Published online Aug 15, 2012. doi: 10.4291/wjgp.v3.i4.85
Revised: August 6, 2012
Accepted: August 10, 2012
Published online: August 15, 2012
AIM: To investigate the relationship between primary afferent neurons, endothelin (ET) and the role of its receptors on ethanol-induced gastric damage in cirrhotic rats.
METHODS: Cirrhosis and portal hypertension were induced in rats by bile duct ligation (BDL) while controls had a sham operation. The association between ET and afferent neurons on the gastric mucosa was evaluated by capsaicin treatment in newborn rats, the use of ET agonists or antagonists, gastric ET-1 and -3 mRNA and synthetic capacity. Ethanol-induced damage was assessed using ex vivo gastric chamber experiments. Gastric blood flow was measured by laser-Doppler flowmetry.
RESULTS: ET-3 and an ETB receptor antagonist significantly reduced the extent of ethanol-induced gastric damage in BDL rats. Gastric ET-1 and -3 levels were 30% higher in BDL rats compared to control rats. Capsaicin treatment restored the gastric resistance and blood flow responses to topical application of ethanol in BDL rats and ET-1 and -3 production to levels observed in controls.
CONCLUSION: Our results suggest that the reduced resistance of the gastric mucosa of cirrhotic rats to ethanol-induced injury is a phenomenon modulated by ET through the ETB receptor and by sensory afferent neurons.
- Citation: Câmara PR, Ferraz GJ, Velloso LA, Zeitune JMR, Suassuna FA, Ferraz JGP. Endothelin and neonatal capsaicin regulate gastric resistance to injury in BDL rats. World J Gastrointest Pathophysiol 2012; 3(4): 85-91
- URL: https://www.wjgnet.com/2150-5330/full/v3/i4/85.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v3.i4.85
Endothelin (ET) is a family of three distinct peptides (ET-1, -2 and -3), best known for its vascular effects. However, it also plays a role in modulating pain, independent from its vascular actions. Data suggest that ET is also a neurotransmitter/neuromodulator and can have a direct nociceptive effect on the peripheral sensory nervous system[1,2].
Pharmacological studies have suggested that ET released in peripheral tissues could act directly on ET A (ETA) receptor-expressing sensory neurons and on ET B (ETB) receptor-expressing satellite cells in dorsal root ganglia or even in nonmyelinating Schwann cells. ETA receptor may play a role in signaling acute or neuropathic pain in peripheral tissues, whereas the ETB receptor may be involved in the transmission of chronic inflammatory pain[3,4]. ET is a strong stimulus for neuropeptide release in neurogenic inflammation, such as substance P, CGRP and catecholamines[5-8], as reported in inflammation of the meninges of rats acting through the ETB receptor[9]. Moreover, ET-1 potentiation of cholinergic nerve-mediated contraction suggests that, in addition to nerves, the vascular territory and inflammatory cells[10], other cell types, such as airway smooth muscle cells and human dermal microvascular endothelial cells, can be targeted[11,12]. Furthermore, ET-1, with the ETA and ETB receptors, has been reported to be present in the rat gastrointestinal tract[13,14] and may be a potent peptide agonist in the liver[15]. Activation of sensory afferent neurons by a luminal irritant plays a major role in regulating the gastric hyperemic response to irritants with consequent impact on resistance to injury[16,17].
The objective of this study was to investigate the relationship between ET and sensory primary afferent neurons in the gastric resistance to ethanol-induced injury in cirrhotic rats, integrating the neurogenic component of regulation of gastric mucosal blood flow and gastric resistance to injury by a topical irritant.
Male Sprague-Dawley rats (200-250 g) provided by the Animal House of the State University of Campinas (UNICAMP) were used in all experiments. They were housed in plastic cages and had free access to water and standard pellet chow. The experimental protocols were approved by the Ethical Principles in Animal Research adopted by the Brazilian College for Animal Experimentation.
Rats were anesthetized using halothane and a midline laparotomy was performed as described previously[18]. Briefly, bile duct ligation (BDL) was performed by isolating the common bile duct and placing two 4-0 sutures proximal/distal to the porta hepatis. Next, a 5-10 mm segment between ligatures was resected. The abdomen was then sutured with 4-0 silk and the animals were allowed to recover. A separate set of animals, referred to as controls, had a sham operation defined as a laparotomy with isolation, but not resection, of the common bile duct, followed by closure of the abdominal wall. Experiments were performed 4 wk following surgery when liver cirrhosis and portal hypertension were established in BDL but not control rats. The 4 wk time point was chosen, given that in our hands, the animals have clear liver cirrhosis and portal hypertension and also based on previous reports that characterized the structural and hemodynamic changes in this particular experimental protocol[16-20].
Mean arterial pressure (mmHg) in conscious control (n = 5) or BDL (n = 5) rats was measured using a rat tail blood pressure monitor (Harvard Apparatus, Holliston, MS). Portal pressure (mmHg) was measured in rats anesthetized with sodium pentobarbitone (25 mg/kg, ip) by cannulation of the superior mesenteric vein with a PE-20 tube connected to a pressure transducer (Transonic, Ithaca, NY). The preparation was equilibrated for 10 min and portal pressure continuously recorded for 10 min on a monitor (Transonic BLF 21D, Ithaca, NY) and analyzed using Win Daq software (DATAQ, Akron, OH).
Gastric resistance to injury induced by topical application of 40% ethanol was investigated using an ex vivo gastric chamber preparation. Rats (n = 5 for each group) were anesthetized with sodium pentobarbitone (25-50 mg/kg ip) and placed over a heating pad connected to a rectal probe (Harvard Apparatus, Holliston, MS) to maintain body temperature at 37 °C[19]. The stomach was exposed following a midline laparotomy and opened with an incision along the greater curvature. It was pinned over a plexiglass platform and clamped with a plexiglass cylinder. This allowed bathing of the mucosa with different solutions. The experiments consisted of six periods of 10 min each. Initially the mucosa was bathed with phosphate buffered saline for two periods, followed by 40% ethanol for one period and then HCl (pH 1.5) for the remaining three periods. All solutions were at 37 °C when added to the chamber and continuously stirred at 200 r/min. The stomach was photographed at the end of experiments and damage measured as % total glandular mucosa using computerized planimetry by an observer blinded to all treatment groups.
Gastric blood flow was measured (n = 5 for each group) using laser-Doppler flowmetry and the ex vivo gastric chamber preparation. A pencil probe (type N, penetration 1 mm, Transonic, Ithaca, NY) connected to a flowmeter (BLF 21D, Transonic, Ithaca, NY) was placed over the gastric body. The preparation was equilibrated for 5 min. Basal gastric blood flow was then recorded for an additional 5 min, after which the experiments were started and performed according to the ex vivo gastric chamber protocol described previously. Basal gastric blood flow was calculated as average flow over the initial 5 min after equilibration was achieved. Gastric blood flow was then continuously recorded throughout the 60 min experimental protocol and maximum changes in flow at each period were calculated and expressed as % change over basal flow.
Animals were treated on the second day of life with capsaicin (30 mg/kg, sc) or vehicle [10% ethanol and 10% Tween 80 in 0.9% (w/v) NaCl solution], as previously described[21]. Adult (60 d) vehicle- and capsaicin-pretreated rats (n > 5 each) were submitted to BDL or sham operation. Ex vivo chamber experiments and measurement of mean arterial and portal pressure were then carried out 4 wk after surgery, as described above. The role of primary sensory afferent neurons and ET receptors was investigated using the systemic administration of the endothelin ETA and ETB receptor agonist ET-1 or ET-3 (0.5 nmol/kg, ip) or using a selective ETA receptor antagonist (BQ 485) or an ETB receptor antagonist (BQ 788, 0.1 mg/kg, ip) 3 min prior to chamber experiments in vehicle- or capsaicin-treated animals (n = 5).
Gastric ET-1 and -3 mRNA expression was examined by reverse-transcription polymerase chain reaction (RT-PCR). Samples were collected from the body at the end of chamber experiments. The RNA was isolated using the TRIZOL method and extracted with chloroform after tissue homogenization (GIBCO BRL, Gaithesburg, MD). It was then recovered from the aqueous phase by precipitation with isopropyl alcohol and suspended in DEPC-treated water. cDNA was synthesized from 10 μg of total RNA using 1 μL of reverse transcriptase (MMLV, GIBCO BRL, Gaithesburg, MD). cDNA samples were stored at -20 °C until use. The nucleotide sequence of the primers for ET-1 and ET-3 was the one previously reported in the literature[22,23]. GAPDH mRNA was the internal control for the PCR reaction. Forty cycles of PCR amplification for ETs isoforms and 33 for GAPDH were chosen following pilot experiments to define amplification conditions. PCR reactions were performed in a final volume of 25 μL containing 2.5 μL cDNA, 2.5 μL 10X Taq buffer, 0.75 μL MgCl2 (1.5 mmol/L), 0.5 μL dNTPs (0.2 mmol/L), 1.5 μL (0.5 μmol/L) of each oligonucleotide pair (ET-1: GCTCCTGCT CCTCCTTGATG-sense, CTCGCTCTATGTAAGTCATGG-antisense and ET-3 GCTGGTGGACTTTATCTGTCC-sense, TTCTCGGGCTCACAGTGACC-antisense) 15.5 μL H2O Milli Q and 0.25 μL Taq DNA polymerase (1.25 U). The amplification cycle was done with denaturation for 1 min at 94 °C, annealing for 45 s at 57 °C and extension for 1.5 min at 72 °C for ET1/ET3 isoforms, respectively. The PCR products (10 μL) previously normalized to give equivalent amounts of the GAPDH control were separated on 1.5% agarose gel containing 10% ethidium bromide. Gels were visualized under UV light and images captured (Chemimager 5500, Alpha-Inotech, San Leandro, CA). The band size for ET-1 and ET-3 was 471 and 477 bp, respectively. Band densitometry was determined to determine the expression of each isoform relative to GAPDH.
Gastric samples (0.5 g wet weight, body) were collected from control and BDL rats, homogenized in the presence of aprotinin (10 μg/mL) and stored in a freezer at -80 °C. They were then thawed and centrifuged at 9000 r/min for 20 min (4 °C). The supernatant was collected, acidified with HCl 2 N and applied to sep-pack C18 cartridge (Waters, USA). The adsorbed peptides were eluted and lyophilized. Competitive enzyme immunoassays for ET-1 and ET-3 were conducted according to the manufacturer’s guide (Peninsula Laboratories Inc. USA).
All drugs were of analytical grade or obtained from Sigma (St. Louis, MO), unless otherwise specified.
Data are expressed as mean ± SE and comparisons among groups (n = 5) were analyzed by one-way ANOVA, followed by the Student’s Newman-Keul’s test for multiple comparisons. Statistical significance was considered when P < 0.05.
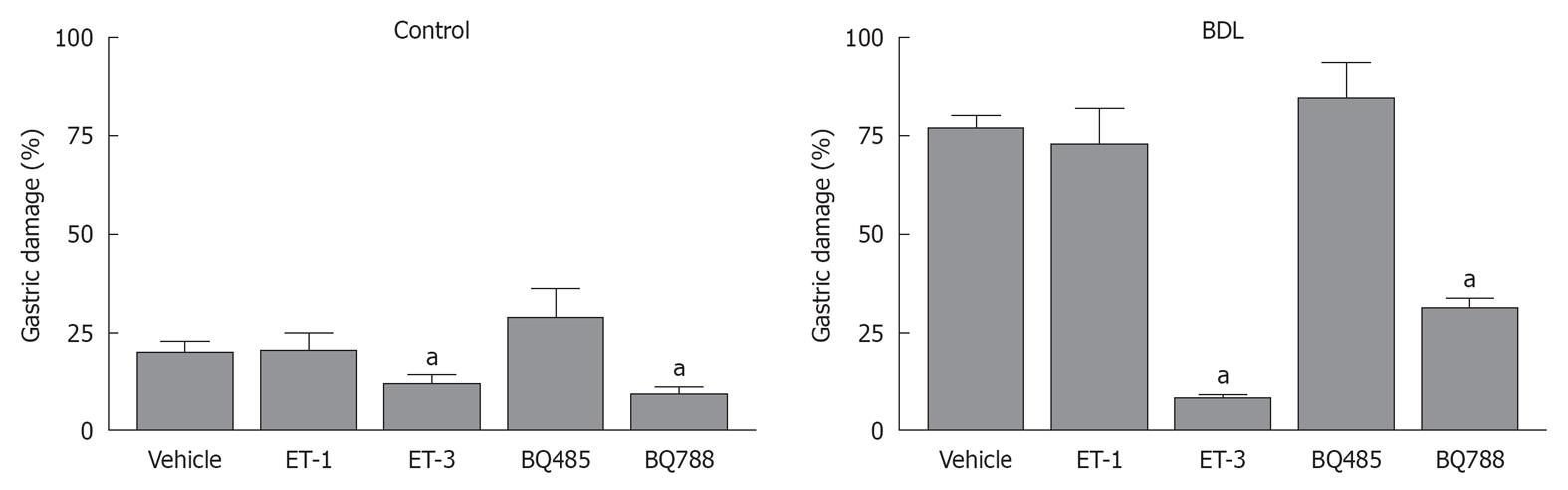
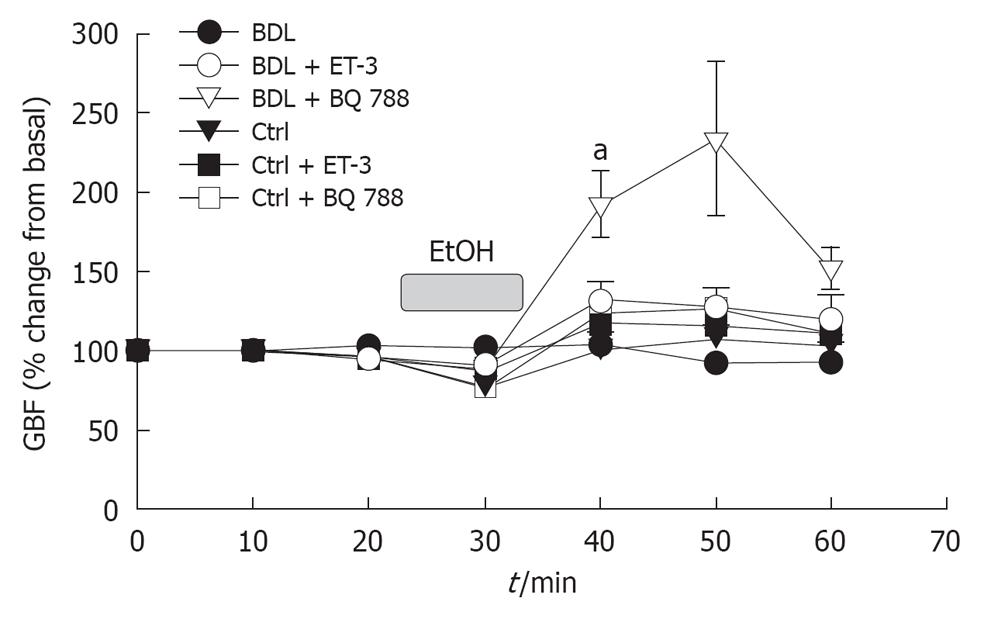
Topical application of ethanol produced a 20% ± 3% damage to the gastric mucosa in control animals while an significantly increased was observed in BDL rats (76% ± 4%, P < 0.001; Figure 1), with associated flat gastric blood flow responses to the luminal irritant (Figures 1 and 2). This phenomenon was associated with a reduction in gastric blood flow, followed by a hyperemic response when the mucosa was bathed with HCl (Figure 3).
Gastric damage was reduced in BDL and controls after administration of ET-3 or the ETB receptor antagonist, BQ788 (Figures 1 and 2). The resistance to injury in ET-3 or BQ788 pretreated rats was increased by at least 50% in the BDL group. A significant increase in gastric blood flow after exposure of the gastric mucosa to 40% ethanol was only noted in BDL rats pretreated with the ETB receptor antagonist (Figure 3). The hyperemic response to ethanol in BQ788-pretreated BDL rats was actually of greater magnitude to that observed in sham-operated controls. ET-1 and the ETA receptor antagonist BQ485 did not affect either ethanol-induced damage (Figure 1) or gastric blood flow (Figure 3) responses in control or BDL animals.
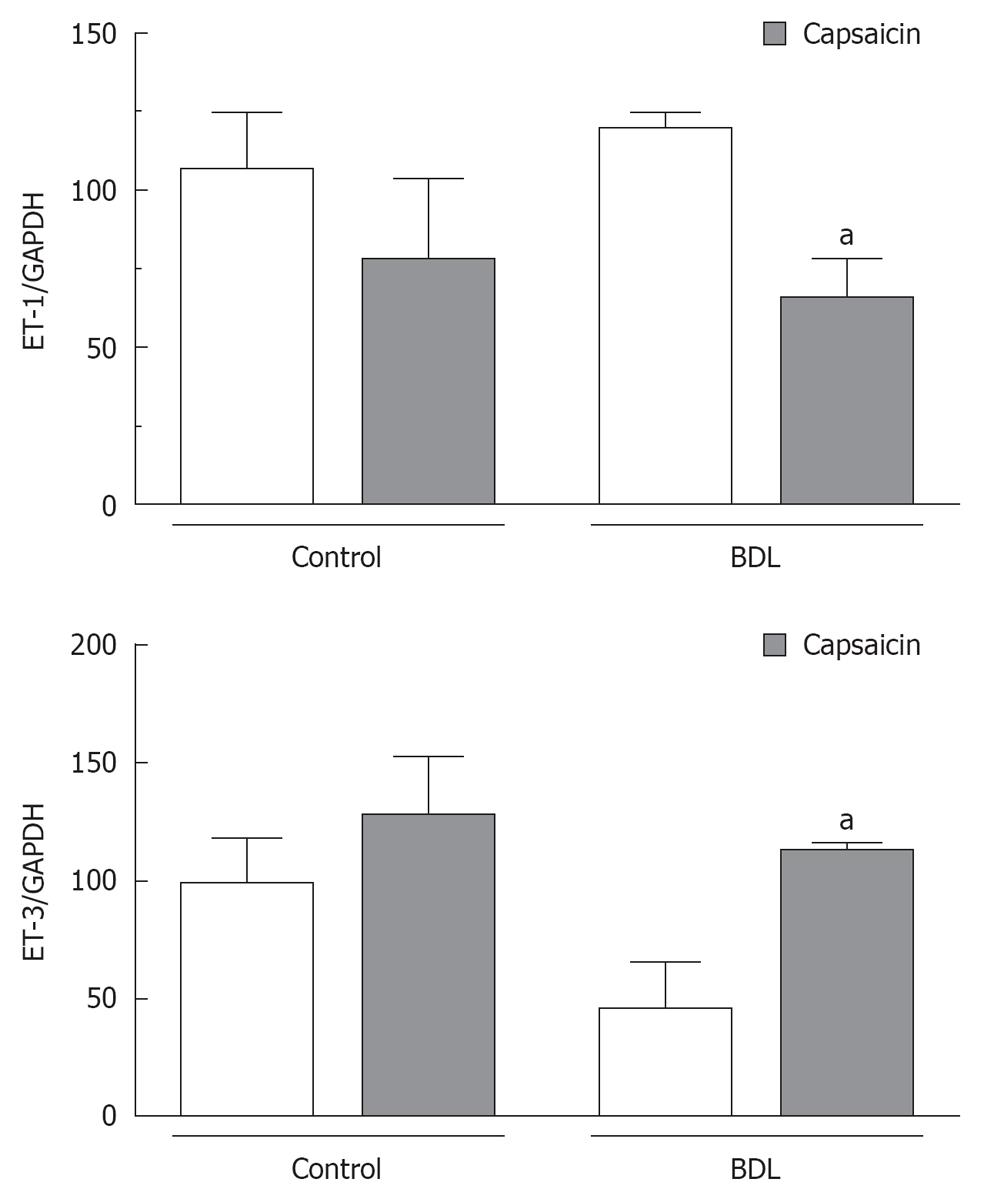
ET-1 mRNA expression was comparable in samples collected from control and BDL rats, while ET-3 mRNA expression was reduced by almost 50% in BDL rats (Figure 4). Ablation of sensory afferent neurons using neonatal treatment with capsaicin produced a significant reduction in ET-1 mRNA expression by the stomach of BDL rats while mRNA expression of ET-3 was detected in levels comparable to controls (Figure 4).
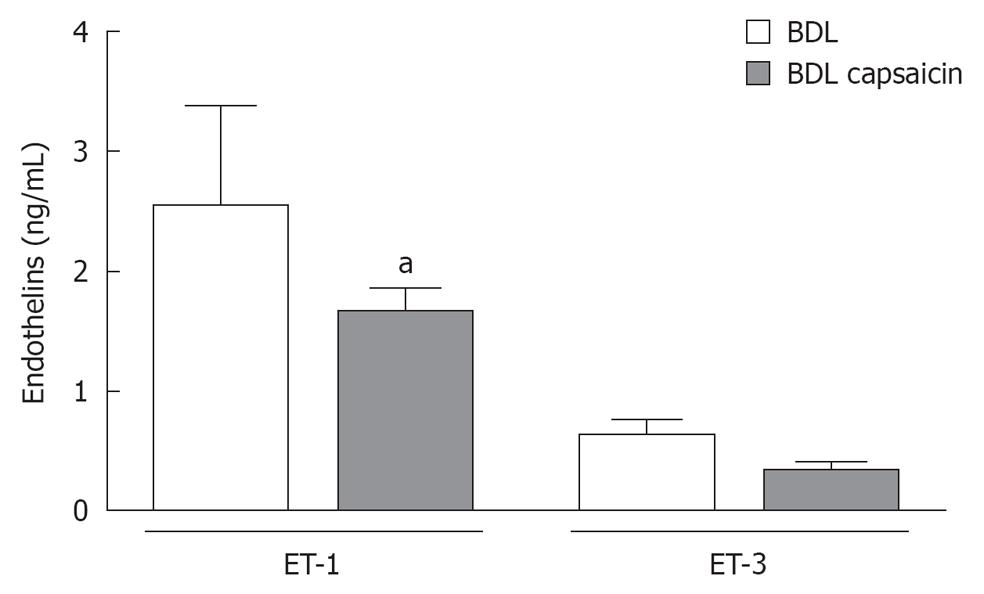
ET-1 and -3 were detected in the stomach of both control and BDL rats. ET-1 was predominant over ET-3 in a 3/1 ratio and its concentration was significantly increased (30% higher, P < 0.05) in the stomach of BDL rats compared to controls (data not shown). Ablation of sensory afferent neurons using neonatal capsaicin did produce a significant reduction in gastric ET-1 in the stomach of BDL rats without significantly changing the approximate 3/1 ratio of gastric ET-1/ET-3 detectable (Figure 5). Capsaicin-treated BDL rats had levels of both ET-1 and -3 similar to that found in controls (data not shown).
The results of the present study show the interaction of ET through its ETB receptor in mediating an increased susceptibility to ethanol-induced gastric damage in bile duct ligated, cirrhotic rats.
It has been previously demonstrated that the development of the hyperdynamic circulation in the context of experimental portal hypertension could be prevented by ablation of primary sensory afferent neurons by capsaicin treatment in newborn rats[19,24]. Capsaicin is the active ingredient of the pungent capsicum pepper and when given acutely to adult animals it activates primary afferent nerves, whereas it permanently ablates the same nerves when given in higher doses neonatally. Ablation of primary afferent neurons in newborn rats has been very useful over the past three decades as a tool to explore the physiology of primary afferent nerves[25]. For example, we have previously demonstrated the involvement of neuropeptides released from these sensory neurons in the development of portal hypertensive gastropathy[17,19].
Our data show that ET-3 administration and ETB receptor blockade result in improved gastric hyperemic response to 40% ethanol in bile duct-ligated rats. This was associated with increased resistance to damage induced by the luminal irritant, whereas ET-1 administration and ETA receptor blockade had no effect. This suggests once again that the regulation of gastric blood flow responses to a luminal irritant plays a major role in the actual resistance of the gastric mucosa to injury. An impaired microcirculatory response or no changes in mucosal resistance to injury following administration of ET-1 or ETA receptor antagonist (BQ485) is puzzling and could be secondary to a desensitization of the ETA receptor to ET-1[26], but not to ET-3 in BDL rats. Indeed, in our hands, both ET-1 and -3 were detected in the stomach and the concentration was significantly higher in the BDL rats for both isoforms, with an abundance of ET-1 over ET-3. The higher gastric levels of ET-1 could have produced desensitization of the ETA receptors within the gastric microcirculation by a mechanism of compensatory adaptation to an increased presence of the peptide. All three ET isoforms have similar affinities for the ETB receptor[1], but the ETA receptor binds ET-l with two- to 10-fold higher affinity than ET-2 and with more than 100-fold higher affinity than ET-3[22]. Therefore, in our experiments, the observed effects of ET-3 treatment on the gastric hyperemic response to ethanol could be secondary to its action on the ETA receptor promoting the increase of gastric blood flow in the context of an absent response to ETA receptor to ET-1. This ultimately increased the resistance of the stomach to injury induced by 40% ethanol in ET-3 treated rats.
ET is a strong stimulus of the release of neuropeptides involved in neurogenic inflammation, such as substance P, CGRP and others. ET may play a role in the repair of damaged neurons[12,27] and several studies have suggested that ETA and ETB receptors are actually expressed in sensory neurons[1-5]. ET-1 predominates in hepatic[15] and gastrointestinal systems[14,28], while ET-3 seems to predominate over ET-1 in neurons of the brain[29]. Sensory afferent neurons in the stomach, when activated by ethanol or luminal irritants, release CGRP that will ultimately release nitric oxide from the gastric microcirculation, producing the gastric hyperemic response so important in the defence against injury[18,29]. Again, our results demonstrate an abundance of ET-1 over ET-3 in the stomach, as measured by ELISA in gastric homogenates with a 30% increase in BDL rats compared to controls. Ablation of capsaicin sensitive neurons in newborn rats prior to inducing cirrhosis and portal hypertension by BDL restored resistance to injury with associated changes in the gastric hyperemic response in our previous study. The data now presented suggest that capsaicin-treated rats had changes in the expression of ET-1 and -3 mRNA, as well as a reduced production of ET-1 by the stomach to levels observed in control, non-cirrhotic rats. Therefore, ablation of sensory afferent neurons by capsaicin resulted in improved gastric resistance to injury by reducing the increased production of ETs by the stomach to levels observed in controls. Whether production/release of ETs by other organs/systems within the hyperdynamic circulation BDL rat model also contributes to what we demonstrated in this study is unclear, was not the goal of this study and remains to be elucidated.
The results of this study suggest that sensory afferent neurons are an integral component of the regulatory mechanisms associated with the gastric hyperemic response to luminal irritants and its effects on mucosal resistance to injury. ET production is modulated by the primary afferent neurons and one could speculate that ET-1 may actually promote tachykinin release from afferent neurons. In turn, binding of released tachykinins (for example, Substance P) to their tachykinin receptor 1 (NK1) on endothelial cells within the gastric microcirculation leads to the final response of increased blood flow by nitric oxide release, namely the gastric hyperemic response. As such, ET-1 could potentially negatively affect gastric mucosal defence in the portal hypertensive gastropathy via ETB receptor activation as ETB is expressed in sensory neurons that promote tachykinin release when activated. Furthermore, as demonstrated by our study, ET-3 treatment produced a reduction in ethanol-induced gastric damage in BDL rats; an effect that was also seen with ETB receptors blocked using BQ-788.
Our results support the notion that studies evaluating the potential role of ETB receptor agonists as well as ET-3 in the treatment of portal hypertensive gastropathy in humans are warranted. The gastric concentration of ET is increased in the stomach of BDL rats, a model of cirrhosis and portal hypertension and its receptors modulate the gastric hyperemic response to topical application of ethanol with consequent impact on the resistance to injury induced by the luminal irritant.
Increased resistance to portal blood flow in the context of liver dysfunction leading to portal hypertension increases the risk of bleeding episodes in cirrhotic patients. It would be helpful to develop strategies to reduce the risk of bleeding in these patients.
Endothelin (ET) has been explored in both basic and clinical research. They play a role in gastric resistance to injury in the bile duct ligation (BDL) model of cirrhosis and portal hypertension in rats. ET production can be modulated by sensory afferent neurons. The effects of ET receptor agonists and antagonists along with ablation of sensory afferent neurons on the resistance of the stomach and the gastric blood flow responses to 40% ethanol were investigated in an effort to integrate the defence mechanisms employed by the stomach in the context of experimental cirrhosis.
Ethanol-induced gastric damage was reduced using an ET-3 agonist and an ETB receptor antagonist in BDL rats. Ablation of sensory afferent neurons by capsaicin in newborn rats restored the gastric resistance to ethanol-induced injury with normalization of gastric ET-1 and -3 synthesis and improved hyperemic response to the topical irritant.
The present study supports the concept that ET-1 and -3 should be investigated in the clinical setting, along with the use of an ET-3 agonist or ETB receptor antagonist. The use of this strategy is warranted in the context of liver cirrhosis, portal hypertension and portal hypertensive gastropathy.
Portal hypertensive gastropathy is a complication found in patients with liver cirrhosis and portal hypertension. It can be associated with clinically significant acute or chronic bleeding episodes. BDL is an experimental model of secondary biliary cirrhosis, liver dysfunction, portal hypertension and ascites, similar to that found in patients with cirrhosis. ET is a family of peptides for experimental use.
This is a study in which the authors analyze the integration between ET and sensory afferent neurons on gastric damage induced by ethanol in rats. The results suggest that an ETB receptor antagonist and ET-3 could be tested in the clinical setting with a goal of increasing the gastric resistance to injury in the context of liver cirrhosis and portal hypertension.
Peer reviewer: Liya Qiao, PhD, Assistant Professor, Department of Physiology and Biophysics, School of Medicine, Virginia Commonwealth University, Richmond, VA 23298, United States
S- Editor Zhai HH L- Editor Roemmele A E- Editor Zheng XM
| 1. | Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW. Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci. 2001;21:999-1006. [PubMed] |
| 2. | Ritz MF, Stuenkel EL, Dayanithi G, Jones R, Nordmann JJ. Endothelin regulation of neuropeptide release from nerve endings of the posterior pituitary. Proc Natl Acad Sci USA. 1992;89:8371-8375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Gokin AP, Fareed MU, Pan HL, Hans G, Strichartz GR, Davar G. Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. J Neurosci. 2001;21:5358-5366. [PubMed] |
| 4. | Dogulu FH, Ozogul C, Akpek S, Kurt G, Emmez H, Ercan S, Baykaner MK. Intra-arterial simultaneous administration of anandamide attenuates endothelin-1 induced vasospasm in rabbit basilar arteries. Acta Neurochir (Wien). 2003;145:579-582. [PubMed] |
| 5. | Zhou QL, Strichartz G, Davar G. Endothelin-1 activates ET(A) receptors to increase intracellular calcium in model sensory neurons. Neuroreport. 2001;12:3853-3857. [PubMed] |
| 6. | Calvo JJ, Gonzalez R, De Carvalho LF, Takahashi K, Kanse SM, Hart GR, Ghatei MA, Bloom SR. Release of substance P from rat hypothalamus and pituitary by endothelin. Endocrinology. 1990;126:2288-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Dymshitz J, Vasko MR. Endothelin-1 enhances capsaicin-induced peptide release and cGMP accumulation in cultures of rat sensory neurons. Neurosci Lett. 1994;167:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Wang DH. The vanilloid receptor and hypertension. Acta Pharmacol Sin. 2005;26:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Brändli P, Löffler BM, Breu V, Osterwalder R, Maire JP, Clozel M. Role of endothelin in mediating neurogenic plasma extravasation in rat dura mater. Pain. 1996;64:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Germonpre PR, Bullock GR, Lambrecht BN, Van De Velde V, Luyten WH, Joos GF, Pauwels RA. Presence of substance P and neurokinin 1 receptors in human sputum macrophages and U-937 cells. Eur Respir J. 1999;14:776-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | D'Agostino B, Advenier C, Falciani M, Gallelli L, Marrocco G, Piegari E, Filippelli A, Rossi F. Endothelin-1 increases cholinergic nerve-mediated contraction of human bronchi via tachykinin synthesis induction. Br J Pharmacol. 2001;134:1447-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Milner P, Loesch A, Burnstock G. Endothelin immunoreactivity and mRNA expression in sensory and sympathetic neurones following selective denervation. Int J Dev Neurosci. 2000;18:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Takahashi K, Jones PM, Kanse SM, Lam HC, Spokes RA, Ghatei MA, Bloom SR. Endothelin in the gastrointestinal tract. Presence of endothelinlike immunoreactivity, endothelin-1 messenger RNA, endothelin receptors, and pharmacological effect. Gastroenterology. 1990;99:1660-1667. [PubMed] |
| 14. | Ohta M, Pai R, Kawanaka H, Ma T, Sugimachi K, Sarfeh IJ, Tarnawski AS. Expression of endothelin-1, and endothelin A and B receptors in portal hypertensive esophagus of rats. J Physiol Pharmacol. 2000;51:57-67. [PubMed] |
| 15. | Gandhi CR, Stephenson K, Olson MS. Endothelin, a potent peptide agonist in the liver. J Biol Chem. 1990;265:17432-17435. [PubMed] |
| 16. | Beck PL, Lee SS, McKnight GW, Wallace JL. Characterization of spontaneous and ethanol-induced gastric damage in cirrhotic rats. Gastroenterology. 1992;103:1048-1055. [PubMed] |
| 17. | Ferraz JG, McKnight W, Sharkey KA, Wallace JL. Impaired vasodilatory responses in the gastric microcirculation of anesthetized rats with secondary biliary cirrhosis. Gastroenterology. 1995;108:1183-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Geraldo J, Ferraz P, Wallace JL. Prostaglandins modulate the responsiveness of the gastric microcirculation of sodium nitroprusside in cirrhotic rats. Hepatology. 1996;23:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Camara PR, Ferraz GJ, Franco-Penteado CF, Sbragia-Neto L, Meirelles LR, Teixeira SA, Muscara MN, Velloso LA, Antunes E, Ferraz JG. Ablation of primary afferent neurons by neonatal capsaicin treatment reduces the susceptibility of the portal hypertensive gastric mucosa to ethanol-induced injury in cirrhotic rats. Eur J Pharmacol. 2008;589:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Câmara PR, Moi GP, Ferraz JG, Zeitune JM. Effect of anesthetics on gastric damage using two models of portal hypertension. World J Gastrointest Pharmacol Ther. 2010;1:81-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Jancsó G, Kiraly E, Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741-743. [PubMed] |
| 22. | David FL, Montezano AC, Rebouças NA, Nigro D, Fortes ZB, Carvalho MH, Tostes RC. Gender differences in vascular expression of endothelin and ET(A)/ET(B) receptors, but not in calcium handling mechanisms, in deoxycorticosterone acetate-salt hypertension. Braz J Med Biol Res. 2002;35:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Terada Y, Tomita K, Nonoguchi H, Yang T, Marumo F. Expression of endothelin-3 mRNA along rat nephron segments using polymerase chain reaction. Kidney Int. 1993;44:1273-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Li Y, Song D, Zhang Y, Lee SS. Effect of neonatal capsaicin treatment on haemodynamics and renal function in cirrhotic rats. Gut. 2003;52:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1150] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 26. | Thakali K, Fink GD, Watts SW. Arteries and veins desensitize differently to endothelin. J Cardiovasc Pharmacol. 2004;43:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Milner P, Bodin P, Guiducci S, Del Rosso A, Kahaleh MB, Matucci-Cerinic M, Burnstock G. Regulation of substance P mRNA expression in human dermal microvascular endothelial cells. Clin Exp Rheumatol. 2004;22:S24-S27. [PubMed] |
| 28. | Migoh S, Hashizume M, Tsugawa K, Tanoue K, Sugimachi K. Role of endothelin-1 in congestive gastropathy in portal hypertensive rats. J Gastroenterol Hepatol. 2000;15:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Tepperman BL, Whittle BJ. Endogenous nitric oxide and sensory neuropeptides interact in the modulation of the rat gastric microcirculation. Br J Pharmacol. 1992;105:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 2.8] [Reference Citation Analysis (0)] |