Published online Jun 15, 2012. doi: 10.4291/wjgp.v3.i3.80
Revised: May 23, 2012
Accepted: June 12, 2012
Published online: June 15, 2012
AIM: To verify whether there is a gender difference in the 13C-urea breath test results in a large cohort.
METHODS: The test results of dyspeptic patients referred for 13C-urea breath testing between January and December, 2007 were evaluated. Testing was carried out at the health insurance organization branches and evaluated at a central laboratory in Israel.
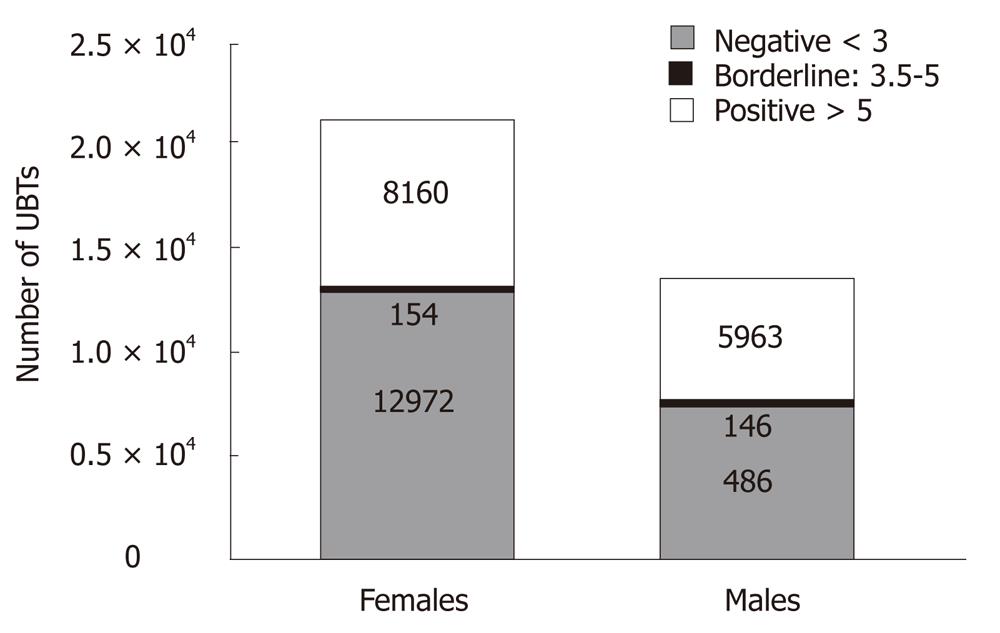
RESULTS: Of a total of 28 746 test results, 18 122 (63.04%) were from females and 10 624 (36.95%) from males. Overall, 10 188 (35.4%) results [expressed as delta over baseline (DOB)] were positive (DOB 13C > 5), 18,326 (63.7%) were negative (DOB 13C < 3.5) and 232 (0.8%) were borderline (DOB 13C 3.5-5). There was a significant difference between the total positive rate among females and males (34.8% vs 37.2%, respectively, P = 0.0003). The mean test value was increased by approximately 10 units for females compared to males (P < 0.01) and this difference was consistent for all age groups (i.e., between 10-80 years of age, P < 0.01).
CONCLUSION: More females were referred to 13C-urea breath testing. More males had positive results. The mean test values were significantly higher among females of all age groups, possibly representing an increased bacterial load among females and suggesting gender-associated differences in Helicobacter pylori host interactions.
-
Citation: Moshkowitz M, Horowitz N, Beit-Or A, Halpern Z, Santo E. Gender-associated differences in urea breath test for
Helicobacter pylori infection referrals and results among dyspeptic patients. World J Gastrointest Pathophysiol 2012; 3(3): 80-84 - URL: https://www.wjgnet.com/2150-5330/full/v3/i3/80.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v3.i3.80
Helicobacter pylori (H. pylori) is the major cause of peptic ulcer disease as well as being implicated in the pathogenesis of gastric cancer[1]. The 13C-urea breath test (13C-UBT) is considered the most accurate non-invasive diagnostic tool for the presence of H. pylori[2,3] and one that is widely used in clinical practice. By measuring the intragastric urease activity, potential advantages of 13C-UBT are threefold: it allows assessment of the H. pylori bacterial load, which, according to several reports, might be a risk factor in the development of peptic ulcer disease[4-7]; it serves to determine the severity of gastritis activity[4-6]; and it influences the efficacy of H. pylori eradication therapy[8-11]. A significant elevation of 13C-UBT values among females infected with H. pylori compared to males was recently reported, suggesting gender-associated differences in H. pylori host interaction[12]. The aim of our current study was to evaluate the pattern of 13C-UBT referrals among a large cohort of dyspeptic males and females and to verify whether or not there is such a difference in 13C-UBT results.
Maccabi Health Services is the second largest health insurance organization (HMO) in Israel, providing health services to approximately 2 million citizens. Its central laboratory provides 13C-UBTs for its subscribers nationwide. The sample for the current study consists of 13C-UBTs collected at the HMO branches and evaluated at MHC’s central laboratory from January to December, 2007. The 13C-UBT was performed with a mass spectrometer (Analytical Precision 2003, UK) using 75 mg of urea labeled with 13C in 200 mL of orange juice. Breath samples were collected twice from each patient (at 0 and 30 min) and the ratio of 12C to 13C was measured at both time points. The difference was calculated by subtraction and termed the excess delta or the delta over the baseline (DOB). A DOB > 5.0 was considered positive for H. pylori infection, a DOB < 3.5 was considered negative for H. pylori infection and a DOB of 3.5-5 was considered as a borderline result. All the study patients were asked to stop the use of H2 antagonists, proton pump inhibitors or any antibiotics one week prior to undergoing the breath test.
Categorical variables were summarized with number and percentage of patients. The χ2 and Fisher exact tests were used to compare categorical variables and the Kruskal-Wallis one-way analysis of variance was used to analyze the demographic data. Significance was set at a P value < 0.05. The data were analyzed using SPSS version 15.0 (SPSS Inc. Chicago, IL).
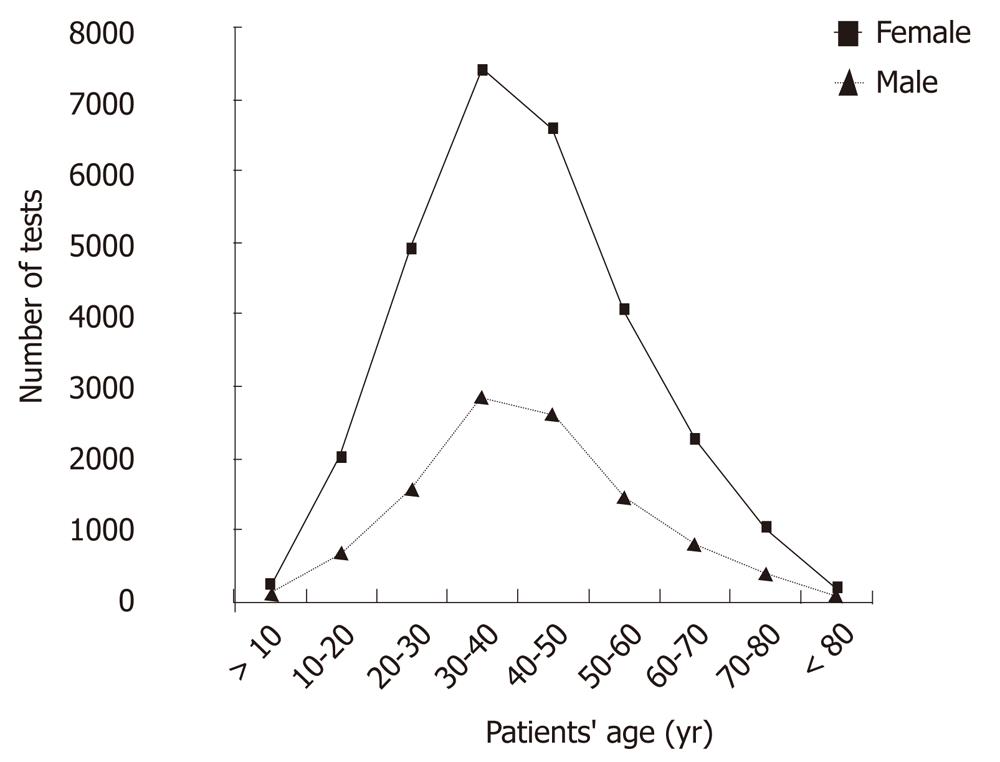
A total of 28 746 13C-UBTs were performed, 18 122 (63.04%) in females and 10 624 (36.95%) in males, during the one year study period. Figure 1 demonstrates the number of 13C-UBT referrals according to the patients’ age. Overall, 10 188 (35.4%) 13C-UBTs were positive (∆13C > 5), 18 326 (63.7%) were negative (∆13C < 3.5) and only 232 (0.8%) were borderline (∆13C 3.5-5). The difference between the total positive rate among females and males (34.8% vs 37.2%) was highly significant (P = 0.0003) (Figure 2).
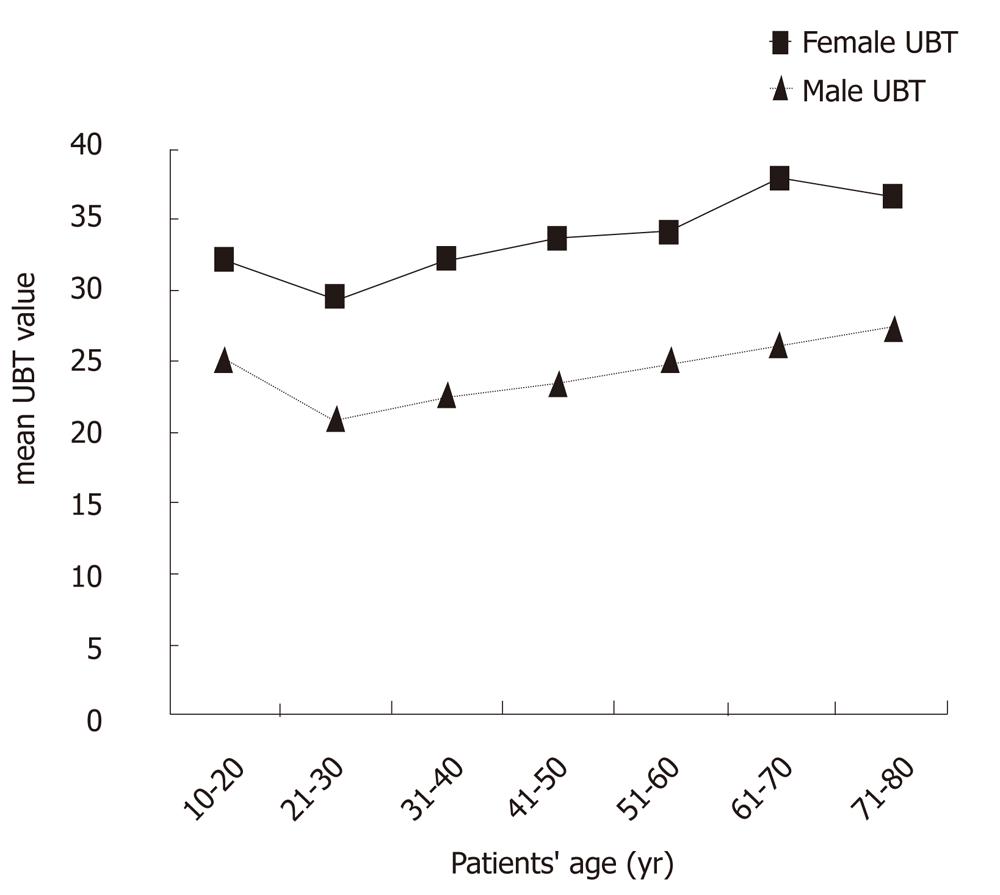
We analyzed the mean 13C-UBT values in both genders according to the patients’ age (Figure 3). There was a significant increase of about 10 units in the mean 13C-UBT value among females compared to males and that difference remained constant for all age groups between 10 years and 80 years of age (P < 0.01 for each).
The main findings of the present study are that more females are referred to 13C-UBTs than males, that the rate of positive results is higher among males, and that there is a highly significant increased mean 13C-UBT value for females in all age groups compared to age-matched males.
The numerical results of the 13C-UBT are the function of total urease activity within the stomach, so the test might serve as a quantitative index of the density of gastric H. pylori colonization. Previous studies have reported inconsistent results about the relationship between 13C-UBT findings and histology-based semi quantitative measures of bacterial infection. Several studies have demonstrated a correlation between the excess of delta (δ) 13CO2 excretion and the H. pylori bacterial load[13-18], while others found that the 13C-UBT value has only qualitative meaning, i.e., either positive or negative for H. pylori infection[19-21]. Kobayashi et al[22] reported that the gastric mucosal density of H. pylori as estimated by real-time polymerase chain reaction was significantly correlated with 13C-UBT results and histological grading. Some groups have shown that 13C-UBT-based or histologically estimated bacterial density in gastric mucosa can predict the extension of gastric inflammation and H. pylori eradication[5,6].
The observation in our study of the significantly increased mean 13C-UBT value found in females of all age groups requires an explanation, whether the 13C-UBT value represents bacterial load or urease enzyme activity. In 13C-UBT, orally administered 13C-labeled urea is hydrolyzed into ammonia and into 13CO2 by urease in the presence of H. pylori infection. The results are expressed as DOB values. Since endogenous 12CO2 production varies with age (i.e., adults more than children), weight, height and sex (i.e., males more than females), individuals with relatively lower body weight and height may produce smaller amounts of endogenous 12CO2, whereupon their DOB values, expressed as a change in the 13CO2/12CO2 ratio, may consequently increase[22]. This, however, can explain only a small part of the increased mean 13C-UBT values among females and not the significantly increased values (approximately 10 units) in all age groups that were found in the present study.
Most H. pylori-related diseases are associated with male gender. The role of gender as a risk factor for H. pylori infection was reviewed by de Martel and Parsonnet in a meta-analysis of large, population-based studies[23]. Those authors found that male gender was significantly associated with H. pylori infection (OR: 1.16, 95% CI: 1.11-1.22) and that this male predominance of H. pylori infection was homogeneous and consistent across adult populations from various countries. They concluded that these findings may partially explain the male predominance of H. pylori-related adult diseases, such as duodenal ulcer and gastric adenocarcinoma.
Gender differences have also been found in response to treatment. Moayyedi et al[24] reported that anti-H. pylori therapy was significantly less successful in women than in men. They hypothesized that this may relate to an increased prevalence of 5-nitroimidazole-resistant organisms in women. Alternatively, there may be gender differences in acid output and gastric blood flow that influence treatment success[25]. The findings of the current study may provide another explanation: that the increased bacterial load among females causes the decreased response to antibiotic therapy.
Several studies have shown that the presence of H. pylori infection is a stronger predictor of gastric cancer in females compared to males[26-29]. Smoking and alcohol consumption were significantly more prevalent in males with gastric cancer than in males without it, and these differences were not present in females. It may therefore be considered that as risk factors, smoking and alcohol consumption have a stronger impact on males than on females. Here again, the finding that females might have an increased bacterial load may provide an explanation for H. pylori infection having been shown to be a stronger predictor of gastric cancer in females compared to males. Ohtani et al[30] have shown an effect of ovarian-dependent female hormones on H. pylori-induced gastric cancer in hypergastrinemic INS-GAS mice and Crabtree and colleagues have demonstrated that there are gender differences in the magnitude of the gastric cytokine responses to H. pylori[31].
An interesting observation in our study is that the number of total 13C-UBT referrals was significantly higher among females than males in almost all age groups, but especially between the third and fifth decades (Figure 2). The increased number of 13C-UBTs among females was also associated with a slightly increased rate of negative test results. Both observations might reflect the increased prevalence of functional dyspepsia among females compared to males[32-34]. It would appear that females, especially those between the third and fifth decades of life, tend to suffer more from functional disorders. This would serve to explain the increased number of females referred to H. pylori13C-UBTs. On the other hand, organic H. pylori-related diseases are more associated with male gender and this would explain the increased rate of positive 13C-UBT results among males.
We found that the number of referrals to 13C-UBTs was greater among females than males, especially among females between the third and fifth decades of life. This could be explained by the increased prevalence of functional dyspepsia among females. The rate of positive 13C-UBT results, however, was greater among males. Another important observation was the significantly increased mean 13C-UBT values among females in all age groups. This may represent an increased bacterial load among females but this gender difference needs to be further investigated before any firm conclusions can be drawn.
Esther Eshkol is thanked for editorial assistance.
Helicobacter pylori (H. pylori) is the major cause of peptic ulcer disease and the 13C-urea breath test (13C-UBT) is considered the most accurate non-invasive diagnostic tool for the presence of H. pylori. A significant elevation of 13C-UBT values among females infected with H. pylori compared to males was recently reported, suggesting gender-associated differences in H. pylori host interaction, and the aim of the current study was to evaluate the pattern of 13C-UBT referrals among a large cohort of dyspeptic males and females and to verify whether or not there is such a difference in 13C-UBT results.
The main findings of the present study are that more females are referred to 13C-UBTs than males, that the rate of positive results is higher among males, and that there is a highly significant increased mean 13C-UBT value for females in all age groups compared to age-matched males.
The authors found significantly increased mean 13C-UBT values among females in all age groups. This may represent an increased bacterial load among females.
H. pylori is a spiral bacterium implicated in gastritis, gastric ulcer and peptic ulcer disease. UBT is a non-invasive diagnostic procedure used to identify infections by H. pylori. It is based upon the ability of the bacterial enzyme urease to convert urea to ammonia and carbon dioxide. UBT is recommended in leading society guidelines as a preferred non-invasive choice for detecting H. pylori before and after treatment.
The study was performed on a large cohort of patients regarding the possibility of a gender difference in the 13C-urea breath test. The analysis is well conducted and the results are interesting.
Peer reviewer: Enzo Ierardi, Professor, Department of Medical Sciences, Section of Gastroenterology, University Gastroenterology Unit, AOU Ospedali Riuniti, 71100 Foggia, Italy
S- Editor Wu X L- Editor Roemmele A E- Editor Wu X
| 1. | Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1348] [Article Influence: 74.9] [Reference Citation Analysis (1)] |
| 2. | Zagari RM, Bazzoli F, Pozzato P, Fossi S, De Luca L, Nicolini G, Berretti D, Roda E. Review article: non-invasive methods for the diagnosis of Helicobacter pylori infection. Ital J Gastroenterol Hepatol. 1999;31:408-415. [PubMed] |
| 3. | Graham DY, Klein PD. Accurate diagnosis of Helicobacter pylori. 13C-urea breath test. Gastroenterol Clin North Am. 2000;29:885-893, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Khulusi S, Mendall MA, Patel P, Levy J, Badve S, Northfield TC. Helicobacter pylori infection density and gastric inflammation in duodenal ulcer and non-ulcer subjects. Gut. 1995;37:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Bayerdörffer E, Lehn N, Hatz R, Mannes GA, Oertel H, Sauerbruch T, Stolte M. Difference in expression of Helicobacter pylori gastritis in antrum and body. Gastroenterology. 1992;102:1575-1582. [PubMed] |
| 6. | Alam K, Schubert TT, Bologna SD, Ma CK. Increased density of Helicobacter pylori on antral biopsy is associated with severity of acute and chronic inflammation and likelihood of duodenal ulceration. Am J Gastroenterol. 1992;87:424-428. [PubMed] |
| 7. | Bor-Shyang S, Chih-Hsein C, Hsiao-Bai Y, Shu-Chu S, Xi-Zhang L. Heavy bacterial loads of H. pylori may precipitate duodenal ulcer bleeding but not bleeding severity. Hepatogastroenterology. 1998;45:2165-2170. [PubMed] |
| 8. | Moshkowitz M, Konikoff FM, Peled Y, Santo M, Hallak A, Bujanover Y, Tiomny E, Gilat T. High Helicobacter pylori numbers are associated with low eradication rate after triple therapy. Gut. 1995;36:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Sheu BS, Yang HB, Su IJ, Shiesh SC, Chi CH, Lin XZ. Bacterial density of Helicobacter pylori predicts the success of triple therapy in bleeding duodenal ulcer. Gastrointest Endosc. 1996;44:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Perri F, Clemente R, Festa V, Quitadamo M, Conoscitore P, Niro G, Ghoos Y, Rutgeerts P, Andriulli A. Relationship between the results of pre-treatment urea breath test and efficacy of eradication of Helicobacter pylori infection. Ital J Gastroenterol Hepatol. 1998;30:146-150. [PubMed] |
| 11. | Maconi G, Parente F, Russo A, Vago L, Imbesi V, Bianchi Porro G. Do some patients with Helicobacter pylori infection benefit from an extension to 2 weeks of a proton pump inhibitor-based triple eradication therapy? Am J Gastroenterol. 2001;96:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Shmuely H, Yahav J, Samra Z, Chodick G, Ofek I. Elevated 13C urea breath test values females infected with Helicobacter pylori. Dig Dis Sci. 2007;52:402-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Suto H, Azuma T, Ito S, Ito Y, Miyaji H, Yamazaki Y, Kohli Y, Kuriyama M. Endoscopic [13C]-urea breath test for quantification of Helicobacter pylori infection. J Gastroenterol Hepatol. 2000;15:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Perri F, Clemente R, Pastore M, Quitadamo M, Festa V, Bisceglia M, Li Bergoli M, Lauriola G, Leandro G, Ghoos Y. The 13C-urea breath test as a predictor of intragastric bacterial load and severity of Helicobacter pylori gastritis. Scand J Clin Lab Invest. 1998;58:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Hilker E, Stoll R, Domschke W. Quantitative assessment of Helicobacter pylori (HP) colonisation of the gastric mucosa by 13C-urea breath test (abstract). Gastroenterology. 1994;106:93. |
| 16. | Epple HJ, Kirstein FW, Bojarski C, Frege J, Fromm M, Riecken EO, Schulzke JD. 13C-urea breath test in Helicobacter pylori diagnosis and eradication: correlation to histology, origin of "false" results, and influence of food intake. Scand J Gastroenterol. 1997;32:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Sheu BS, Lee SC, Yang HB, Lin XZ. Quantitative result of 13C urea breath test at 15 minutes may correlate with the bacterial density of H. pylori in the stomach. Hepatogastroenterology. 1999;46:2057-2062. [PubMed] |
| 18. | Lee MS, Hong SJ, Cho YD. 13C-urea breath test reflect gastric Helicobacter pylori test as a predictor of intragastric bacterial load and histological findings (abstract). Gut. 1999;45 Suppl 5. |
| 19. | Logan RP, Dill S, Bauer FE, Walker MM, Hirschl AM, Gummett PA, Good D, Mossi S. The European 13C-urea breath test for the detection of Helicobacter pylori. Eur J Gastroenterol Hepatol. 1991;3:915-921. |
| 20. | Logan RP, Polson RJ, Misiewicz JJ, Rao G, Karim NQ, Newell D, Johnson P, Wadsworth J, Walker MM, Baron JH. Simplified single sample 13Carbon urea breath test for Helicobacter pylori: comparison with histology, culture, and ELISA serology. Gut. 1991;32:1461-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 149] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Auroux J, Lamarque D, Tankovic J, Benamouzig R, Mahé S, Chaumette MT, Delchier JC. [Comparison of quantifying Helicobacter pylori gastric infection by culture, histology and C13 urea breath test]. Gastroenterol Clin Biol. 1998;22:407-412. [PubMed] |
| 22. | Kobayashi D, Eishi Y, Ohkusa T, Ishige T, Minami J, Yamada T, Takizawa T, Koike M. Gastric mucosal density of Helicobacter pylori estimated by real-time PCR compared with results of urea breath test and histological grading. J Med Microbiol. 2002;51:305-311. [PubMed] |
| 23. | de Martel C, Parsonnet J. Helicobacter pylori infection and gender: a meta-analysis of population-based prevalence surveys. Dig Dis Sci. 2006;51:2292-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Moayyedi P, Feltbower R, Crocombe W, Mason S, Atha P, Brown J, Dowell AC, Richards ID, Axon AT. The effectiveness of omeprazole, clarithromycin and tinidazole in eradicating Helicobacter pylori in a community screen and treat programme. Leeds Help Study Group. Aliment Pharmacol Ther. 2000;14:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Prewett EJ, Smith JT, Nwokolo CU, Sawyerr AM, Pounder RE. Twenty-four hour intragastric acidity and plasma gastrin concentration profiles in female and male subjects. Clin Sci (Lond). 1991;80:619-624. [PubMed] |
| 26. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2738] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 27. | Kikuchi S, Wada O, Nakajima T, Nishi T, Kobayashi O, Konishi T, Inaba Y. Serum anti-Helicobacter pylori antibody and gastric carcinoma among young adults. Research Group on Prevention of Gastric Carcinoma among Young Adults. Cancer. 1995;75:2789-2793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Hansen S, Melby KK, Aase S, Jellum E, Vollset SE. Helicobacter pylori infection and risk of cardia cancer and non-cardia gastric cancer. A nested case-control study. Scand J Gastroenterol. 1999;34:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Janulaityte-Günther D, Kupcinskas L, Pavilonis A, Valuckas K, Percival Andersen L, Wadström T. Helicobacter pylori antibodies and gastric cancer: a gender-related difference. FEMS Immunol Med Microbiol. 2005;44:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Ohtani M, García A, Rogers AB, Ge Z, Taylor NS, Xu S, Watanabe K, Marini RP, Whary MT, Wang TC. Protective role of 17 beta -estradiol against the development of Helicobacter pylori-induced gastric cancer in INS-GAS mice. Carcinogenesis. 2007;28:2597-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Crabtree JE, Court M, Aboshkiwa MA, Jeremy AH, Dixon MF, Robinson PA. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori infection in Mongolian gerbils. J Pathol. 2004;202:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Welén K, Faresjö A, Faresjö T. Functional dyspepsia affects women more than men in daily life: a case-control study in primary care. Gend Med. 2008;5:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Flier SN, Rose S. Is functional dyspepsia of particular concern in women? A review of gender differences in epidemiology, pathophysiologic mechanisms, clinical presentation, and management. Am J Gastroenterol. 2006;101:S644-S653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Ahlawat SK, Cuddihy MT, Locke GR. Gender-related differences in dyspepsia: a qualitative systematic review. Gend Med. 2006;3:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |