Published online Dec 15, 2011. doi: 10.4291/wjgp.v2.i6.138
Revised: November 18, 2011
Accepted: December 3, 2011
Published online: December 15, 2011
AIM: To determine tolerance to fiber supplementation of semi-elemental tube feeds in critically ill patients and measure its effect on colonic microbiota and fermentation.
METHODS: Thirteen intensive care unit patients receiving jejunal feeding with a semi-elemental diet for predominantly necrotizing pancreatitis were studied. The study was divided into 2 parts: first, short-term (3-9 d) clinical tolerance and colonic fermentation as assessed by fecal short chain fatty acid (SCFA) concentrations and breath hydrogen and methane was measured in response to progressive fiber supplementation increasing from 4 g tid up to normal requirement levels of 8 g tid; second, 4 patients with diarrhea were studied for 2-5 wk with maximal supplementation to additionally assess its influence on fecal microbiota quantitated by quantitative polymerase chain reaction (qPCR) of microbial 16S rRNA genes and Human Intestinal Tract Chip (HITChip) microarray analysis. Nearly all patients were receiving antibiotics (10/13) and acid suppressants (11/13) at some stage during the studies.
RESULTS: In group 1, tolerance to progressive fiber supplementation was good with breath hydrogen and methane evidence (P = 0.008 and P < 0.0001, respectively) of increased fermentation with no exacerbation of abdominal symptoms and resolution of diarrhea in 2 of 4 patients. In group 2 before supplementation, fecal microbiota mass and their metabolites, SCFA, were dramatically lower in patients compared to healthy volunteers. From qPCR and HITChip analyses we calculated that there was a 97% reduction in the predominant potential butyrate producers and starch degraders. Following 2-5 wk of fiber supplementation there was a significant increase in fecal SCFA (acetate 28.4 ± 4.1 μmol/g to 42.5 ± 3.1 μmol/g dry weight, P = 0.01; propionate 1.6 ± 0.5 vs 6.22 ± 1.1, P = 0.006 and butyrate 2.5 ± 0.6 vs 5.9 ± 1.1, P = 0.04) and microbial counts of specific butyrate producers, with resolution of diarrhea in 3 of 4 patients.
CONCLUSION: Conventional management of critically ill patients, which includes the use of elemental diets and broad-spectrum antibiotics, was associated with gross suppression of the colonic microbiota and their production of essential colonic fuels, i.e., SCFA. Our investigations show that fiber supplementation of the feeds has the potential to improve microbiota mass and function, thereby reducing the risks of diarrhea due to dysbiosis.
- Citation: O’Keefe SJ, Ou J, DeLany JP, Curry S, Zoetendal E, Gaskins HR, Gunn S. Effect of fiber supplementation on the microbiota in critically ill patients. World J Gastrointest Pathophysiol 2011; 2(6): 138-145
- URL: https://www.wjgnet.com/2150-5330/full/v2/i6/138.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v2.i6.138
Diarrhea is a frequent complication in critically ill patients and often results in interruption of feeding and exacerbation of malnutrition when it is associated with tube feeding. For example, we recently reported our experience with acute pancreatitis where 43% of tube-fed patients developed persistent diarrhea[1,2]. Whilst diarrhea in tube-fed patients was previously attributed to feed-intolerance and malabsorption by the small intestine, it has become increasingly recognized that the major cause is antibiotic disturbance of the microbiota, termed “antibiotic-associated diarrhea” or “dysbiosis”[3-5]. This disturbance may precipitate diarrhea by at least two mechanisms: by suppressing fermentation and reducing the production of the primary energy source and epithelial regulator of the colonic mucosa - the short chain fatty acid (SCFA), butyrate, and by permitting the overgrowth of pathogens. Perhaps the best recognized example of the second mechanism is Clostridium difficile (C. difficile) infection. In our clinical study mentioned above, 50% of the cases of diarrhea were associated with C. difficile infections[2]. The emergence of C. difficile as a notorious “superbug” responsible for epidemics of hospital-acquired infections worldwide has attracted major media concern (e.g., “Stomach Bug Crystallizes an Antibiotic Threat”, New York Times, April 13, 2009). There is good evidence that C. difficile infection is a consequence of dysbiosis as it thrives in a “permissive” environment[4,5] devoid of butyrate[6], indeed its presence may be a biomarker of the severity of the dysbiosis.
We recently hypothesized that current intensive care unit (ICU) management which invariably includes broad-spectrum antibiotic therapy, proton pump inhibitors (PPI) and elemental tube feeds, forms an ideal environment for the proliferation of C. difficile infection[7]. We are concerned that research into enteral nutrition has focused on the needs of the upper gastrointestinal (GI) tract with the development of specialized feeds that enhance enterocyte function but starve the colon as they are fully absorbed in the small intestine (i.e. non-residual, elemental). “Topical” nutrition is essential for health in not only the small intestine, but also the large, where undigested complex carbohydrates support microbiota health and balance, which in turn produce SCFAs and butyrate, which maintain mucosal function and health. As there is good evidence that fiber supplementation of tube feeds can reduce diarrhea in critically ill septic patients in the ICU receiving antibiotics[8] and in patients receiving enteral nutrition for severe acute pancreatitis[9,10], we conducted the following study to (1) test tolerance to progressive fiber supplementation; and (2) examine the effect this had on the microbiota and their production of SCFAs in critically ill patients, predominantly with acute pancreatitis, needing enteral feeding.
The study was divided into 2 parts. In the first group of critically ill patients (group 1), short-term (3-9 d) clinical tolerance and colonic fermentation responses to progressive fiber supplementation of their elemental tube feeds was measured. In the second part, a smaller number of “high-risk” severely ill ventilated patients, all dependent on jejunal feeding and all with diarrhea (group 2), were followed for a longer period of time (2-5 wk) to assess not only tolerance but also the associated changes in microbiota composition. Results were evaluated by comparison to age and sex-matched healthy volunteers consuming a normal diet.
Patient selection: Adult critically ill ICU patients referred to our nutrition support service with impaired gastric emptying for jejunal feeding with semi-elemental diets were selected. Patients were only included if it was estimated that they would need long term feeding, i.e. more than 2 wk. As reflected by our practice, most of the patients needed jejunal feeding for acute pancreatitis complicated by cystic swelling or necrosis, causing gastro-duodenal compression. Details of our nutritional management of this group of patients with a double-lumen gastric decompression and jejunal feeding tube has recently been published[11-13].
Controls: To evaluate the fermentation responses to fiber and fecal microbiota composition, 5 age-matched healthy subjects, body mass index range 23-27 kg/m2, served as controls. Breath hydrogen responses to drinks containing 10 g of soluble fiber were measured hourly for 6 h following the drink. Fresh morning stool samples were obtained from the same individuals consuming normal food for characterization of the microbiota composition and activity. For fecal SCFA concentrations, we used our recently published data from twenty-three 50-65-year-old healthy male and female Americans also consuming a normal diet[14].
Enteral feeding: Jejunal feeding was commenced and managed as previously described[11]. Transnasal endoscopy (Olympus 5.8 mm diameter upper GI endoscope) was used to access the jejunum to place a guide wire down the jejunum, permitting tube placement after withdrawal of the endoscope[12]. A double-lumen nasogastric decompression-jejunal feeding tube was used in all cases (Kangaroo-Dobbhoff tube system, Sherwood Medical Co., St Louis, MI; 16 Fr outer gastric tube, 9 Fr internal jejunal feeding tube). Feeding was commenced using a semi-elemental formula diet (Peptamen AF, Nestle Nutrition, NJ, United States, which contains 4 g soluble fiber (as oligofructose and inulin) per liter at 25 mL/h for 24 h, then increased to 50 mL/h for day 2, and then to goal calculated as the volume needed to deliver 1.5 g protein/kg ideal body weight/day. It is important to note that the recommended intake of fiber for Americans is 25 g/d for women and 38 g/d for men[15].
Fiber supplementation: In both groups, fiber supplementation was only commenced after goal enteral nutrition infusion rates had been achieved. In group 1, baseline breath responses to conventional tube feeding were measured at hourly intervals during the day (roughly 9 am to 5 pm) before fiber supplementation. On the following day, the response to bolus injection of 10 g of the fiber supplement (Benefiber, Novartis, United States, a wheat dextrin, dissolved in 25 mL water) down the jejunal tube was measured at hourly intervals for 6 h. Progressive fiber supplementation of the tube feeds was then commenced at 4 g tid, increasing after successive 3-d periods with tolerance to 8 g tid and up to a maximum of 12 g tid if diarrhea continued. Tolerance was assessed clinically with particular attention to abdominal distress, namely nausea, vomiting, diarrhea, abdominal distention, gas and pain. Ventilated patients were assessed by abdominal examination for pain and gaseous distention and diarrhea. Diarrhea was defined by our own practice protocol as the passage of more than 3 liquid stools > 500 mL/d; severe diarrhea was defined as the passage of > 1000 mL/d. Fermentation was monitored by daily measurement of breath hydrogen and methane at hourly intervals between 9 am and 5 pm. Fermentation was also assessed by measurement of fecal SCFA concentrations before and on the last day of fiber supplementation. In group 2, progressive fiber supplementation was given in a similar way but for a longer duration (2-5 wk) and as a more physiological continuous infusion piggy-backed into the jejunal feeding tube.
Most fecal samples, and in particular those collected from ventilated patients, were obtained from rectal tubes. Samples were contained in 10 g airtight sterile containers and frozen at -80°C immediately after defecation. End expiratory breath was sampled and analyzed as previously[16].
The microbiota was assessed in all patients in group 2 before and after fiber supplementation by estimating counts of total and specific bacteria by real time quantitative polymerase chain reaction (qPCR) of the 16S ribosomal RNA gene using Bacteria domain primers (broad primers Uni331F and Uin797R)[17] and Bifidobacteria (primers Bif164F, Bif662R) (Sigma Aldrich, CA). All PCR experiments were done in triplicate with a reaction volume of 10 μL using MicroAmp optical 384-well reaction plates. Following amplification, a dissociation step was included to analyze the melting profile of the amplified products. Ten-fold dilution series of the plasmid standard for the respective bacterial group or species was run along with the samples. Quantification of unknowns was made by using standard curves obtained from the amplification profile of known concentrations of the plasmid DNA containing the respective amplicon for each set of primers. Data were analyzed with SDS v2.3 software supplied by Applied Biosystems.
In 2 patients and 2 of the controls (1 male, 1 female), full analysis of microbial composition was determined by phylogenetic microarray using the Human Intestinal Tract Chip (HITChip) at Wageningen University in The Netherlands. This provides information on the proportional composition on over 1100 intestinal bacterial phylotypes[18]. Proportions were converted to counts by measuring counts of total bacteria in fecal samples as above. Confirmation of these calculations was made by also measuring counts of specific bacterial taxa by qPCR. SCFA were measured in duplicate samples as previously described[14] by gas chromatography (Agilent Technologies 6890N Network GC System with flame-ionization detection).
Baseline values from patients were evaluated employing computer software (SPSS 17 for Windows) by comparison to healthy controls by Student’s unpaired t test if normally distributed and by Mann-Whitney U test if not. Changes after supplementation were evaluated by Students paired t test or Wilcoxon Rank Sum test as appropriate.
Thirteen patients were enrolled, 9 in group 1 and 4 in group 2. Demographic details are summarized in Table 1. The majority needed jejunal feeding for the management of complicated severe necrotizing pancreatitis and organ failure (all with Apache II scores > 20) where gastric feeding was impossible because of gastro-duodenal compression by the inflammatory mass, and distal elemental diet feeding was appropriate to maintain gut function without pancreatic stimulation[19,20]. One patient had multiple traumas due to a motor vehicle accident and another was started on tube feeding for C. difficile colitis and gastroparesis. All but one were commenced on fiber supplementation within the first week of commencement of feeding. All but 3 patients were receiving prophylactic broad-spectrum antibiotics during the fermentation measurements. All but 2 were also receiving acid suppressants, 9 on PPI and 2 on H-2 antagonists. Six made an otherwise uneventful recovery, were weaned back onto a normal diet and discharged home, but 7 continued to need tube feeding due to continued gastric outlet compression, and were transferred once in a stable condition to a rehabilitation unit or specialized nursing facility.
| Patient | Diagnosis | Age (yr) | Sex | BMI (kg/m2) | Duration of fiber suppl (d) | Maximum fiber (g/d) | Medications, gastrointestinal symptoms before feeding, outcome |
| Group 1: bolus injections: short term | |||||||
| 1 | Trauma | 59 | M | 34.7 | 3 | 15 | Metronidazole, lanzoprazole, diarrhea, d/c to snf |
| 2 | SAP | 65 | M | 38.0 | 8 | 29 | Cefepime, lanzoprazole, diarrhea, discharge home |
| 3 | SAP | 85 | F | 31.0 | 3 | 24 | Omeprazole, diarrhea, discharge home |
| 4 | SAP | 89 | F | 29.4 | 6 | 32 | Fluconazole, lanzoprazole, transfer rehab |
| 5 | SAP | 43 | M | 34.7 | 9 | 12 | Fluconazole, vancomycin, pantoprazole, discharge home |
| 6 | SAP | 34 | M | 44.8 | 3 | 18 | Ertapenem, omeprazole, diarrhea, d/c to snf |
| 7 | SAP | 81 | F | 35.5 | 3 | 15 | Omeprazole, discharge home |
| 8 | SAP | 62 | M | 27.0 | 7 | 22 | Famotidine, metronidazolel, discharge home |
| 9 | SAP | 56 | M | 28.7 | 6 | 24 | Trimethoprim-sulfamethoxazole, voriconazole, pantoprazole, discharge home |
| Group 2: continuous infusions: long term | |||||||
| 10 | SAP | 88 | F | 30.3 | 19 | 35 | Metronidazole, aztreonam, famotidine, diarrhea transfer to snf |
| 11 | SAP | 65 | F | 27.6 | 36 | 36 | Piperacillin-tazobactam, doripenem, metronidazole, pantoprazole, cefuroxime, diarrhea, distension, pain. d/c to snf |
| 12 | SAP | 47 | F | 31.0 | 33 | 18 | Piperacillin-tazobactam, pantoprazole, fluconazole, metronidazole, vancomycin, diarrhea, distention, d/c to snf |
| 13 | Chronic sepsis C diff | 79 | F | 34.9 | 23 | 24 | Metronidazole, vancomycin, lanzoprazole, diarrhea, distension, pain. Clostridium difficile. d/c to snf |
Tolerance: Tolerance to bolus fiber supplementation overall was good, with a median supplementation rate of 22 g/d, range 12-32 g/d. For the 5 who had no diarrhea before commencement of supplementation, supplementation rates of 15-32 g/d were tolerated without the induction of GI symptoms. In the 4 patients who had diarrhea at commencement of fiber supplementation, 2 improved with supplementation of 18 and 24 g/d and 2 remained unchanged with supplementation of 15 and 29 g/d. Gas was not a complaint and increasing abdominal distension was not detected clinically.
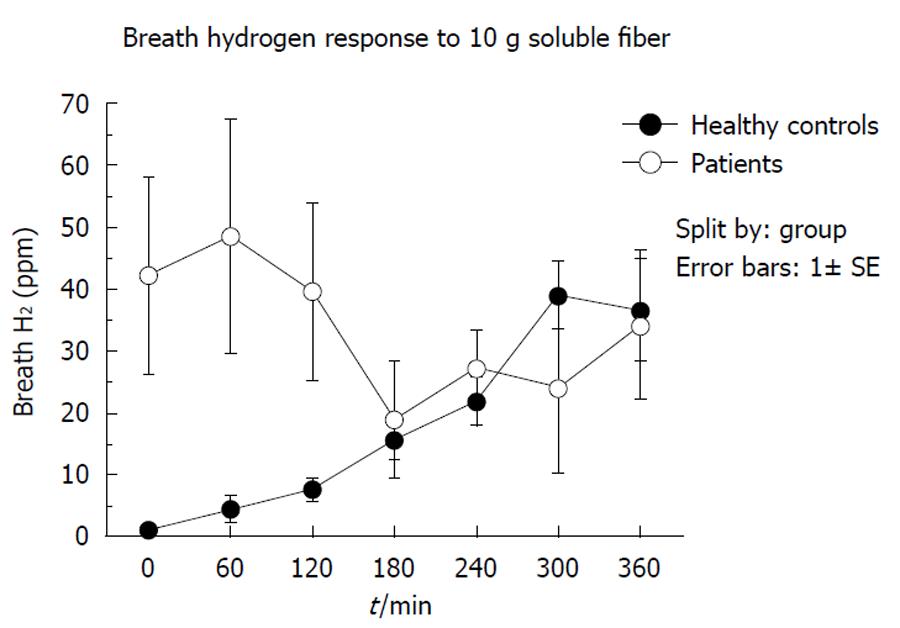
Fermentation: Figure 1 shows a comparison between the normal breath hydrogen responses to consumption of 10 g soluble fiber in the 5 healthy volunteers and the 9 patients in group 1 at the commencement of bolus supplementation. The normal increase in breath hydrogen over 6 h was not seen, chiefly because initial hydrogen concentrations were very high in 2 subjects (186 and 113 ppm), suggesting premature fermentation of tube feeds and soluble fiber by bacterial overgrowth of the small intestine[21].
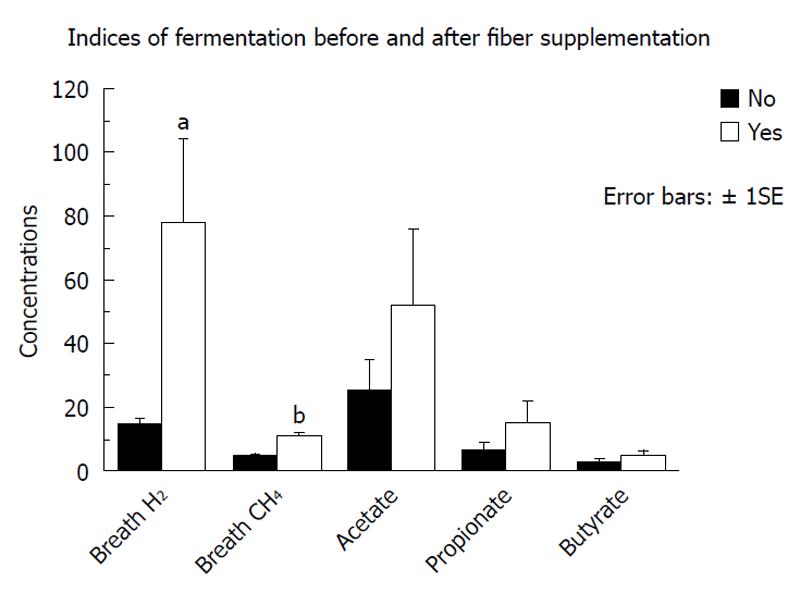
Figure 2 summarizes the fecal SCFA results of the responses to maximal fiber supplementation in group 1. Although the group mean fecal SCFA concentrations increased after supplementation, the individual changes were variable and therefore not statistically significant. Both breath hydrogen and breath methane increased significantly (P = 0.008 and P < 0.0001 respectively).
Effects of 2-5 wk fiber supplementation: All 4 of these patients had diarrhea at commencement of supplementation. In 3, the diarrhea improved with progressive supplementation to 18, 24, and 35 g/d. In the fourth patient (No. 11), with severe acute necrotizing pancreatitis, the diarrhea continued despite progressive supplementation to 36 g/d. This patient differed from the others as she continued to need broad-spectrum IV antibiotics (cefuroxime) and PPI (pantoprazole) for suspected sepsis and high gastric juice drainage in contrast to 2 of the other patients (No. 10 and No. 13) who were weaned off antibiotics and PPI before the end of the study. The third patient (No. 12), whose diarrhea settled on 18 g/d fiber supplementation, was still on IV vancomycin after diarrhea resolution.
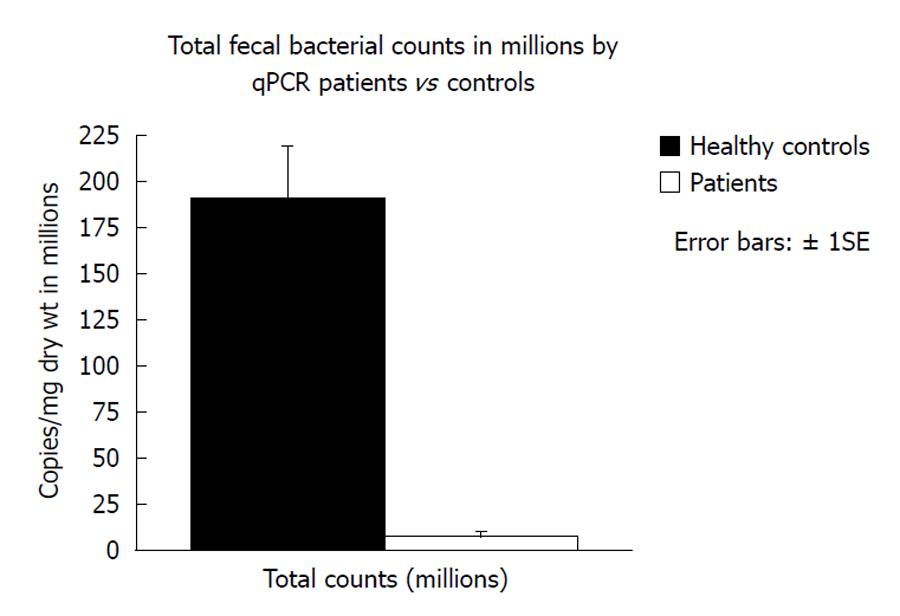
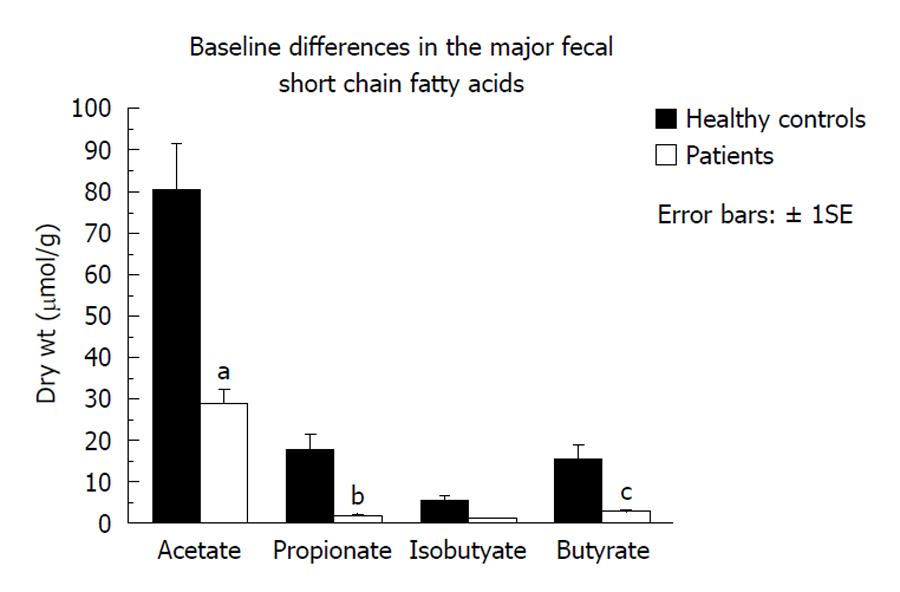
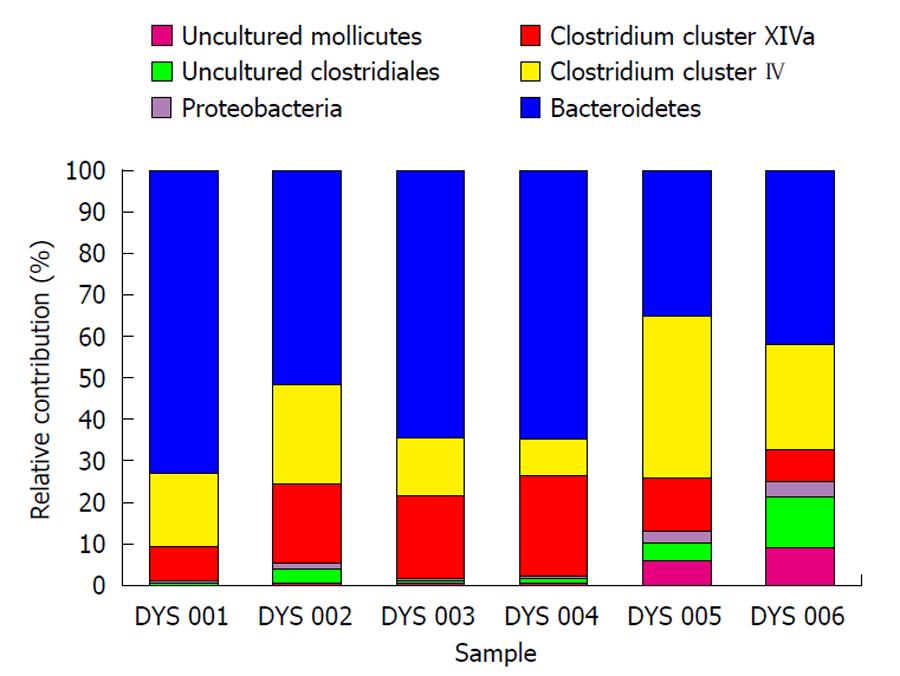
Fecal microbiota and SCFAs: Figure 3 shows that total fecal bacterial counts estimated by qPCR were orders of magnitude lower in the 4 patients compared to healthy controls. Commensurate with this, fecal SCFA were also significantly lower in patients (Figure 4). Composition analysis by HITChip in 2 of the 4 patients (No. 10 and No. 11) and 2 healthy controls showed that the composition of the remaining microbiota was also different (Figure 5). The difference in Bacteroidetes composition was striking, with this phylum making up 35% of the microbiota in healthy subjects and 60% in patients. Conversely, there was a reduction of the proportion of Firmicutes, which contain the major butyrate-producers, in patients (50%) compared to controls (30%). Converting the proportions of phyla to numbers by combining the qPCR and HITChip analyses, we calculate that there was a 97% reduction in the predominant potential butyrate producers and starch degraders, at the genus level, from Clostridia clusters XIVa [Eubacterium rectale (E. rectale), Roseburia intestinalis (R. intestinalis)] and IV [Faecalibacterium prausnitzii (F. prausnitzii), Ruminococcus bromii (R. bromii) and Ruminococcus obeum (R. obeum)] before fiber supplementation. Taken together with the lower fecal SCFA concentrations, this demonstrates a general suppression of colonic fermentation.
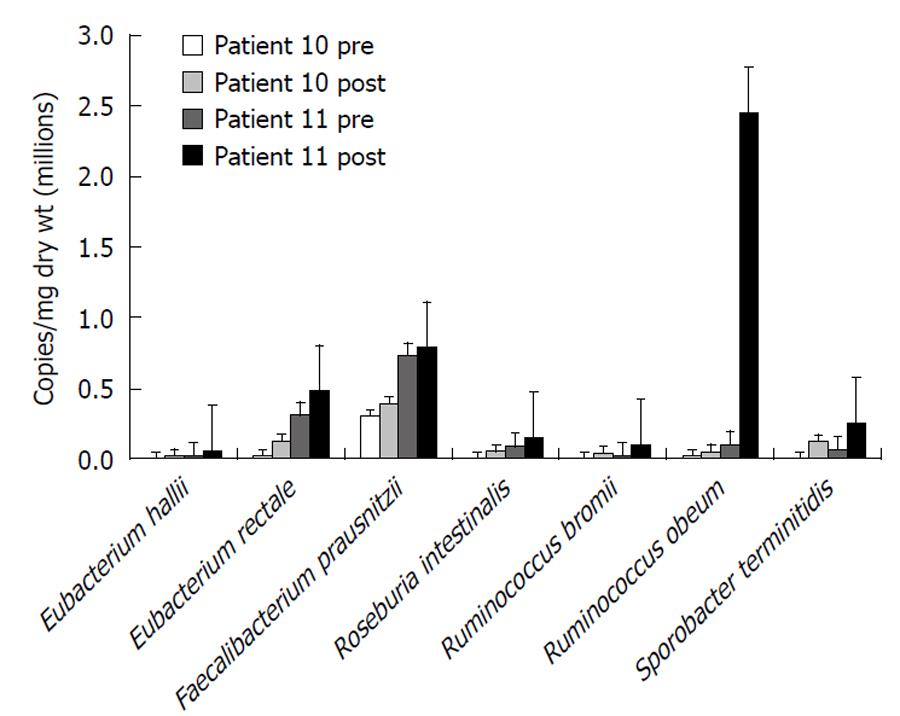
Following 2-5 wk fiber supplementation in group 2, there was a 6-fold increase in Firmicutes accompanied by a significant increase in fecal SCFA (acetate 28.4 ± 4.1 μmol/g to 42.5 ±3.1 μmol/g dry weight, P = 0.01; propionate 1.6 ± 0.5 vs 6.22 ± 1.1, P = 0.006; and butyrate 2.5 ± 0.6 vs 5.9±1.1, P = 0.04), indicating an increase in carbohydrate fermentation. The responses of a select number of bacteria known to maintain colonic mucosal health are illustrated in Figure 6. In general, all of these bacteria increased in numbers after supplementation, more so in patient 11 than in patient 10 - which might be explained by the fact that patient 10 was still receiving antibiotics during the repeat measurement during fiber supplementation. Specifically, microbial counts increased with fiber supplementation in the major potential butyrate producers, E. rectale, E. hallii and R. intestinalis, all members of Clostridia cluster XIVa. There were similar increases in R. bromii, R. obeum and in Sporobacter terminitidis, organisms that degrade starch and other complex carbohydrates. F. prausnitzii counts also increased in both patients. This bacterium is not only involved in starch degradation but may also have a specific role in preventing colitis[22]. The “probiotic” genus, Bifidobacterium spp. also increased towards normal (1.5 × 105 to 5.9 × 105, control 8.4 × 105 by qPCR).
The present data demonstrate that, in the patient population studied, progressive supplementation of elemental formula diets with up to 35 g soluble fiber per day fed into the jejunum was well tolerated, with no increase in abdominal symptoms and resolution of diarrhea in 5 of 8 patients. Secondly, detailed investigation in a few of these subjects fed for 3-5 wk revealed that initial fecal bacterial counts were dramatically reduced compared to healthy volunteers and that fiber supplementation increased the proportions and counts of recognized butyrate-producers as well as fecal butyrate concentrations. However, it was important to note that levels remained lower than normal, possibly because of the effects of concomitant antibiotic therapy. We acknowledge that our microbiota findings must be considered provisional, as the few patients we studied - because of cost of analysis - may not be representative of all ICU patients. Nonetheless, it is very likely that the widespread use of antibiotics and fiber-deficient diets have a devastating effect on microbiota composition and function placing patients at high risk of diarrheal disease.
Most of the patients studied were dependent on jejunal tube feeding because of complications of severe acute pancreatitis as this population reflects the bulk of our nutritional support practice commonly needing prolonged ICU support and tube feeding[1,2]. The results demonstrate that nearly all were managed with broad-spectrum antibiotics and acid suppressants, all received low residue semi-elemental tube feeds and most (8/11) had diarrhea when first studied. In a systematic review of the literature, Petrov and Whelan identified only 5 suitable randomized controlled trials (RCT) comparing the frequency of diarrhea in enteral and parenterally fed patients with acute pancreatitis[9]. Diarrhea was significantly higher in enterally fed patients: 29% vs 7%; but this difference is to be expected if the gut is not being used. There is only one RCT evaluating the value of fiber supplementation of the enteral feeds, that of Karakan et al[10] who randomized 30 patients with severe acute pancreatitis (mean Apache II score of 9.4-9.6) to supplementation with 24 g/d of soluble (0.7 g/100 mL) and insoluble fiber (0.8 g/100 mL). Remarkably, supplementation was associated with a significant reduction in hospital stay, duration of nutrition therapy, acute phase response and overall complications.
The maintenance of microbiotal balance is pivotal for colonic health as they provide essential nutrients for the colonic mucosa[23]. Ever since Roediger[24] first identified butyrate as the preferred energy substrate for colonocytes and Harig et al[25] showed that depletion of colonic SCFA lead to the development of acute colitis (“diversion colitis”), there has been intense, but chiefly experimental, research into the importance of SCFA synthesis in the maintenance of colonic health. We have recently reviewed some of the more recent evidence, which indicates that sufficient colonic butyrate is essential for the maintenance of cellular homeostasis and a normal colonocyte phenotype; that SCFA have anti-inflammatory effects, either directly by regulating the release of prostaglandin E2, cytokines and chemokines from human immune cells, or indirectly by their ability to support the growth of probiotic species, such as Bifidobacteria and Lactobacilli; that SCFA also possess immune-modulating and anti-inflammatory actions by binding certain G-coupled receptors, which may stimulate the normal resolution of inflammatory responses in colonocytes; and finally, that SCFA production enhances colonic blood flow as well as fluid and electrolyte uptake[26].
However, butyrate production is not the only essential function of the microbiota. Water-soluble vitamins, such as folate, biotin, B-12, are also synthesized and utilized by the mucosa[22] and different microbial species support each other with cross-feeding, thus maintaining a disciplined society preventing overgrowth with pathogens such as C. difficile. For example, starch degraders such as R. bromii and Bifidobacterium adolescentis cross-feed to produce acetate, which is the major energy source for the major butyrate-producers E. rectale, Roseburia spp. and F. prausnitzii[27]. Bifidobacteria also process starch and soluble fiber into lactate, which is released into the lumen and fuels other butyrate-producers such as E. hallii[27]. Furthermore, the lactate reduces the luminal pH, favoring the growth of butyrate-producers and suppressing pathogens. The real-life situation is likely far more complex than we currently recognize, bearing in mind that the microbial population outnumbers our own cells by 10:1 and that their genetic library outnumbers ours by 100:1[28]. For example, specific bacteria may secrete specific anti-inflammatory substances as illustrated by Sokol et al[22], with the observation that F. prausnitzii exhibits anti-inflammatory effects on cellular and TNBS colitis models, partly due to secreted metabolites able to block nuclear factor κB activation and interleukin-8 production.
Although we did not directly measure microbial activity in the small intestine, we performed soluble fiber-breath tests, which provide indirect information on the distribution of fermenters in the small and large intestine. The high baseline and early increase in breath hydrogen levels after injection of soluble fiber into the jejunum shown in Figure 1, is characteristic of small bowel bacterial overgrowth, as the tube feed and fiber is fermented before it can be absorbed in the small intestine or fermented in the colon. The most likely explanation for bacterial overgrowth of the small intestine is the virtual routine use of PPI in critically ill patients. Earlier studies of ours, in 20 patients with peptic ulcer disease before and after PPI therapy (omeprazole 20 mg/d), showed that duodenal bacterial counts increased in all patients following treatment, geometric mean counts increasing from 330 CFU/mL to 95 000 CFU/mL, and that this was accompanied by an increase in intestinal transit and diarrhea[21].
Our investigations raise serious concern that the normal microbiota balance within us is grossly disturbed in critically ill patients managed with semi-elemental tube feeds, acid suppressants and antibiotics. Not only are the microbiota killed or suppressed by broad-spectrum antibiotics, but the remaining colonies are starved by the use of non-residue diets. This combination inevitably leads to dysbiosis and increased risk of colitis and diarrhea. Further studies are needed to separate out the relative risks due to antibiotics and colonic starvation and to determine whether the incremental improvement in microbial growth and function with fiber supplementation will be sufficient to counteract the effects of antibiotic therapy. At the same time, the use of unproven antibiotic (as in patients with acute pancreatitis[29]) and PPI prophylaxis[30-32] should be withheld, or modified, so that it spares colonic health-promoting bacteria, as in the case of the new anti-C. difficile drug, fidaxomicin[33].
We thank Hans Heilig (Zoetendal lab, Wageningen) and Mei Wang, PhD (Gaskins lab, UIUC) for technical assistance with the HITChip and qPCR analysis of the fecal samples, respectively.
Diarrhea is a common problem in critically ill patients dependent on tube feeding for prolonged periods. There is concern that the diarrhea results from the combined use of elemental formulae diets which deprive the colonic microbiota of their nutrition, which in turn reduce the production of short chain fatty acids (SCFAs) which maintain the health of the colonic mucosa, and the regular use of broad spectrum antibiotics which further suppress microbial fermentation.
Preservation of colonic microbiota composition and function is likely to reduce the morbidity associated with conventional intensive care unit (ICU) management.
The studies have demonstrated that progressive supplementation of jejunal tube-feeds with soluble fiber is well tolerated by critically ill patients, increasing colonic fermentation and reducing diarrhea. High throughput technology was employed to investigate the nature of the underlying dysbiosis. First, a phylogenetic microarray using the Human Intestinal Tract Chip provided information on the proportional composition on over 1100 intestinal bacterial phylotypes. Second, proportions within the microbiota composition were converted to counts by measurement of the total bacterial counts in fecal samples by quantitative polymerase chain reaction analysis.
The results indicate that patients dependent on semi-elemental diets for any length of time should be given regular fiber supplementation to maintain colonic health and function.
Fiber is a complex carbohydrate that is indigestible by small intestinal enzymes but fermentable by colonic microbes to form SCFAs, which are the primary energy source and epithelial regulators for the colonic mucosa.
This is a unique study examining the interactions between the conventional ICU management, including semi-elemental diets and antibiotics, and the composition and function of the colonic microbiota.
Peer reviewer: Maxim S Petrov, Dr., Department of Surgery, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand
S- Editor Wu X L- Editor Roemmele A E- Editor Zheng XM
| 1. | Rolniak S, Raina A, Hegazi R, Centa-Wagner PK, Kandil HM, Hughes SJ, Lee KK, James A, Moser J, Graham TO. Simultaneous nasogastric decompression with mid-jejunal feeding avoids total parenteral nutrition (TPN) and early surgery in the management of complicated acute pancreatitis and gastric outlet obstruction. Gastroenterology. 2009;136:A-76. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Rolniak S, Centa-Wagner P, Kandil H, Graham T, O’Keefe S. A year’s experience with mid-jejunal enteral feeding in patients with acute pancreatitis and gastric outlet obstruction. JPEN J Parenter Enteral Nutr. 2010;34:182A. |
| 3. | Young VB, Schmidt TM. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol. 2004;42:1203-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | De La Cochetière MF, Durand T, Lalande V, Petit JC, Potel G, Beaugerie L. Effect of antibiotic therapy on human fecal microbiota and the relation to the development of Clostridium difficile. Microb Ecol. 2008;56:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, Young VB. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 793] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 6. | May T, Mackie RI, Fahey GC, Cremin JC, Garleb KA. Effect of fiber source on short-chain fatty acid production and on the growth and toxin production by Clostridium difficile. Scand J Gastroenterol. 1994;29:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | O'Keefe SJ. Tube feeding, the microbiota, and Clostridium difficile infection. World J Gastroenterol. 2010;16:139-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Spapen H, Diltoer M, Van Malderen C, Opdenacker G, Suys E, Huyghens L. Soluble fiber reduces the incidence of diarrhea in septic patients receiving total enteral nutrition: a prospective, double-blind, randomized, and controlled trial. Clin Nutr. 2001;20:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Petrov MS, Whelan K. Comparison of complications attributable to enteral and parenteral nutrition in predicted severe acute pancreatitis: a systematic review and meta-analysis. Br J Nutr. 2010;103:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Karakan T, Ergun M, Dogan I, Cindoruk M, Unal S. Comparison of early enteral nutrition in severe acute pancreatitis with prebiotic fiber supplementation versus standard enteral solution: a prospective randomized double-blind study. World J Gastroenterol. 2007;13:2733-2737. [PubMed] |
| 11. | O'Keefe SJ. A guide to enteral access procedures and enteral nutrition. Nat Rev Gastroenterol Hepatol. 2009;6:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | O'Keefe SJ, Foody W, Gill S. Transnasal endoscopic placement of feeding tubes in the intensive care unit. JPEN J Parenter Enteral Nutr. 2003;27:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | O’Keefe SJD, Rolniak S, Raina A, Graham T, Hegazi R, Center-Wagner P. Enteral feeding patients with gastric outlet obstruction. Nutr Clin Pract. 2011;In press. |
| 14. | O'Keefe SJ, Ou J, Aufreiter S, O'Connor D, Sharma S, Sepulveda J, Fukuwatari T, Shibata K, Mawhinney T. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr. 2009;139:2044-2048. [PubMed] |
| 15. | Institute of Medicine, Food, and Nutrition Board. Dietary Reference Intakes. Washington, DC: National Academies Press 2002; . |
| 16. | O'Keefe SJ, Chung D, Mahmoud N, Sepulveda AR, Manafe M, Arch J, Adada H, van der Merwe T. Why do African Americans get more colon cancer than Native Africans? J Nutr. 2007;137:175S-182S. [PubMed] |
| 17. | Yu Z, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques. 2004;36:808-812. [PubMed] |
| 18. | Rajilić-Stojanović M, Heilig HG, Molenaar D, Kajander K, Surakka A, Smidt H, de Vos WM. Development and application of the human intestinal tract chip, a phylogenetic microarray: analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ Microbiol. 2009;11:1736-1751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 347] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 19. | Kaushik N, Pietraszewski M, Holst JJ, O'Keefe SJ. Enteral feeding without pancreatic stimulation. Pancreas. 2005;31:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | O’Keefe SJD. Jejunal feeding is the best approach to early enteral feeding in patients with acute pancreatitis. AGA Perspectives. 2006;2:5-19. |
| 21. | Lewis SJ, Franco S, Young G, O'Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10:557-561. [PubMed] |
| 22. | Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731-16736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2747] [Cited by in RCA: 3199] [Article Influence: 188.2] [Reference Citation Analysis (0)] |
| 23. | O'Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol. 2008;24:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424-429. [PubMed] |
| 25. | Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 513] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 26. | Greer JB, O'Keefe SJ. Microbial induction of immunity, inflammation, and cancer. Front Physiol. 2011;1:168. [PubMed] |
| 27. | Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol. 2007;9:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 419] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 28. | Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 2278] [Article Influence: 119.9] [Reference Citation Analysis (0)] |
| 29. | Jafri NS, Mahid SS, Idstein SR, Hornung CA, Galandiuk S. Antibiotic prophylaxis is not protective in severe acute pancreatitis: a systematic review and meta-analysis. Am J Surg. 2009;197:806-813. [PubMed] |
| 30. | Cook DJ, Reeve BK, Guyatt GH, Heyland DK, Griffith LE, Buckingham L, Tryba M. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. JAMA. 1996;275:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Choudhry MN, Soran H, Ziglam HM. Overuse and inappropriate prescribing of proton pump inhibitors in patients with Clostridium difficile-associated disease. QJM. 2008;101:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Cunningham R, Dial S. Is over-use of proton pump inhibitors fuelling the current epidemic of Clostridium difficile-associated diarrhoea? J Hosp Infect. 2008;70:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Tannock GW, Munro K, Taylor C, Lawley B, Young W, Byrne B, Emery J, Louie T. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology. 2010;156:3354-3359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |