oesophagus | |
| Drewes et al[26], World J Gastroenterol, 2008 | 62-channel EPs; 12 HV and 8 CPP | Higher activity in theta band |
| Electrical stimulation of the oesophagus | The main theta components oscillated with 4.4 Hz in the patients and 5.5 Hz in the controls | |
| Olesen et al[27], Pancreatology, 2010 | Topographic matching pursuit was used to extract the EEG information in the early brain activation after stimulation | The energy in the delta band was higher in healthy volunteers |
| 62-channel EPs; 14 HV and 24 CPP | EP latencies at frontal electrodes were prolonged | |
| Electrical stimulation of the sigmoid | The insular dipoles were localised more posterior | |
| Patients’ daily pain experience was recorded in a pain diary | The shift of insular dipole localisation was negatively correlated with the patients’ clinical pain scores | |
| Amplitudes, latencies, and brain sources of the EPs were analysed | ||
| Correlation analysis was done between patients’ pain scores and the changes in brain sources | ||
| Olesen et al[9], Clin Gastroenterol Hepatol, 2010 | 62-channel EPs; 15 HV and 25 CPP | Increased latency of P1 |
| Olesen et al[21], Eur J Gastroenterol Hepatol, 2011 | Electrical stimulation of the sigmoid | Increased activity in delta, theta and alpha bands, and decreased beta band activity |
| Amplitudes and latencies of the EPs were analysed | ||
| 62-channel spontaneous EEG; 15 HV and 31 CPP | ||
| Wavelet frequency analysis was used to retrieve amplitude strengths of the EEG | Differences in theta activity were located over centro-frontal brain regions, whereas differences in other frequency bands were located over frontal regions | |
| The amplitude strengths were summarized in frequency bands with corresponding topographies | ||
| Olesen et al[28], Aliment Pharmacol Ther, 2011 | 62-channel EPs | No differences in any of the EP characteristics between pregabalin and placebo groups |
| 31 CPP randomly assigned to receive increasing doses of placebo or pregabalin over 3 wk | ||
| Electrical stimulation of the sigmoid | ||
| Amplitudes, latencies, and brain sources of the EPs were analysed | ||
| Olesen et al[7], Eur J Pain, 2013 | Three sequences of 62-channel CHEPs; 15 HV and 15 CPP | During successive stimulation of the pancreatic area, N2/P2 amplitude increased 25% in CP patients, while it decreased 20% in healthy volunteers |
| Upper abdominal region (pancreatic ”viscerotome”) and the right forearm (heterologous area) were stimulated. | After stimulation of the forearm, N2/P2 amplitudes increased 3% in CP patients compared to a decrease of 20% in healthy volunteers | |
| Habituation was calculated as the relative change in CHEPs amplitudes between the first and the third stimulation sequence |
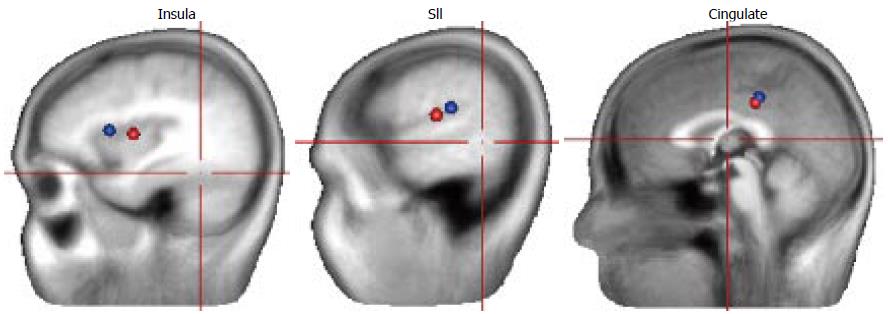
In a study by Dimcevski et al[8], where painful EPs at three stimulation sites (oesophagus, gut and duodenum) were utilised, a decrease in latencies of the EPs was seen. The authors argued that the decrease in latencies most likely reflects central nervous system changes such as hyperexcitability and reorganization. The central reorganization was confirmed by source analysis where the bilateral insular sources were localized more medial in all three stimulation sites and the cingulate source was localized more posterior in patients for the oesophageal stimulation. Since insula is suggested to play an important role in integrating visceral sensory and motor activity together with the limbic integration[25], the authors concluded that the reorganization within the insular cortex in CP patients likely mirrors disruption in the coordination and processing of visceral pain. The change within cingulate cortex, although more discrete than the change within insula, was interpreted as reflecting changes in the cognitive and effective pain components as a result of the long-lasting frequent pain attacks in CP. Another study using the same dataset for oesophageal EPs as Drewes et al[26] but looking at data in frequency domain was done. The authors found that patients showed higher activity in theta frequency band and theta activity was centered around 4.4 Hz in patients and 5.5 Hz in healthy controls. The authors interpreted the increased theta activity in patients as possibly reflecting a thalamocortical dysrhythmia like discussed above for the resting EEG. Additionally, the power in delta band was higher in healthy controls than in CP patients. Due to a major spread between subjects regarding delta activity, it could not be clearly concluded whether the changes in delta band were due to expected delta increase due to pain in healthy volunteers or whether the decrease in delta activity in patients is a consequence of chronic pain. Hence, further studies addressing this should be conducted. Recently, we have done several CP studies where rectal EPs were applied[9,27,28]. We observed that the rectal evoked potential latencies were prolonged and this was confined to the frontal scalp electrodes[27]. This is in contrast to the previous oesophageal CP study and this could be due to different stimulation sites. Since the prolongation of latency was only seen at the frontal electrodes, this likely reflects an alteration in cerebral pain processing. This was confirmed by analysis of underlying sources showing that insular dipoles were localised more posterior in the patients than in healthy subjects (please see Figure 3) and this shift was negatively correlated to the patient symptom scores. These findings suggest a pain-associated adaptive cortical reorganization in CP patients. In another rectal EP study, it was also seen that the first positive component (P1) was prolonged in the CP patient group, likely reflecting reorganization of central pain pathways[9]. In a clinical study, where thirty-one patients with CP were randomly assigned to receive increasing doses of pregabalin or placebo for three consecutive weeks, no differences in rectal EP characteristics or their sources were seen, neither after pregabalin or placebo treatment[28]. However, since pregabalin was an effective drug significantly increasing patients’ pain thresholds, the lack of changes in EP characteristics likely implies that visceral pain is mediated through subcortical mechanisms in patients with CP.
There is a lack of studies with somatic EPs in CP patients. However, recently contact heat evoked potentials (CHEPs) have received attention due to the stimulation being non-invasive and the relative selective activation of nociceptors. One study was done with CHEPs to investigate whether habituation was abnormal in CP patients[7]. The stimulation sites were the upper abdominal region (pancreatic “viscerotome”) and right forearm (heterologous area). It was seen that during the repetitive stimuli, the CHEPs amplitudes increased in patients, although more prominently after stimulation of the upper abdominal region (25% as compared to 3% after arm stimulation), whereas in healthy controls, the amplitudes decreased during the repetitive stimuli by 20% (as expected). As the upper abdominal area shares spinal innervation with the pancreatic gland, these findings likely reflect abnormalities in cerebral pain processing distinctive of CP. Brain source analysis was done for these CHEPs data (unpublished) and a posterior shift of the operculum (representing insula and secondary somatosensory cortex) source and an anterior shift of the cingulate source were observed following the stimulation of upper abdominal area. The operculum shift was positively correlated to the patient symptom score. No changes were seen in CP patients following stimulation of the arm. Since source position changes were only seen after stimulation of the area sharing spinal innervation with the pancreatic gland and these changes correlated to the patients’ pain scores, the results are probably a reflection of maladaptive neuroplastic changes characteristic of CP.
The focus of pain treatment in CP has for many years concentrated on pathological findings in the pancreatic gland or extra pancreatic causes of pain. However, as we learn more about the mechanisms underlying pain in CP, it is clear that many patients will also benefit from treatments targeting central pain mechanisms[1]. A major obstacle to achieve successful treatment responses is to identify patients who will specifically benefit from these treatments (i.e., patients with evidence of abnormal central pain processing). Therefore, simple and objective diagnostic methods to identify abnormal central pain processing are highly desirable.
As discussed in this review, the electrophysiological research points to three cortical phenomena in CP: (1) thalamocortical dysrhythmia, as evident in increase of theta band activity in resting-state EEG and esophageal EPs; (2) cortical hyperexcitability as reflected in patients’ lack of habituation to contact heat stimulation. The cortical hyperexcitability phenomenon could also be a consequence of thalamocortical dysrhythmia as happens in migraine[29]; and (3) reorganization within insular cortex following visceral and contact-heat EPs.
The electrophysiological methods reviewed in the present paper may be used as tools to unravel the origin of pain in patients with CP. Thereby patients with abnormalities in central pain processing can be identified prior to treatment assignment and thus the methods can assist clinicians when establishing treatment indications for pancreatitis pain in the individual patient (e.g., invasive procedures vs medical treatment). However, further longitudinal clinical studies are needed to establish the value of the methods in the management of CP pain.
P- Reviewers: Grizzi F, Pehl C S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Pasricha PJ. Unraveling the mystery of pain in chronic pancreatitis. Nat Rev Gastroenterol Hepatol. 2012;9:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Forsmark CE. Management of chronic pancreatitis. Gastroenterology. 2013;144:1282-1291.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Drewes AM, Krarup AL, Detlefsen S, Malmstrøm ML, Dimcevski G, Funch-Jensen P. Pain in chronic pancreatitis: the role of neuropathic pain mechanisms. Gut. 2008;57:1616-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, Müller MW, Giese T, Büchler MW, Giese NA. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177-186.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 5. | Ceyhan GO, Demir IE, Rauch U, Bergmann F, Müller MW, Büchler MW, Friess H, Schäfer KH. Pancreatic neuropathy results in „neural remodeling“ and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Am J Gastroenterol. 2009;104:2555-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Bouwense SA, Olesen SS, Drewes AM, Frøkjær JB, van Goor H, Wilder-Smith OH. Is altered central pain processing related to disease stage in chronic pancreatitis patients with pain? An exploratory study. PLoS One. 2013;8:e55460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Olesen SS, Hansen TM, Graversen C, Valeriani M, Drewes AM. Cerebral excitability is abnormal in patients with painful chronic pancreatitis. Eur J Pain. 2013;17:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Dimcevski G, Sami SA, Funch-Jensen P, Le Pera D, Valeriani M, Arendt-Nielsen L, Drewes AM. Pain in chronic pancreatitis: the role of reorganization in the central nervous system. Gastroenterology. 2007;132:1546-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Olesen SS, Brock C, Krarup AL, Funch-Jensen P, Arendt-Nielsen L, Wilder-Smith OH, Drewes AM. Descending inhibitory pain modulation is impaired in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2010;8:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2099] [Cited by in RCA: 2177] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 11. | Mayer EA, Aziz Q, Coen S, Kern M, Labus JS, Lane R, Kuo B, Naliboff B, Tracey I. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol Motil. 2009;21:579-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clin Neurophysiol. 2004;115:2195-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1208] [Cited by in RCA: 1154] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 13. | Baliki MN, Geha PY, Apkarian AV. Spontaneous pain and brain activity in neuropathic pain: functional MRI and pharmacologic functional MRI studies. Curr Pain Headache Rep. 2007;11:171-177. [PubMed] |
| 14. | Olejniczak P. Neurophysiologic basis of EEG. J Clin Neurophysiol. 2006;23:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Hughes JR, John ER. Conventional and quantitative electroencephalography in psychiatry. J Neuropsychiatry Clin Neurosci. 1999;11:190-208. [PubMed] |
| 16. | Constant I, Sabourdin N. The EEG signal: a window on the cortical brain activity. Paediatr Anaesth. 2012;22:539-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Wiech K, Preissl H, Birbaumer N. Neuroimaging of chronic pain: phantom limb and musculoskeletal pain. Scand J Rheumatol Suppl. 2000;113:13-18. [PubMed] |
| 18. | Dorchy H. To: Hyllienmark L, Maltez J, Dandenell A, Ludvigsson J, Brismar T (2005) EEG abnormalities with and without relation to severe hypoglycaemia in adolescents with type 1 diabetes. Diabetologia 48: 412-419. Diabetologia. 2005;48:2191-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Stern J, Jeanmonod D, Sarnthein J. Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage. 2006;31:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 20. | Tayama J, Sagami Y, Shimada Y, Hongo M, Fukudo S. Effect of alpha-helical CRH on quantitative electroencephalogram in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:471-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Olesen SS, Hansen TM, Graversen C, Steimle K, Wilder-Smith OH, Drewes AM. Slowed EEG rhythmicity in patients with chronic pancreatitis: evidence of abnormal cerebral pain processing? Eur J Gastroenterol Hepatol. 2011;23:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 335] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 23. | Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842-1857. [PubMed] |
| 24. | Lelic D, Olesen SS, Valeriani M, Drewes AM. Brain source connectivity reveals the visceral pain network. Neuroimage. 2012;60:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229-244. [PubMed] |
| 26. | Drewes AM, Gratkowski M, Sami SA, Dimcevski G, Funch-Jensen P, Arendt-Nielsen L. Is the pain in chronic pancreatitis of neuropathic origin? Support from EEG studies during experimental pain. World J Gastroenterol. 2008;14:4020-4027. [PubMed] |
| 27. | Olesen SS, Frøkjær JB, Lelic D, Valeriani M, Drewes AM. Pain-associated adaptive cortical reorganisation in chronic pancreatitis. Pancreatology. 2010;10:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Olesen SS, Graversen C, Olesen AE, Frøkjaer JB, Wilder-Smith O, van Goor H, Valeriani M, Drewes AM. Randomised clinical trial: pregabalin attenuates experimental visceral pain through sub-cortical mechanisms in patients with painful chronic pancreatitis. Aliment Pharmacol Ther. 2011;34:878-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Magis D, Vigano A, Sava S, d’Elia TS, Schoenen J, Coppola G. Pearls and pitfalls: electrophysiology for primary headaches. Cephalalgia. 2013;33:526-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |