Published online Dec 15, 2011. doi: 10.4291/wjgp.v2.i6.114
Revised: August 12, 2011
Accepted: August 19, 2011
Published online: December 15, 2011
Inflammatory bowel diseases (IBD) are a group of chronic inflammatory disorders most commonly affecting young adults. Currently available therapies can result in induction and maintenance of remission, but are not curative and have sometimes important side effects. Advances in basic research in IBD have provided new therapeutic opportunities to target the inflammatory process involved. Gene and cell therapy approaches are suitable to prevent inflammation in the gastrointestinal tract and show therefore potential in the treatment of IBD. In this review, we present the current progress in the field of both gene and cell therapy and future prospects in the context of IBD. Regarding gene therapy, we focus on viral vectors and their applications in preclinical models. The focus for cell therapy is on regulatory T lymphocytes and mesenchymal stromal cells, their potential for the treatment of IBD and the progress made in both preclinical models and clinical trials.
- Citation: Marel SVD, Majowicz A, Deventer SV, Petry H, Hommes DW, Ferreira V. Gene and cell therapy based treatment strategies for inflammatory bowel diseases. World J Gastrointest Pathophysiol 2011; 2(6): 114-122
- URL: https://www.wjgnet.com/2150-5330/full/v2/i6/114.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v2.i6.114
Inflammatory bowel diseases (IBD) are chronic inflammatory diseases most commonly affecting young adults[1-3]. The exact pathogenesis is unknown, but it is widely accepted that IBD result from an inappropriate response of a defective mucosal immune system to the intestinal flora and other luminal antigens[4-6].
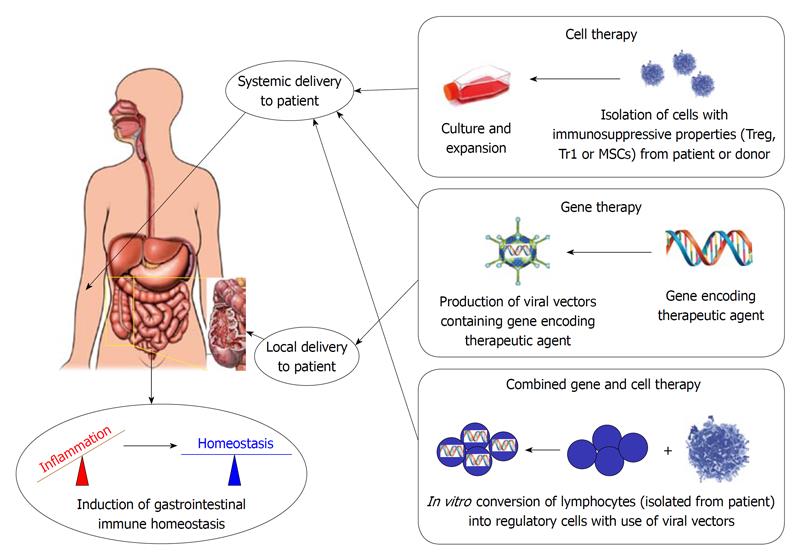
IBD include two major disorders: ulcerative colitis (UC) and Crohn’s disease (CD). These disorders have distinct and overlapping pathologic and clinical characteristics[7]. UC is a relapsing non-transmural inflammatory condition that is limited to the colon[8]. Patients characteristically present with bloody diarrhoea, passage of pus, mucus, or both, and abdominal cramping[8]. CD is a relapsing, transmural inflammatory disease of the gastrointestinal (GI) mucosa that can involve the entire GI tract from the mouth to the anus[8]. Patients characteristically present with discontinuous involvement of various portions of the GI tract and the development of complications including strictures, abscesses, or fistulas[8]. IBD are associated with a considerable reduction in quality of life of the patients[9-11] and currently no curative treatment options are available. Conventional therapeutics cannot prevent complications in IBD and although novel treatment strategies, including TNF-neutralizing antibodies, have greatly increased the therapeutic armamentarium, many patients still have to undergo surgery[12]. For this reason, the development of new treatments is required to prevent initiation of inflammation and, more importantly, allow for long-term remission. Gene and cell therapy approaches are more and more considered in relation to the prevention of inflammation in the GI tract. Gene therapy consists of the insertion or alteration of genes within an individual's cells to treat disease. Cell therapy describes the process of introducing new cells into a tissue in order to treat a disease. Both approaches have been applied successfully in a clinical setting for a broad range of diseases either separately or together, including early stage clinical development for IBD[13-23]. Here we discuss current progress in the field and future treatment prospects in the context of IBD.
To facilitate the uptake and the expression of the transgene in the target cell, a vector is required. Vectors can be non-viral or viral. The choice of a safe and reliable vector that can mediate long-term gene transfer to both dividing and non-dividing cells is of vital importance for a gene therapy approach. Although viral vectors are created from pathogenic viruses, they are modified in such a way as to minimize their pathogenicity. This usually involves the deletion of a part of the viral genome critical for viral replication. Such a virus can efficiently infect cells and has the potential for long term stable gene expression. In the gene therapy section of this review we will focus on viral vectors that have been used successfully in gene therapy applications in recent years[14,15], are able to target the gut[24-32] and can therefore be considered for gene delivery in the GI tract, namely retro-, lenti-, adeno- and adeno associated viral vectors (for an overview see: Tables 1-3 or Figure 1).
| Viral vector | Gut targeting | Reference for gut targeting | Status of development | Reference for status of development | |
| Gene therapy | Retro- and lentiviral | Yes | [24-27] | Not performed | N/A |
| Adenoviral | Yes | [28-30] | Preclinical | [28-30] | |
| AAV | Yes | [31,32] | Not performed | N/A |
| Applied strategy | Viral vector used | Reference for viral vector used | Status of development | Reference for status of development | |
| Combined gene and cell therapy | Ex vivo generated Treg/Tr1 | Retrovirus | [113,115] | Preclinical | [113,115] |
| T cell receptor transgenic Treg | Retrovirus | [116] | Preclinical | [116] |
For an overview of non-viral delivery methods to the intestine we recommend the review from O’Neill et al[33].
Retroviral vectors were used for the first time in a clinical setting over 20 years ago[34-36] and are among the most commonly used vectors in gene therapy. Retroviral particles require disruption of the nuclear membrane to gain access and therefore need cell division for entering the cell[37]. Retroviruses have been demonstrated to be able to transduce intestinal epithelial cells[24-26], although at a low efficiency. Alternatively, intestinal epithelial cells can be transduced efficiently by lentiviruses[27] which are a sub-class of retroviruses. The lentiviruses have an advantage over retroviruses as vectors in gene therapy because of their ability to transduce non-dividing cells[38,39]. Furthermore the lentivirus did not induce mucosal damage or distribute beyond the distal colon[27] and appeared therefore as a potential vector for gene delivery in the treatment of IBD.
However, a safety issue to be considered with both retro- and lentiviral vectors is their potential to integrate at many sites in the human genome[40,41]. Those genomic integrations can result in insertional mutagenesis causing cancer development as has been observed in clinical trials[19,42-44]. Even though significant improvements in lentiviral vector safety have been achieved in recent years[45], the concern for random integration remains and needs to be addressed[46,47] before these vectors can be considered as safe tools for gene therapy applications in IBD.
Despite the fact that adenoviruses are pathogenic viruses and can cause morbidity, especially in immune-compromised patients[48], adenoviral vectors have been frequently used in gene therapy due to their broad tissue tropism and lack of integration into the host genome[49]. Gene therapy using adenoviral vectors has shown potential in the treatment of colitis in preclinical models[28-30]. For example, a single systemic injection of an adenoviral vector carrying the interleukin-10 (IL-10) transgene was sufficient not only to prevent the onset of colitis but also to induce clinical and histological remission in mice with established disease[29]. Additionally Schmiedlin-Ren et al[50] demonstrated that intestinal epithelial cells of IBD patients can be efficiently transduced ex vivo by adenoviral vectors. All together, these results suggest that targeting of the inflamed intestine through the luminal route can be possible using adenoviral vectors[50].
However, hematologic and hepatic toxicities were observed in animal studies after injection with high vector doses[51-53], which imply that further development in generating a new type of adenoviral vector is necessary before considering clinical applications. Recently a gutted adenovirus, devoid of all viral coding sequences, was shown to induce less toxicity[54] after delivery. However, this finding, if promising for future therapeutic applications, needs further exploration.
The non-pathogenic, replication-deficient adeno-associated virus (AAV) holds promise for gene therapy. The AAV vector has a good safety profile as it remains predominantly episomal[55]. In general, 99% of recombinant AAV are maintained as episomal copies[56], indicating a very low risk of insertional mutagenesis compared with retroviral vectors. Furthermore, AAV vectors are able to transduce both dividing and quiescent cells[57,58] and were demonstrated to be effective as gene therapy vectors in several promising preclinical models for autoimmune- and inflammatory disorders[59-66]. The therapeutic potential of the AAV as a vector in gene therapy has also been demonstrated in a clinical setting in recent studies[67-77].
AAV vectors were shown to be able to target the GI tract[31,32] and long term transgene expression post AAV treatment was reported which, in relation with the high turn-over of intestinal cells, suggests that transduction of the slow-dividing intestinal stem cells was achieved[31,32]. However, no data are presently available about the treatment of experimental colitis with AAV vectors.
Cell-based therapies aim to introduce new cells into a tissue in order to treat a disease and can permit the replacement of function[78], or restore the homeostasis of the immune system[79]. In the last 50 years hematopoietic stem cell transplantation has been developed as a curative option for inherited disorders and hematologic or lymphoid cancers[13,80], leading the way toward innovative therapies for other illnesses. Recent results obtained from animal models and early human clinical trials in graft versus host disease but also CD showed that either regulatory T lymphocytes or mesenchymal stromal cells (MSCs) may be of clinical relevance for the treatment of IBD (for an overview see: Tables 1-3 or Figure 1).
The immune system contains a population of T cells, called regulatory T lymphocytes that are specialized in immune suppression[81,82]. Low level autoimmunity may occur in the intestine as a result of the presence of the microbial flora or auto-reactive T cells. Regulatory T lymphocytes are generated in the mesenteric lymph nodes and subsequently migrate and expand in the gut[83], thereby preventing progress to chronic autoimmune disease[84,85]. These cells are able to suppress an immune response both by cell contact [e.g. killing or functional modulation of antigen presenting cells (APCs) or effector T cells] and soluble factor dependent mechanisms (e.g. secretion of immunosuppressive cytokines or deprivation of cytokines necessary for the expansion/survival of responder T cells)[86,87]. Antigen specific regulatory T lymphocytes have been described as having more therapeutic efficacy than polyclonal regulatory T cells[88-90]. In IBD the antigenic targets are not totally defined[6] and cell therapy would have to be restricted to polyclonal cells. However, regulatory T lymphocytes don’t need to be antigen specific in order to suppress immune responses as a result of bystander suppression and infectious tolerance[91,92]. These are general mechanisms through which regulatory T lymphocytes are able to create a regulatory milieu in vivo[91,92] and could introduce tolerance in IBD.
Regulatory T lymphocytes were shown to be effective in both the cure and the prevention of experimental colitis in multiple animal models[93-96]. It was shown, for example, that transfer of regulatory T lymphocytes into mice with colitis led to resolution of the lamina propria infiltrate in the intestine and reappearance of normal intestinal architecture[96]. Therefore regulatory T lymphocytes could be used as a therapeutic tool in IBD where their homeostasis is disturbed[97,98].
Among the different T cells with suppressive activity the CD4+CD25highFOXP3+ regulatory T cell (Treg)[82] and the type 1 regulatory T cell (Tr1)[81] subsets are the most well-defined so far. The Tr1 is typically characterized based on the cytokine production profile (IL-10high)[81] and Treg by the expression of the transcription factor Forkhead box p3 (FOXP3 in humans/Foxp3 in mice), which appears to function as the master regulator in their development and function[99,100].
Treg and Tr1 have the potential to prevent or cure colitis[93-96] and a favourable safety profile was demonstrated in phase I clinical trials[21,101]. Tr1 were shown to have a preliminary efficacy signal in patients in a phase I clinical trial for refractory CD (unpublished data, UEGW 2010-ABS-577). Currently the efficacy of Treg and Tr1 based cell therapy awaits further confirmation from phase II/III clinical trials but overall these results emphasize that both Treg and Tr1 are promising tools for therapeutic applications in IBD.
MSCs are non-haematopoietic stromal cells exhibiting multi-lineage differentiation capacity and the ability to mediate immunosuppressive and anti-inflammatory effects[102-105]. The exact mechanism by which MSCs suppress the immune system is not fully understood. It is known, however, that MSCs have immunosuppressive features in common with regulatory T lymphocytes, as for example preventing the maturation of APCs[102] or physically hindering T cells from contacting APCs[103]. Additionally, it was shown that FOXP3 expression confers a greater immunosuppressive potential to MSCs[106].
MSCs can be isolated from various tissues[107-109] and were shown to ameliorate experimental colitis[110,111]. In humans, MSCs obtained from adipose tissue induced healing in perianal fistulas in patients with CD[17]. Furthermore, in a phase I clinical trial, administration of autologous bone marrow-derived MSCs was shown to be safe and feasible in the treatment of refractory CD[22]. Additionally it was demonstrated that ex vivo expanded autologous bone marrow-derived MSCs are a safe and feasible approach for intrafistular injections in patients with CD[23]. These results[17,22,23] show potential and await further verification in phase II/III clinical trials which are currently being conducted.
The phenotype and function of lymphocytes can be modified using viral vectors, to create tools for a cell therapy approach in the treatment of autoimmune-, and inflammatory disorders[112] and by consequent IBD[113-116]. It was shown that the ex vivo targeting of spleen derived CD4+ T cells by a retroviral vector expressing IL-10 was able to generate Tr1 that prevented colitis in an experimental model of IBD[113].
By the same approach, fully functional Treg were generated by transduction of T cells with a Foxp3 transgene. These cells were able to suppress autoimmunity and graft rejection in vivo[89,115,117,118]. Furthermore, Hori et al[115] showed that the in vitro generated Treg prevented colitis in a mice model of IBD.
Additionally it was demonstrated that Treg can be efficiently transduced to express functional antigen-specific receptors[116]. Adoptive transfer of small numbers of these transduced Treg was associated with antigen-specific, dose-dependent amelioration of experimental colitis in mice[116].
The route of therapeutic delivery is important when considering gene or cell therapy in relation with IBD. The mucus lining in the intestine is a barrier for gene transfer via the luminal route[119] and the clearance of viral particles by the liver represents a problem for the systemic delivery[120]. Nonetheless, as described above it has been shown that transduction via these routes is possible and that long term transgene expression can be achieved. Possible viral vectors, as for example the AAV based viral vectors seem to have the potential to transduce the GI tract, but the optimization of gene targeting to the gut needs to be further explored. This could be achieved by testing different AAV serotypes[121] or modifying the AAV capsid[122]. A promising method is the socalled DNA shuffling method. DNA shuffling is a method whereby genes are rearranged to form hybrid genes with new properties[123]. This can be done using polymerase chain reactions, as described by Cohen[123]. If this approach is used for genes encoding AAV capsid proteins it can allow for the development of cell type specific vectors[124] and thereby shows promise for creating a gut targeting AAV. Furthermore, chemical redirection of the AAV capsid shows potential in engineering vectors with novel tissue tropisms[125]. Chemical engineering refers to a process whereby the amino acids on the surface of the AAV capsid are changed[125]. This method has proved to be successful in redirecting the AAV from liver to skeletal and cardiac muscle following systemic administration in mice[125] and could therefore have potential in directing the AAV to the GI tract.
Due to the presence of stem cells in intestinal crypts[126] the gut is suggested to be an interesting target for therapeutic gene transfer. Every crypt in the intestine contains four to six independent stem cells[126]. Stem cells are believed to divide very rarely[126]. Therefore, these cells could have the potential to permit long term, stable transgene expression after transduction. It has been shown that intestinal stem cells can be transduced in vitro using a retroviral vector[127]. Long term transgene expression observed in the gut after AAV vector delivery in mice suggests that transduction of intestinal stem cells is possible in vivo[31,32].
Knowledge of the pathophysiology of IBD is growing and it has become clear that significant genetic as well as phenotypic heterogenecity exists within both CD and UC[128]. These findings offer opportunities for more specifically targeted interventions. Gene or cell therapy based treatment strategies can be adapted and targeted exclusively at certain subgroups within the IBD patient population with characterized genetic defects linked to the impairment of their gut physiology.
Strategies to optimize gene therapy approaches include the use of a tissue specific promoter enabling site specific expression of a transgene. Recently, gut specific promoters have been described[129-131]. The A33-antigen promoter for example strictly depends on the presence of the intestine-specific transcription factor Cdx1 which is essential for the unique intestinal expression pattern of the A33-antigen gene[129,131]. Therefore this promoter is a promising candidate to induce intestine specific expression of a transgene[131].
IBD are a group of chronic inflammatory disorders most commonly affecting young adults and currently there is no curative treatment available. A gene therapy approach for the local expression of therapeutic agents in the gut or a cell therapy approach using regulatory T cells or MSCs may offer an alternative treatment for GI inflammation. Both gene and cell therapy approaches have shown promising results in preclinical models of IBD. Cell therapy approaches have been translated to a clinical setting and currently phase II/III clinical trials for the treatment of refractory CD are in progress. Concerning gene therapy, further development of viral vector delivery to the gut as well as long term efficacy are still needed, but pre-clinical data are promising.
Overall, both gene and cell therapy have the potential to become important players in the next generation of therapeutic agents that will be aimed at unmet medical needs such as those that exist in IBD.
Peer reviewers: Julio Chebli, Professor, Department of Medicine, Federal University of Juiz de Fora, 296 Maria Jose Leal, Juiz de Fora 36036247, Brazil; Alkiviadis Efthymiou, Dr., Bioclinic Private Hospital, 75 Ermou Street, Thessaloniki 54623, Greece; I Michael Leitman, Dr., Chief of General Surgery, Department of Surgery, Albert Einstein College of Medicine-Beth Israel Medical Center, 10 Union Square East, 2M, New York, NY 10003, United States
S- Editor Wu X L- Editor Hughes D E- Editor Zheng XM
| 1. | Haug K, Schrumpf E, Barstad S, Fluge G, Halvorsen JF. Epidemiology of ulcerative colitis in western Norway. Scand J Gastroenterol. 1988;23:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Haug K, Schrumpf E, Halvorsen JF, Fluge G, Hamre E, Hamre T, Skjøllingstad R. Epidemiology of Crohn's disease in western Norway. Study group of Inflammatory Bowel Disease in Western Norway. Scand J Gastroenterol. 1989;24:1271-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Loftus EV, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 304] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 4. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1506] [Article Influence: 83.7] [Reference Citation Analysis (2)] |
| 5. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2200] [Article Influence: 137.5] [Reference Citation Analysis (6)] |
| 6. | Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573-621. [PubMed] |
| 7. | Waterman M, Xu W, Stempak JM, Milgrom R, Bernstein CN, Griffiths AM, Greenberg GR, Steinhart AH, Silverberg MS. Distinct and overlapping genetic loci in Crohn's disease and ulcerative colitis: correlations with pathogenesis. Inflamm Bowel Dis. 2011;17:1936-1942. [PubMed] |
| 8. | Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1356] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 9. | Graff LA, Walker JR, Clara I, Lix L, Miller N, Rogala L, Rawsthorne P, Bernstein CN. Stress coping, distress, and health perceptions in inflammatory bowel disease and community controls. Am J Gastroenterol. 2009;104:2959-2969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Graff LA, Vincent N, Walker JR, Clara I, Carr R, Ediger J, Miller N, Rogala L, Rawsthorne P, Lix L. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1882-1889. [PubMed] |
| 11. | Lix LM, Graff LA, Walker JR, Clara I, Rawsthorne P, Rogala L, Miller N, Ediger J, Pretorius T, Bernstein CN. Longitudinal study of quality of life and psychological functioning for active, fluctuating, and inactive disease patterns in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Cannom RR, Kaiser AM, Ault GT, Beart RW, Etzioni DA. Inflammatory bowel disease in the United States from 1998 to 2005: has infliximab affected surgical rates? Am Surg. 2009;75:976-980. [PubMed] |
| 13. | Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1657] [Cited by in RCA: 1758] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 14. | Aiuti A, Bachoud-Lévi AC, Blesch A, Brenner MK, Cattaneo F, Chiocca EA, Gao G, High KA, Leen AM, Lemoine NR. Progress and prospects: gene therapy clinical trials (part 2). Gene Ther. 2007;14:1555-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Alexander BL, Ali RR, Alton EW, Bainbridge JW, Braun S, Cheng SH, Flotte TR, Gaspar HB, Grez M, Griesenbach U. Progress and prospects: gene therapy clinical trials (part 1). Gene Ther. 2007;14:1439-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698-2703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 735] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 17. | Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 566] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 18. | Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, Scaramuzza S, Andolfi G, Mirolo M, Brigida I. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 711] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 19. | Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC, Martinache C, Rieux-Laucat F, Latour S, Belohradsky BH. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2010;363:355-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 435] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 20. | Gaudet D, de Wal J, Tremblay K, Déry S, van Deventer S, Freidig A, Brisson D, Méthot J. Review of the clinical development of alipogene tiparvovec gene therapy for lipoprotein lipase deficiency. Atheroscler Suppl. 2010;11:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061-1070. [PubMed] |
| 22. | Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut. 2010;59:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 470] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 23. | Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788-798. [PubMed] |
| 24. | Lau C, Soriano HE, Ledley FD, Finegold MJ, Wolfe JH, Birkenmeier EH, Henning SJ. Retroviral gene transfer into the intestinal epithelium. Hum Gene Ther. 1995;6:1145-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Lozier JN, Yankaskas JR, Ramsey WJ, Chen L, Berschneider H, Morgan RA. Gut epithelial cells as targets for gene therapy of hemophilia. Hum Gene Ther. 1997;8:1481-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Noel RA, Shukla P, Henning SJ. Optimization of gene transfer into intestinal epithelial cells using a retroviral vector. J Pediatr Gastroenterol Nutr. 1994;19:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Matsumoto H, Kimura T, Haga K, Kasahara N, Anton P, McGowan I. Effective in vivo and ex vivo gene transfer to intestinal mucosa by VSV-G-pseudotyped lentiviral vectors. BMC Gastroenterol. 2010;10:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Barbara G, Xing Z, Hogaboam CM, Gauldie J, Collins SM. Interleukin 10 gene transfer prevents experimental colitis in rats. Gut. 2000;46:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Lindsay JO, Ciesielski CJ, Scheinin T, Hodgson HJ, Brennan FM. The prevention and treatment of murine colitis using gene therapy with adenoviral vectors encoding IL-10. J Immunol. 2001;166:7625-7633. [PubMed] |
| 30. | Lindsay J, Van Montfrans C, Brennan F, Van Deventer S, Drillenburg P, Hodgson H, Te Velde A, Sol Rodriguez Pena M. IL-10 gene therapy prevents TNBS-induced colitis. Gene Ther. 2002;9:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Polyak S, Mah C, Porvasnik S, Herlihy JD, Campbell-Thompson M, Byrne BJ, Valentine JF. Gene delivery to intestinal epithelial cells in vitro and in vivo with recombinant adeno-associated virus types 1, 2 and 5. Dig Dis Sci. 2008;53:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | During MJ, Xu R, Young D, Kaplitt MG, Sherwin RS, Leone P. Peroral gene therapy of lactose intolerance using an adeno-associated virus vector. Nat Med. 1998;4:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | O'Neill MJ, Bourre L, Melgar S, O'Driscoll CM. Intestinal delivery of non-viral gene therapeutics: physiological barriers and preclinical models. Drug Discov Today. 2011;16:203-218. [PubMed] |
| 34. | Blaese RM, Culver KW, Chang L, Anderson WF, Mullen C, Nienhuis A, Carter C, Dunbar C, Leitman S, Berger M. Treatment of severe combined immunodeficiency disease (SCID) due to adenosine deaminase deficiency with CD34+ selected autologous peripheral blood cells transduced with a human ADA gene. Amendment to clinical research project, Project 90-C-195, January 10, 1992. Hum Gene Ther. 1993;4:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Culver KW, Osborne WR, Miller AD, Fleisher TA, Berger M, Anderson WF, Blaese RM. Correction of ADA deficiency in human T lymphocytes using retroviral-mediated gene transfer. Transplant Proc. 1991;23:170-171. [PubMed] |
| 36. | Anderson WF, Blaese RM, Culver K. The ADA human gene therapy clinical protocol: Points to Consider response with clinical protocol, July 6, 1990. Hum Gene Ther. 1990;1:331-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Miller DG, Adam MA, Miller AD. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239-4242. [PubMed] |
| 38. | Bukrinsky MI, Haffar OK. HIV-1 nuclear import: in search of a leader. Front Biosci. 1999;4:D772-D781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 649] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 40. | Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1010] [Cited by in RCA: 991] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 41. | Schröder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1324] [Cited by in RCA: 1350] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 42. | Fehse B, Roeder I. Insertional mutagenesis and clonal dominance: biological and statistical considerations. Gene Ther. 2008;15:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1926] [Cited by in RCA: 1697] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 44. | Marshall E. Clinical research. Gene therapy a suspect in leukemia-like disease. Science. 2002;298:34-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1112] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 46. | Wanisch K, Yáñez-Muñoz RJ. Integration-deficient lentiviral vectors: a slow coming of age. Mol Ther. 2009;17:1316-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 47. | Kay MA. State-of-the-art gene-based therapies: the road ahead. Nat Rev Genet. 2011;12:316-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 502] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 48. | Gustafson I, Lindblom A, Yun Z, Omar H, Engstrom L, Lewensohn-Fuchs I, Ljungman P, Broliden K. Quantification of adenovirus DNA in unrelated donor hematopoietic stem cell transplant recipients. J Clin Virol. 2008;43:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Petrus I, Chuah M, VandenDriessche T. Gene therapy strategies for hemophilia: benefits versus risks. J Gene Med. 2010;12:797-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Schmiedlin-Ren P, Kesisoglou F, Mapili JA, Sabek SE, Barnett JL, Chey WD, Roessler B, Zimmermann EM. Increased transduction of human intestinal epithelial cells by adenoviral vectors in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:464-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Brunetti-Pierri N, Nichols TC, McCorquodale S, Merricks E, Palmer DJ, Beaudet AL, Ng P. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum Gene Ther. 2005;16:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | McCormack WM, Seiler MP, Bertin TK, Ubhayakar K, Palmer DJ, Ng P, Nichols TC, Lee B. Helper-dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia A model. J Thromb Haemost. 2006;4:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 54. | Toietta G, Mane VP, Norona WS, Finegold MJ, Ng P, McDonagh AF, Beaudet AL, Lee B. Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci U S A. 2005;102:3930-3935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Engelhardt JF. AAV hits the genomic bull's-eye. Nat Biotechnol. 2006;24:949-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Schnepp BC, Clark KR, Klemanski DL, Pacak CA, Johnson PR. Genetic fate of recombinant adeno-associated virus vector genomes in muscle. J Virol. 2003;77:3495-3504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 57. | Monahan PE, Samulski RJ. Adeno-associated virus vectors for gene therapy: more pros than cons? Mol Med Today. 2000;6:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 58. | Stilwell JL, Samulski RJ. Adeno-associated virus vectors for therapeutic gene transfer. Biotechniques. 2003;34:148-50, 152, 154 passim. [PubMed] |
| 59. | Buff SM, Yu H, McCall JN, Caldwell SM, Ferkol TW, Flotte TR, Virella-Lowell IL. IL-10 delivery by AAV5 vector attenuates inflammation in mice with Pseudomonas pneumonia. Gene Ther. 2010;17:567-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Nomoto T, Okada T, Shimazaki K, Yoshioka T, Nonaka-Sarukawa M, Ito T, Takeuchi K, Katsura KI, Mizukami H, Kume A. Systemic delivery of IL-10 by an AAV vector prevents vascular remodeling and end-organ damage in stroke-prone spontaneously hypertensive rat. Gene Ther. 2009;16:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, Roncal CA, Glushakova OY, Chiodo VA, Atkinson MA. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol. 2005;16:3651-3660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 62. | Chen B, Kapturczak MH, Joseph R, George JF, Campbell-Thompson M, Wasserfall CH, Atkinson MA, Tisher CC, Flotte TR, Agarwal A. Adeno-associated viral vector-mediated interleukin-10 prolongs allograft survival in a rat kidney transplantation model. Am J Transplant. 2007;7:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Smith JR, Verwaerde C, Rolling F, Naud MC, Delanoye A, Thillaye-Goldenberg B, Apparailly F, De Kozak Y. Tetracycline-inducible viral interleukin-10 intraocular gene transfer, using adeno-associated virus in experimental autoimmune uveoretinitis. Hum Gene Ther. 2005;16:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Carter JD, Ellett JD, Chen M, Smith KM, Fialkow LB, McDuffie MJ, Tung KS, Nadler JL, Yang Z. Viral IL-10-mediated immune regulation in pancreatic islet transplantation. Mol Ther. 2005;12:360-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Apparailly F, Millet V, Noël D, Jacquet C, Sany J, Jorgensen C. Tetracycline-inducible interleukin-10 gene transfer mediated by an adeno-associated virus: application to experimental arthritis. Hum Gene Ther. 2002;13:1179-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Yang Z, Chen M, Wu R, Fialkow LB, Bromberg JS, McDuffie M, Naji A, Nadler JL. Suppression of autoimmune diabetes by viral IL-10 gene transfer. J Immunol. 2002;168:6479-6485. [PubMed] |
| 67. | Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, Glader B, Chew AJ, Tai SJ, Herzog RW. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 685] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 68. | Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1459] [Cited by in RCA: 1645] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 69. | Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 769] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 70. | Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1392] [Cited by in RCA: 1451] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 71. | Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105:15112-15117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 548] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 72. | Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240-2248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1661] [Cited by in RCA: 1648] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 73. | Stroes ES, Nierman MC, Meulenberg JJ, Franssen R, Twisk J, Henny CP, Maas MM, Zwinderman AH, Ross C, Aronica E. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol. 2008;28:2303-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 74. | Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, Rouhani F, Conlon TJ, Calcedo R, Betts MR. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A. 2009;106:16363-16368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 75. | Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 76. | Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 665] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 77. | Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G, Craenen JM, Lewis S, Malik V, Shilling C. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009;66:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 78. | Hussain MA, Theise ND. Stem-cell therapy for diabetes mellitus. Lancet. 2004;364:203-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 79. | Duijvestein M, van den Brink GR, Hommes DW. Stem cells as potential novel therapeutic strategy for inflammatory bowel disease. J Crohns Colitis. 2008;2:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | de la Morena MT, Gatti RA. A history of bone marrow transplantation. Immunol Allergy Clin North Am. 2010;30:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 894] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 82. | Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1671] [Cited by in RCA: 1839] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 83. | Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Müller W, Sparwasser T, Förster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237-246. [PubMed] |
| 84. | Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 849] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 85. | Amarnath S, Costanzo CM, Mariotti J, Ullman JL, Telford WG, Kapoor V, Riley JL, Levine BL, June CH, Fong T. Regulatory T cells and human myeloid dendritic cells promote tolerance via programmed death ligand-1. PLoS Biol. 2010;8:e1000302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1345] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 87. | Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3904] [Article Influence: 229.6] [Reference Citation Analysis (0)] |
| 88. | Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells--ex vivo expansion and therapeutic potential. Semin Immunol. 2006;18:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 89. | Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 90. | Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 995] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 91. | Huibregtse IL, van Lent AU, van Deventer SJ. Immunopathogenesis of IBD: insufficient suppressor function in the gut? Gut. 2007;56:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 781] [Cited by in RCA: 781] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 93. | Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 400] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 94. | Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112-6119. [PubMed] |
| 95. | Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2752] [Cited by in RCA: 2709] [Article Influence: 96.8] [Reference Citation Analysis (2)] |
| 96. | Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939-3943. [PubMed] |
| 97. | Li Z, Arijs I, De Hertogh G, Vermeire S, Noman M, Bullens D, Coorevits L, Sagaert X, Schuit F, Rutgeerts P. Reciprocal changes of Foxp3 expression in blood and intestinal mucosa in IBD patients responding to infliximab. Inflamm Bowel Dis. 2010;16:1299-1310. [PubMed] |
| 98. | Veltkamp C, Anstaett M, Wahl K, Möller S, Gangl S, Bachmann O, Hardtke-Wolenski M, Länger F, Stremmel W, Manns MP. Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNFα treatment. Gut. 2011;60:1345-1353. [PubMed] |
| 99. | Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5576] [Cited by in RCA: 5859] [Article Influence: 266.3] [Reference Citation Analysis (0)] |
| 100. | Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2198] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 101. | Trzonkowski P, Bieniaszewska M, Juścińska J, Dobyszuk A, Krzystyniak A, Marek N, Myśliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 537] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 102. | Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 748] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 103. | Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722-3729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1165] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 104. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3277] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 105. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2455] [Cited by in RCA: 2363] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 106. | Sundin M, D'arcy P, Johansson CC, Barrett AJ, Lönnies H, Sundberg B, Nava S, Kiessling R, Mougiakakos D, Le Blanc K. Multipotent mesenchymal stromal cells express FoxP3: a marker for the immunosuppressive capacity? J Immunother. 2011;34:336-342. [PubMed] |
| 107. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5012] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 108. | Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D, Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 325] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 109. | Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1077] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 110. | Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 497] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 111. | González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 112. | June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9:704-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 113. | Van Montfrans C, Rodriguez Pena MS, Pronk I, Ten Kate FJ, Te Velde AA, Van Deventer SJ. Prevention of colitis by interleukin 10-transduced T lymphocytes in the SCID mice transfer model. Gastroenterology. 2002;123:1865-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 114. | Van Montfrans C, Hooijberg E, Rodriguez Pena MS, De Jong EC, Spits H, Te Velde AA, Van Deventer SJ. Generation of regulatory gut-homing human T lymphocytes using ex vivo interleukin 10 gene transfer. Gastroenterology. 2002;123:1877-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 115. | Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6077] [Cited by in RCA: 6388] [Article Influence: 290.4] [Reference Citation Analysis (0)] |
| 116. | Elinav E, Adam N, Waks T, Eshhar Z. Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology. 2009;136:1721-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 117. | Chai JG, Xue SA, Coe D, Addey C, Bartok I, Scott D, Simpson E, Stauss HJ, Hori S, Sakaguchi S. Regulatory T cells, derived from naïve CD4+CD25- T cells by in vitro Foxp3 gene transfer, can induce transplantation tolerance. Transplantation. 2005;79:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 118. | Peng J, Dicker B, Du W, Tang F, Nguyen P, Geiger T, Wong FS, Wen L. Converting antigen-specific diabetogenic CD4 and CD8 T cells to TGF-beta producing non-pathogenic regulatory cells following FoxP3 transduction. J Autoimmun. 2007;28:188-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | Sandberg JW, Lau C, Jacomino M, Finegold M, Henning SJ. Improving access to intestinal stem cells as a step toward intestinal gene transfer. Hum Gene Ther. 1994;5:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 743] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 121. | Kotin RM. Prospects for the use of adeno-associated virus as a vector for human gene therapy. Hum Gene Ther. 1994;5:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 169] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 122. | Mitchell AM, Nicolson SC, Warischalk JK, Samulski RJ. AAV's anatomy: roadmap for optimizing vectors for translational success. Curr Gene Ther. 2010;10:319-340. [PubMed] |
| 123. | Cohen J. How DNA shuffling works. Science. 2001;293:237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 124. | Li W, Asokan A, Wu Z, Van Dyke T, DiPrimio N, Johnson JS, Govindaswamy L, Agbandje-McKenna M, Leichtle S, Redmond DE. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Mol Ther. 2008;16:1252-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 125. | Horowitz ED, Weinberg MS, Asokan A. Glycated AAV vectors: chemical redirection of viral tissue tropism. Bioconjug Chem. 2011;22:529-532. [PubMed] |
| 126. | Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 482] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 127. | Kawaguchi AL, Dunn JC, Fonkalsrud EW. In vivo growth of transplanted genetically altered intestinal stem cells. J Pediatr Surg. 1998;33:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 128. | Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1149] [Cited by in RCA: 1092] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 129. | Johnstone CN, White SJ, Tebbutt NC, Clay FJ, Ernst M, Biggs WH, Viars CS, Czekay S, Arden KC, Heath JK. Analysis of the regulation of the A33 antigen gene reveals intestine-specific mechanisms of gene expression. J Biol Chem. 2002;277:34531-34539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 130. | Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275-33283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 658] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 131. | Mayer K, Iolyeva ME, Meyer-Grahle U, Brix K. Intestine-specific expression of green fluorescent protein-tagged cathepsin B: proof-of-principle experiments. Biol Chem. 2008;389:1085-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 132. | Feng G, Nadig SN, Bäckdahl L, Beck S, Francis RS, Schiopu A, Whatcott A, Wood KJ, Bushell A. Functional regulatory T cells produced by inhibiting cyclic nucleotide phosphodiesterase type 3 prevent allograft rejection. Sci Transl Med. 2011;3:83ra40. [PubMed] |
| 133. | Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3:83ra41. [PubMed] |
| 134. | Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3:83ra42. [PubMed] |
| 135. | Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 585] [Article Influence: 24.4] [Reference Citation Analysis (0)] |