Published online Aug 15, 2013. doi: 10.4291/wjgp.v4.i3.53
Revised: June 17, 2013
Accepted: July 18, 2013
Published online: August 15, 2013
Processing time: 117 Days and 10.4 Hours
AIM: To investigate tumor necrosis factor-α (TNF-α), syndecan 1 and basic fibroblast growth factor (bFGF) balance in Crohn’s disease (CD) strictures.
METHODS: Our study was performed on 24 surgical specimens of CD fibrotic stenosis. Ten histological normal surgical samples were retrieved for both the large and small bowel from patients with benign conditions and healthy tissue represented control collection. Sex and age in controls did not differ from CD group. Three endoscopic biopsy specimens taken after informed consent in subjects with normal colon were also used as negative controls. TNF-α, syndecan 1 and bFGF were detected by both reverse transcriptase reverse transcriptase polymerase chain reaction after mRNA extraction (results expressed as fold-change) and immunohistochemistry.
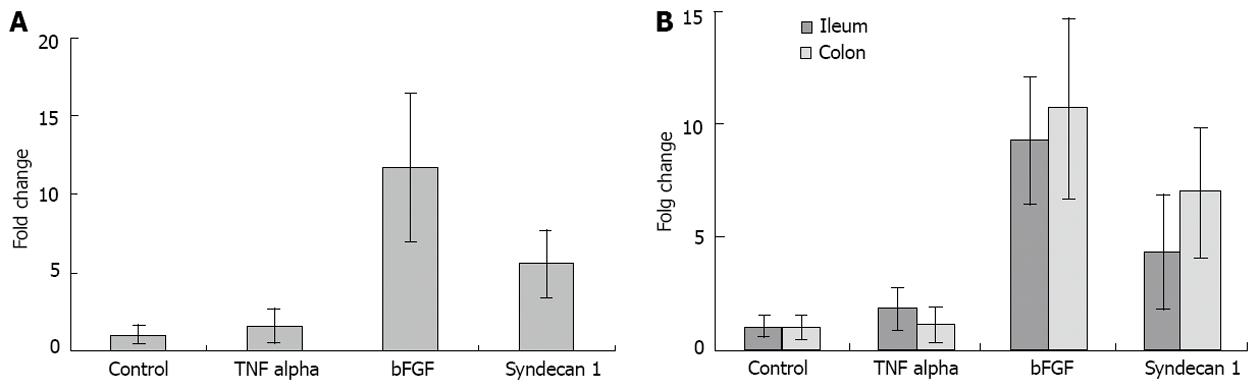
RESULTS: TNF-α did not show any significant difference between CD and control specimens (1.54 ± 1.19; P > 0.05). Very high levels of bFGF were observed in CD (11.76 ± 4.65; P < 0.001) unlike syndecan 1 which showed a moderate increase (5.53 ± 2.18; P < 0.005). analysis of variance (ANOVA) plus Student-Neumann-Keuls showed: bFGF > syndecan 1 > TNF-α = control. Immunoreactivity for bFGF was observed in epithelial, stromal, endothelial cells and even in the muscular layer, whilst in normal tissue it was almost unexpressed. Syndecan 1 and TNF-α staining was confined to mucosal epithelial and stromal cells, while in controls syndecan 1 was found in its normal site, i.e., basolateral area of the crypts and TNF-α very poorly expressed.
CONCLUSION: Fibrotic stenosis of CD may be the final result of an irreversible transformation of different cells into fibrogenic phenotype no longer inhibited by post-transcriptional regulation.
Core tip: The present manuscript reports a study of molecular pattern in the course of stenotic complication of Crohn’s disease. We have studied the interaction among the main cytokine [tumor necrosis factor-α (TNF-α)], an adhesion molecule (syndecan 1) and a growth factor basic fibroblast growth factor which are strictly involved in damage repair and mucosal healing, as showed in a previous study. In this study we demonstrated that a deep dysregulation of interaction of these three factors may support stenotic fibrosis in Crohn’s disease and suggest that this condition needs to be investigated before a biological treatment since TNF-α lack downregulation could further stimulate fibrogenesis.
- Citation: Ierardi E, Giorgio F, Piscitelli D, Principi M, Cantatore S, Fiore MG, Rossi R, Barone M, Di Leo A, Panella C. Altered molecular pattern of mucosal healing in Crohn’s disease fibrotic stenosis. World J Gastrointest Pathophysiol 2013; 4(3): 53-58
- URL: https://www.wjgnet.com/2150-5330/full/v4/i3/53.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v4.i3.53
Considerable downregulation of tumor necrosis factor-α (TNF-α) may occur after infliximab therapy in inflammatory bowel diseases (IBD) in those patients who achieve disorder remission (responders: about the 70%)[1]. Such downregulation may be associated with a dramatic regression of endoscopic lesions with a feature suitable of “mucosal healing’’[2].
TNF-α downregulation presumably interacts with the adhesion molecules (syndecan 1)[3] and growth factors [basic fibroblast growth factor (bFGF)][4] in the process of mucosal repair. Indeed, syndecan 1 is located in the basolateral region of the columnar epithelium and plays a relevant role in the course of IBD damage reversal[5-7]. This role seems to be due to its ability to change bFGF morphology and modulate the structure of its receptors, allowing its binding to repair dedicated epithelial and stromal cells located at the margins of ulcerative lesions[8-10]. The relevance of syndecan 1 also may be due to its aptitude to inhibit bFGF proteolysis, which restricts tissue repair, thus maintaining mucosal damage[9]. In normal tissues bFGF is present in basement membranes and in the subendothelial extracellular matrix of blood vessels. It stays membrane-bound as long as there is no signal peptide[11]. In intestinal inflammation both molecules are found in either stromal cells and matrix[12] with an increased expression.
In a previous study, we have analyzed the mucosal levels of TNF-α as well as bFGF/syndecan 1 link in a selected group of ulcerative colitis (UC) responder patients designed for an observational 6-mo “post-hoc’’ investigation[13]. Our results showed that the cytokine decrease induced by infliximab treatment is accompanied by a simultaneous decrease in both adhesion molecule and growth factor in the presence of mucosal healing. Additionally, we have found that syndecan 1 overexpression in stromal cells and apical epithelium is reversed after infliximab successful therapy on mucosal lesions.
Therefore, infliximab therapy downregulation of bFGF/syndecan 1 link could represent a possible molecular pathway of mucosal healing in ulcerative colitis despite our results do not clarify completely the reliability of the hypothesis, since the parallel pattern of TNF-α, syndecan 1 and bFGF could only be a simultaneous consequence of the control of inflammation.
Similarly, Verspaget et al[14] reported that healing of fistulizing/perianal Crohn’s disease (CD) seems to be reflected by a decrease in high serum bFGF as well as in immunohistochemical mucosal labeling index. Nevertheless, bFGF levels do not correlate with the therapeutic response in active disease, when indicated by Crohn’s disease activity index (CDAI) score. Authors explained this result with the inclusion of several subjective parameters in the overall CDAI score, which may mask the real inflammatory bowel picture.
We performed the present study in order to verify the simultaneous configuration of TNF-α, bFGF and syndecan 1 in patients with a severe complication of CD, i.e., intestinal resection for severe fibrotic stenosis. Aim of the work was to investigate whether a dysregulation of their balance observed in mucosal healing could indicate a molecular pattern for stenotic complication.
Our study was performed on surgical specimens from 24 patients undergoing intestinal resection for fibrotic stenosis. Baseline demographic and clinical characters are reported in Table 1. Ten histological normal surgical samples were respectively retrieved for both the large and small bowel from patients operated for large adenomas (3 patients), acute diverticular perforation (2 patients), intestinal volvulus (2 patients), internal hernia passing into the bursa omentalis for mesenteric malformation (1 patient), acute mesenteric ischemia (1 patient) and post-surgical small bowel obstruction (1 patient). Strikingly, healthy tissue surrounding diseased one was taken and represented the control collection, which did not differ for sex and age distribution from the CD group. Additionally, 3 endoscopic biopsy specimens taken after informed consent in subjects undergoing colonoscopy for intestinal cancer familiarity were used as negative controls in each assay.
| Crohn’s disease (n = 24) | |
| Age (yr) | 36 ± 17 |
| Gender (male) | 15 (62) |
| CDAI | 197 ± 41 |
| Steroids | 7 (29) |
| AZA/6-MP | 7 (29) |
| Infliximab | 5 (20) |
| ADA | 6 (25) |
| Extraintestinal manifestations | 5 (20) |
| Intestinal fistola | 3 (8) |
| Previous surgical interventions | 0 (0) |
| Disease duration (mo) | 59 ± 62 |
As reported above[15], reverse transcriptase polymerase chain reaction has the ability to reflect the altered pattern of the expression of genes dedicated to the synthesis of a specific molecule and quantify its transcription levels. Therefore, in this study, the technique allowed the amount of mRNA codifying for the synthesis of TNF-α, syndecan 1 and bFGF to be estimated. The amount was expressed by a numerical value (i.e., the fold change compared with controls)[16]. The relative expression of the studied gene levels was calculated using the 2-CT method. RNA was extracted from at least five sections of 10 μm of paraffin blocks, using the RNeasy paraffin-embedded Formalin-Fixed and Parrffin-Embedded (FFPE) Kit (Qiagen, GmbH, Germany). The choice of this kit was suggested, since it has been specifically designed for the purification of total RNA from (FFPE) tissue sections. Briefly, 10 μm thick sections were cut (at least 5) using a specific microtome (Leica Microsystems, Wetzlar, Germany). Five hundred microlitres of xylene were added to the sections to yield a solution that was then vortexed for 10 s and then incubated for 10 min at room temperature (25 °C). The next step was the addition of 500 μL of absolute ethanol, the novel solution was again vortexed vigorously for 10 s and centrifuged for 2 min at 11000 g. The supernatant was carefully removed by pipetting without disturbing the pellet. Then, any residual xylene/ethanol was carefully removed using a fine pipette tip.
The lid was kept open until air induced the drying of the pellet (about 5 min at room temperature: 25 °C). After deparaffinisation, the method proceeded according to the manufacturer’s instructions for RNA extraction. In detail, final mRNA concentrations were estimated by ultraviolet absorbance at 260/280 nm. Aliquots of total mRNA (1 mg) were reverse-transcripted using random hexamers and TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA) with 3.125 U/mL of MultiScribe Reverse Transcriptase in a final volume of 50 mL. A series of six serial dilutions (from 20 to 0.1 ng/mL) of colon tissue DNA (cDNA) was used as a template. Two-step reverse transcription polymerase chain reaction (PCR) was performed using first-strand with a final concentration of 13 TaqMan gene expression assay, that is, the analysed molecules plus reference gene (glyceraldehyde-3-phosphate-dehydrogenase) (Applied Biosystems). The final reaction volume was 25 mL, and this was analysed in triplicate (all experiments were repeated twice). A non-template control (Rnase-free water) was included on every plate. Our method was validated further, moreover, by enclosing in each assay fresh samples from three normal patients and frozen at -90 °C until the analysis, as above reported. These samples were treated with the same technique as for paraffin-embedded samples with the exclusion of paraffin removal and rehydration procedures. Specific thermal cycler conditions were employed using a realtime PCR System (Applied Biosystems). A standard curve plus validation experiment were performed for each primer/probe set.
Syndecan 1 immunohistochemical staining was additionally performed for each sample from both patients and controls in order to establish and semiquantitatively evaluate its location. Its stromal expression was reflected by the percentage of positive cells, evaluated in three randomised fields for a total of at least 1000 cells, while its epithelial expression of basolateral and apical amount was reflected by a subjective score already used by our group for other studies[17]. Syndecan-1 expression was assessed on paraffin-embedded sections using a monoclonal mouse antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:80 in buffer phosphate.
Immunohistochemistry for bFGF was performed as follows. The primary antibody was a polyclonal antibody directed against bFGF (code No. sc-79-G; Santa Cruz Biotechnology Inc., Santa Cruz, CA). This rabbit affinity-purified antibody to bFGF was raised against the amino-terminal domain of human bFGF. Secondary biotinylated antibodies (code No. E 0432), i.e., goat antirabbit immunoglobulin G (IgG), were purchased from Dako, Denmark. The colour reaction was developed by 3-amino-9-ethylcarbazole in acetate buffer containing H2O2. Specificity of the immunohistochemical staining was assessed by neutralization incubation of the primary antibodies with a 5- to 10-fold (by weight) excess of blocking peptide (code no. sc-79-P) also obtained from Santa Cruz Biotechnology, Inc., and incubation of preimmune serum or buffer instead of the primary antibody, all showing negative staining.
Statistical analysis was performed using analysis of variance (ANOVA) plus the Student-Newman-Keuls test in order to compare numerical values indicating TNF-α, syndecan 1 and bFGF expression either in patients with complicated IBD and controls. Statistical analyses were performed using specific software (Statsoft 6.0 program for Windows 98).
Figure 1A reports the levels of the three investigated molecules in specimens with complicated CD and controls. The levels of TNF-α did not show a significant difference between the two groups (Crohn’s value: 1.54 ± 1.19; P > 0.05). However, very high levels of bFGF were observed in CD (11.76 ± 4.65; P < 0.001), while syndecan 1 showed a moderate increase, as characteristic of inflamed tissue (5.53 ± 2.18; P < 0.005). ANOVA plus Student-Neumann-Keuls showed: bFGF > syndecan 1 > TNF-α = control. The pattern remained unchanged even when the samples were divided into large and small bowel specimens as demonstrated in Figure 1B.
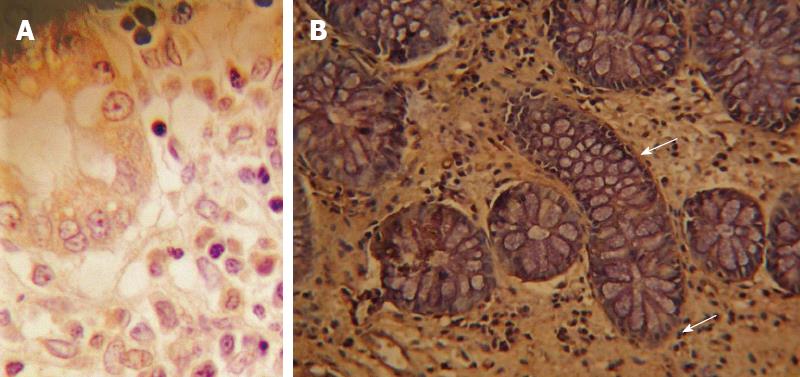
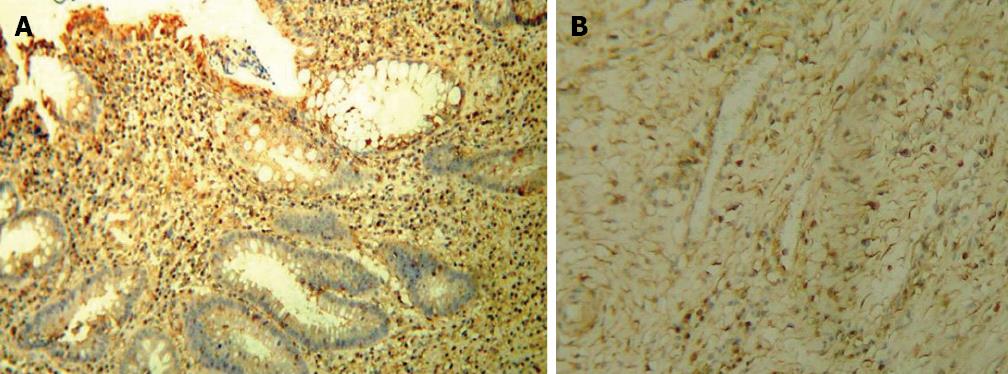
As reported in Figure 2A, immunohistochemistry showed a syndecan 1 pattern reflecting that of inflammation even if confined at mucosal layer (i.e., diffusion to stromal cells and to the apical surface of epithelium). In detail, the labeling index in stromal cells was 42.57 ± 9.21. In normal tissue no stromal cell was positive for the molecule as demonstrated in Figure 2B. Additionally, in inflamed mucosa epithelium was diffusely stained, whilst the staining was almost absent in the basolateral crypt area. However, in controls a mild staining confined to basal area of the crypts represented the only expression of this molecule. Immunoreactivity for bFGF was observed in epithelial, stromal and endothelial cells. In areas with signs of severe inflammation, the bFGF reaction in the extracellular matrix was relatively intense (Figure 3A). A positive staining for bFGF was finally observed even in the muscular layer (Figure 3B). In controls, it was almost unexpressed.
In this study we investigated the pattern of TNF-α, syndecan 1 and bFGF in patients with CD complicated by fibrotic stenosis undergoing surgical resection. The basis of our investigation was represented by our previous finding showing that infliximab therapy downregulation of bFGF/syndecan 1 link could represent a possible molecular pathway of mucosal healing in ulcerative colitis[13]. Indeed, the link between the adhesion molecule and the growth factor is essential for mucosal repair since syndecan 1 has the ability to change bFGF morphology and modulate the structure of its receptors, allowing its binding to repair dedicated epithelial and stromal cells. Moreover, syndecan 1 has a peculiar aptitude to inhibit bFGF proteolysis, which restricts tissue repair, thus maintaining mucosal inflammatory damage[5-7,10]. We found that the decrease of TNF-α induced by infliximab treatment is accompanied by a decrease in both adhesion molecule and growth factor in the presence of mucosal healing. Nevertheless, our results did not completely clarify whether infliximab therapy downregulation of bFGF/syndecan 1 link could represent a molecular pathway of mucosal healing, since the parallel pattern of TNF-α, syndecan 1 and bFGF could only be a simultaneous consequence of the control of inflammation. Furthermore, we experienced the “timing’’ of TNF-α decrease and bFGF/syndecan 1 reversal to normal levels and sites in cultured biopsy samples taken from patients with IBD and incubated in a medium containing an amount of infliximab similar to that reached in the serum of treated patients[18,19]. After 24 h we assayed TNF-α, syndecan 1 and bFGF in tissue homogenates. The final finding was that TNF-α decreased, while syndecan 1 and bFGF levels were still high when evaluated by both molecular method and immunohistochemistry[20].
In the present study we surprisingly observed that TNF-α mucosal levels were not significantly increased in patients with fibrotic stenosis. A possible explanation of this finding may be that an overgrowth of fibrotic tissue may become evident as a successive step after active inflammation, characterized by TNF-α raise. Therefore, at the stage of fibrotic stenosis requiring surgery, inflammatory mucosal changes may be an irrelevant phenomenon in most patients. Nevertheless, we cannot exclude that TNF-α decrease could have been also affected by immunosuppressive therapies assumed by most patients of our series, as shown in Table 1.
Syndecan 1 levels were significantly increased with a pattern similar to what observed in the active phase of inflammatory bowel diseases, i.e., almost absent at the baso-lateral crypt level and strongly evident in stromal cells and apical surface of epithelium[5]. A possible explanation for this pattern is that the molecule location, despite limited to mucosal layer, shows a configuration which reflects the attempt of bFGF modulation for tissue damage reversal. However, this function cannot be completely and effectively articulated for the overexpression of bFGF and especially for its location along the whole intestinal wall, i.e., outside the district in which syndecan 1 could operate.
Indeed, the main result of this study was the bFGF overexpression enclosing all intestinal wall layers. The process is known to involve various cells (epithelial, fibroblasts, myofibroblasts, monocytes, macrophages and neutrophils and endothelial cells)[21]. Additionally, its extension reached the smooth muscle layer. The explanation of this aspect may be argued from different likely reasons. First, it is presumable that the low levels of TNF-α may provoke a failure in cytokine induced bFGF proteolysis. A further enlightenment may be that the presence of syndecan 1 is limited to the mucosal layer with a consequent only partial regulation of bFGF binding to specific receptors dedicated to tissue repair. A final explanation could be an irreversible transformation of different type of cells into the fibrogenic phenotype, thus provoking the prevalence of fibrotic on inflammatory stenotic lesions[22]. During this process the exceeding of extracellular matrix cannot be inhibited by the regulatory mechanisms of the phenomenon according to what hypothesized by Pucilowska et al[23,24].
A speculative clinical consideration of our results may be the need of an accurate evaluation in the case of stenosis in the course of CD using all diagnostic available tools (histology, ultrasonography with Doppler evaluation of resistance index, biochemical indices of inflammation) in order to distinguish inflammatory from fibrotic stenosis. This could allow addressing anti-TNF-α therapy only to the first case avoiding that the cytokine decrease may be a factor supporting the fibrotic complication[25].
Downregulation of tumor necrosis factor-α (TNF-α) interacts with syndecan 1 and basic fibroblast growth factor (bFGF) in inducing mucosal healing in inflammatory bowel disease. In detail, after TNF-α fall, syndecan 1 and bFGF decrease and disappear from stromal area returning to the original location, i.e., basolateral area of the crypts and basement membranes and in subendothelial extracellular matrix of blood vessels, respectively.
The pattern of TNF-α, syndecan 1 and bFGF remains unknown when a complication occurs in inflammatory bowel disease. In detail, the pathway of fibrotic stenosis of Crohn’s disease (CD) requiring a surgical operation may be an interesting research hotspot for the possibility of investigating a possible alteration of the molecular configuration occurring in mucosal healing.
The main result was the bFGF overexpression enclosing all intestinal wall layers until smooth muscle. The explanation of this aspect may be argued from different likely reasons. First, it is presumable that the low levels of TNF-α may provoke a failure in cytokine induced bFGF proteolysis. A further enlightenment may be that the presence of syndecan 1 is limited to the mucosal layer with a consequent only partial regulation of bFGF binding to specific receptors dedicated to tissue repair. A final explanation could be an irreversible transformation of different type of cells into the fibrogenic phenotype, thus provoking the prevalence of fibrotic on inflammatory stenotic lesions. During this process the exceeding of extracellular matrix cannot be inhibited by the regulatory mechanisms
A speculative clinical consideration of our results may be the need of an accurate evaluation in the case of stenosis in the course of CD using all diagnostic available tools in order to distinguish inflammatory from fibrotic stenosis. This could allow addressing anti-TNF-α therapy only to the first case avoiding that the cytokine decrease may be a factor supporting the fibrotic complication.
CD is the main chronic inflammatory bowel disease involving the whole intestinal wall; fibrotic stenosis is a stricture requiring a surgical resection in order to avoid or resolve an intestinal occlusion; TNF-α is the main cytokine involved in inflammatory bowel disease related damage; syndecan 1 is an adhesion molecule implicated in tissue repair; bFGF is a strong fibrogenesis stimulator aimed to restore damaged tissues.
This is a retrospective study in which authors analyze the simultaneous configuration of TNF-α, bFGF and syndecan 1 in patients with a severe complication of CD, i.e., intestinal resection for severe fibrotic stenosis. A deep dysregulation of their balance was observed thus indicating a molecular pattern for stenotic complication.
P- Reviewer Guo H S- Editor Zhai HH L- Editor A E- Editor Li JY
| 1. | Hassan C, Ierardi E, Burattini O, De Francesco V, Zullo A, Stoppino G, Panella C, Morini S. Tumour necrosis factor alpha down-regulation parallels inflammatory regression in ulcerative colitis patients treated with infliximab. Dig Liver Dis. 2007;39:811-817. [PubMed] |
| 2. | Chevaux JB, Vavricka SR, Rogler G, Lakatos PL, Schoepfer A, Peyrin-Biroulet L. Mucosal healing with anti-TNF antibodies. Digestion. 2012;86 Suppl 1:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Mali M, Jaakkola P, Arvilommi AM, Jalkanen M. Sequence of human syndecan indicates a novel gene family of integral membrane proteoglycans. J Biol Chem. 1990;265:6884-6889. [PubMed] |
| 4. | Kim HS. Assignment1 of the human basic fibroblast growth factor gene FGF2 to chromosome 4 band q26 by radiation hybrid mapping. Cytogenet Cell Genet. 1998;83:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Day R, Forbes A. Heparin, cell adhesion, and pathogenesis of inflammatory bowel disease. Lancet. 1999;354:62-65. [PubMed] |
| 6. | Day R, Ilyas M, Daszak P, Talbot I, Forbes A. Expression of syndecan-1 in inflammatory bowel disease and a possible mechanism of heparin therapy. Dig Dis Sci. 1999;44:2508-2515. [PubMed] |
| 7. | Saunders S, Jalkanen M, O’Farrell S, Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556. [PubMed] |
| 8. | Beck PL, Podolsky DK. Growth factors in inflammatory bowel disease. Inflamm Bowel Dis. 1999;5:44-60. [PubMed] |
| 9. | Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol Cell Biol. 1997;17:4015-4023. [PubMed] |
| 10. | Rahmoune H, Chen HL, Gallagher JT, Rudland PS, Fernig DG. Interaction of heparan sulfate from mammary cells with acidic fibroblast growth factor (FGF) and basic FGF. Regulation of the activity of basic FGF by high and low affinity binding sites in heparan sulfate. J Biol Chem. 1998;273:7303-7310. [PubMed] |
| 11. | Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:REVIEWS3005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1222] [Cited by in RCA: 1339] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 12. | Florkiewicz RZ, Shibata F, Barankiewicz T, Baird A, Gonzalez AM, Florkiewicz E, Shah N. Basic fibroblast growth factor gene expression. Ann N Y Acad Sci. 1991;638:109-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Ierardi E, Giorgio F, Zotti M, Rosania R, Principi M, Marangi S, Della Valle N, De Francesco V, Di Leo A, Ingrosso M. Infliximab therapy downregulation of basic fibroblast growth factor/syndecan 1 link: a possible molecular pathway of mucosal healing in ulcerative colitis. J Clin Pathol. 2011;64:968-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Verspaget HW, van der Zon AM, Gao Q, van Hogezand RA. Infliximab for Crohn’s disease. Aliment Pharmacol Ther. 2001;15:2041-2042. [PubMed] |
| 15. | Ierardi E, Giorgio F, Rosania R, Zotti M, Prencipe S, Della Valle N, De Francesco V, Panella C. Mucosal assessment of tumor necrosis factor alpha levels on paraffined samples: a comparison between immunohistochemistry and real time polymerase chain reaction. Scand J Gastroenterol. 2010;45: 1007-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169-193. [PubMed] |
| 17. | Principi M, Day R, Marangi S, Burattini O, De Francesco V, Ingrosso M, Pisani A, Panella C, Forbes A, Di Leo A. Differential immunohistochemical expression of syndecan-1 and tumor necrosis factor alpha in colonic mucosa of patients with Crohn’s disease. Immunopharmacol Immunotoxicol. 2006;28:185-195. [PubMed] |
| 18. | Moorghen M, Chapman M, Appleton DR. An organ-culture method for human colorectal mucosa using serum-free medium. J Pathol. 1996;180:102-105. [PubMed] |
| 19. | Dame MK, Bhagavathula N, Mankey C, DaSilva M, Paruchuri T, Aslam MN, Varani J. Human colon tissue in organ culture: preservation of normal and neoplastic characteristics. In Vitro Cell Dev Biol Anim. 2010;46:114-122. [PubMed] |
| 20. | Della Valle N, Giorgio F, Cantatore S, Zotti M, Panella C, Ierardi E. Tumor Necrosis Factor alpha, syndecan 1 and basic fibroblast growth factor levels and site in cultured biopsy specimens of patients with inflammatory bowel diseases after incubation with infliximab. Dig Liver Dis. 2013;45:S107. |
| 21. | Rieder F, Brenmoehl J, Leeb S, Schölmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Sappino AP, Schürch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144-161. [PubMed] |
| 23. | Pucilowska JB, McNaughton KK, Mohapatra NK, Hoyt EC, Zimmermann EM, Sartor RB, Lund PK. IGF-I and procollagen alpha1(I) are coexpressed in a subset of mesenchymal cells in active Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1307-G1322. [PubMed] |
| 24. | Pucilowska JB, Williams KL, Lund PK. Fibrogenesis. IV. Fibrosis and inflammatory bowel disease: cellular mediators and animal models. Am J Physiol Gastrointest Liver Physiol. 2000;279:G653-G659. [PubMed] |
| 25. | Sorrentino D, Avellini C, Beltrami CA, Pasqual E, Zearo E. Selective effect of infliximab on the inflammatory component of a colonic stricture in Crohn’s disease. Int J Colorectal Dis. 2006;21:276-281. [PubMed] |