Published online Dec 15, 2011. doi: 10.4291/wjgp.v2.i6.103
Revised: September 20, 2011
Accepted: October 14, 2011
Published online: December 15, 2011
Despite advances in treatment and the declining incidence, gastric cancer remains the second leading cause of cancer-related deaths in the world. Understanding the progression from inflammation to cancer in the stomach is crucial in the development of novel therapies and strategies for treating this disease. Chronic inflammation of the stomach is typically caused by Helicobacter pylori (H. pylori) and resulting lesions may lead to gastric cancer. During the progression from inflammation to cancer, the stomach epithelium changes with evidence of the disruption of normal epithelial cell differentiation and infiltrating inflammatory cells. Coincident with the development of atrophic gastritis and metaplasia, is the loss of the gastric morphogen Sonic Hedgehog (Shh). Given its critical role as a regulator of gastric tissue homeostasis, the disruption of Shh expression during inflammation correlates with the loss of normal epithelial cell differentiation, but this has only recently been rigorously tested in vivo using a unique mouse model of targeted gastric Shh deletion. While pre-neoplastic lesions such as atrophic gastritis and intestinal metaplasia are associated with the loss of Shh within the acid-secreting glands of the stomach, there is a clear link between elevated Shh and signaling to gastric cancers. The current review focuses on the effects of aberrant Shh expression and its role in the development of gastric cancer, specifically in response to H. pylori infection.
- Citation: Sherman AE, Zavros Y. Role of Sonic Hedgehog signaling during progression from inflammation to cancer in the stomach. World J Gastrointest Pathophysiol 2011; 2(6): 103-108
- URL: https://www.wjgnet.com/2150-5330/full/v2/i6/103.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v2.i6.103
Despite advances in treatment and declining incidence, stomach cancer remains the second leading cause of cancer-related deaths in the world[1]. Understanding the progression from inflammation to cancer in the stomach is crucial in the development of novel therapies and strategies for treating this disease. The Hedgehog family of proteins, comprised of Sonic Hedgehog (Shh), Indian Hedgehog (Ihh) and Desert Hedgehog (Dhh), has been implicated in a variety of solid tumors including stomach cancer[2-4]. This review will focus on the role of Shh in the progression from inflammation to tumorigenesis of gastric carcinomas and its role in stomach cancer.
Hedgehog was given its name from the “spiny” phenotype found in Drosophila embryos with a mutation in the gene[5]. Three mammalian homologs have since been discovered: Shh, Ihh and Dhh. Ihh and Dhh have mostly tissue-specific expression while Shh has widespread expression of which deficiency leads to neural, limb growth and foregut defects[6]. Hedgehog is generated as a pre-protein before its signal sequence is cleaved, yielding an approximately 45 kDa precursor. The precursor is internally cleaved and a cholesterol moiety is attached to a glycine residue on the nascent carboxy-terminus[7]. This covalent attachment of cholesterol is a unique modification among secreted ligands and is a requirement for tissue specificity and localization of the molecule. In vertebrates, Shh binds and inhibits Patched-1 (Ptc), consequently activating Smoothened (Smo), which transduces the Hedgehog signal into the cytoplasm, activating the Gli family of transcription factors[8,9]. Despite this information, the events leading to Gli activation, especially in the mammalian stomach, are poorly understood.
Shh is a peptide morphogen produced in gastric epithelium; however, expression in specific cell types has been controversial. Recently, a study utilizing a Shh-LacZ reporter mouse line identified all major cell lineages of the corpus expressing Shh; these included surface pit, mucous neck, zymogenic and parietal cells[10]. Some stromal cells express signal transduction components in addition to Shh; these include the 12-transmembrane receptor, Ptc, Smo, 7-transmembrane receptor and Gli transcription factor family. The Hedgehog ligand activates Smo which leads to transcriptional activation of Gli1, Gli2 becoming an activator and Gli3 no longer acting as a repressor[11]. Therefore, Hedgehog signaling results in an increase of activator Gli and a decrease in repressor Gli. These events are crucial in the development and maintenance of multiple organs and the role of Shh as a key morphogen has been well documented[5,12].
Shh has important roles in the development of multiple organ systems including neuronal and gastrointestinal systems[13,14]. Shh in the mammalian stomach has been found to be hormonally regulated by gastrin and requires an acidic environment for proper processing[15]. The acid-activated pepsin A protease was found to mediate the processing of the 45kDa precursor molecule to the active Shh molecule. These findings indicate the importance of the acidic gastric environment for maintenance of Shh in the stomach.
Much of what we know about Shh’s role in mammals has come from studies in animal models. Shh-knockout mice display key features that support the importance of proper Shh homeostasis for normal development and physiological function.
Shh -/- mouse embryos displayed multiple gut abnormalities including overt gut malrotation and intestinal transformation of the stomach[16]. Additionally, neurons of the enteric nervous system underwent abnormal differentiation compared to wildtype. While this system provided evidence for the role of Hedgehog in the coordination of developmental genes, these mice die at or shortly after birth.
In order to study the effects of Shh signaling in the stomach, Xiao et al[17] developed a mouse model expressing a parietal cell-specific deletion of Shh (HKCre/ShhKO). This study found evidence of hypochlorhydria, hypergastrinemia and foveolar hyperplasia by 8 mo of age. Additionally, a delay in the differentiation of zymogen cells, demonstrated by an increase in immature cells expressing markers for both mucous neck and zymogen cells, was documented in the stomach of HKCre/ShhKO mice[17]. The deletion of Shh from parietal cells led to alterations in stomach morphology and gastric cell differentiation; however, there was no evidence of parietal cell atrophy, a key step in the development of stomach cancer in Helicobacter pylori (H. pylori) infection[18,19]. The absence of parietal cell atrophy suggests that the loss of Shh expression alone is not sufficient to trigger the development of stomach cancer.
H. pylori infection is globally widespread and a major cause of chronic atrophic gastritis with persistent infection in 50% of the global population[20,21]. While infection with H. pylori has been directly linked to the development of gastric cancer, the mechanism of tumorigenesis is still unclear. There are many factors that have been considered in Helicobacter-mediated carcinogenesis; however, the focus of this review will be to discuss its contribution by modulating Shh expression. H. pylori infection primarily contributes to gastric cancer development through two mechanisms: loss of Shh by destruction of parietal cells and induction of a chronic inflammatory gastric environment.
The ability of H. pylori to evade the primarily Th1-mediated host immune response contributes to the development of chronically elevated interleukin-1β (IL-1β) and other inflammatory cytokines in the stomach[10,22]. A study of H. pylori associated with chronic atrophic gastritis found upregulation of a gene encoding cysteine-rich protein A, which induces Th1 cytokines interferon (IFN)-γ and IL-12[23]. Additionally, H. pylori-associated atrophic gastritis has been found to be more frequent in patients with pro-inflammatory polymorphisms of genes for IL-1β and tumor necrosis factor (TNF)-α[24,25]. Specifically, it has been shown that elevated IL-1β leads to the suppression of Shh, which is necessary for the growth and differentiation of the gastric mucosa during cell restitution during H. pylori infection[10,26].
The loss of parietal cells through Helicobacter infection and subsequent autoimmunity has been well documented[22,27]. Molecular mimicry between the H+,K+-ATPase localized to parietal cells canaliculi and H. pylori leads to the selective destruction of parietal cells during the immune response to infection. There is evidence that Shh is expressed and secreted from parietal cells and that the loss of parietal cells results in lower expression of Shh[15,28,29]. Additionally, the higher luminal pH in the stomach during Helicobacter infection may interfere with Shh signaling as it has been shown that gastric Shh processing is pH-dependent[15]. The loss of Shh, coupled with an increase in the proliferation and differentiation of gastric stem cells, contributes to improper regeneration of gastric epithelium. Shh acts via an autocrine loop in the stomach and is implicated in stem cell restitution of damaged gastric mucosa during H. pylori chronic infection[30]. Despite its crucial role in gastric homeostasis, the loss of Shh alone is not sufficient for neoplastic transformation[17]. An additional study examining the role of Shh in the presence of Helicobacter demonstrated the importance of the chronic inflammatory environment induced by infection for the induction of gastric atrophy associated with Shh suppression[10].
H. pylori infection in Mongolian gerbils has been found to induce inflammation and a loss of Shh expression[31-33]. Infection resulted in a smaller region of Shh-expressing cells in the stomach and hyperproliferation of Shh-negative cells after 51 wk. In humans, the loss of Shh expression coincided with the induction of caudal type homeobox 2 (Cdx2), intestine-specific transcription factors in the stomach[34]. In fact, Cdx2-transgenic mice developed gastric polyps with invasive gastric adenocarcinoma, implicating intestinal metaplasia in gastric carcinogenesis[35]. Further studies of this mouse model demonstrated Cdx2 transcriptionally down-regulating Shh through its promoter, inducing metaplasia through the expression of intestinal metaplastic mucosa and loss of gastric phenotype[35]. Cyclopamine, an inhibitor of Shh signaling, has been shown in vivo to reduce the expression of parietal cell-specific H+,K+-ATPase[36]. The lack of an appropriate level of Shh signaling leads to improper gastric morphogenesis during healing and ultimately gastric mucosal atrophy, predisposing the individual to gastric cancer[30].
With an estimated annual 989 600 new cases and 738 000 deaths, stomach cancer is the second leading cause of cancer-related deaths worldwide[1]. The importance of Shh in the development and maintenance of proper gastric function is established; however, there are alternative explanations for its role in carcinogenesis.
The aforementioned studies hypothesize that lower Shh expression in H. pylori infection leads to impaired differentiation and morphogenesis during healing secondary to inflammatory signals. Other studies suggest H. pylori infection selects for cells that are resistant to cell death and that these cells produce higher levels of Shh[37,38]. The anti-apoptotic properties of these cells may contribute to the tumorigenesis in these human gastric carcinoma cells[38]. It is important to consider that an in vitro study may not be the most appropriate representation of the development of human disease due to the lack of environmental factors, including inflammatory cytokines, signaling and morphogenic factors from parietal cells, and the contribution of progenitor cells.
Previous data suggests the involvement of CD44+ cells in the repair of gastric mucosa in H. pylori infection[39]. In fact, this study found that both IFN-γ and TNF-α increased CD44 expression; however, elevated levels of IL-1β and IFN-γ may cause transformation of some of these recruited stem cells into cancer stem cells (CSCs). CD44 has been identified as a marker of CSCs, thus supporting the idea that gastric CSCs exist and possess tumorigenic properties both in vitro and in vivo. In fact, Takaishi et al[40,41] found that CD44 expression correlated with dysplastic gastric glands and invasive gastric lesions in Helicobacter-infected mice. In a mouse model of activated gastric Wnt and prostaglandin E2 signaling, CD44 was upregulated in gastric tumors[42]. Wnt signaling is a downstream effector of Hedgehog signaling; therefore, this finding provides additional evidence that CD44 may play a role in gastric tumorigenesis, possibly through Shh signaling[43,44]. In another study, CD44+ cells isolated from these tumors had high expression of CD133, another marker used in identifying CSCs, and low expression of MUCA5C, a marker of gastric epithelial differentiation[42,45]. Furthermore, Shh has been found to be upregulated in CD44+ cells and inhibition of Shh signaling by cyclopamine blocks the chemoresistant and self-renewing properties of these proposed CSCs[46]. In humans, CD44+ tumors are also associated poorer survival in patients with intestinal-type gastric adenocarcinoma[47].
The seemingly paradoxical disappearance and reappearance of Shh expression during gastric carcinogenesis requires further elucidation. The loss of parietal cells during H. pylori infection may explain the initial deficit of Shh expression, while the repopulation of the gastric mucosa by stem cells expressing Shh may explain the high levels of expression found in gastric adenocarcinomas. Of particular interest are bone-marrow-derived mesenchymal stem cells (MSCs) and their potential role in tumorigenesis. These cells are recruited to sites of tissue injury and have been hypothesized to be the link between chronic inflammation and stomach cancer[48,49]. Houghton et al[48,49] transplanted marrow-derived cells tracked by X-galactosidase in a lethally irradiated mouse model of chronic Helicobacter infection. They found that the mice developed metaplasia and dysplasia, and that the marrow-derived cells were proliferating in the dysplastic glands of the stomach. Furthermore, this MSC engraftment could only be demonstrated with concurrent Helicobacter infection, supporting the idea that chronic inflammation is necessary for the development of parietal cell atrophy and subsequent neoplastic transformation. Furthermore, it has been shown that inhibition of Hedgehog signaling by cyclopamine decreases MSC cell proliferation and clonogenecity, suggesting a role of Hedgehog in the maintenance of this cell population in the periphery[50]. Emerging data provides further evidence for the role of MSCs in gastric carcinogenesis. MSCs have been isolated and characterized from patients undergoing radical gastrectomy for stomach cancer[51,52]. These cells expressed CD44, had a larger population in S phase compared to control, but lacked tumorigenic properties when transplanted into BALB/c nude mice. Further studies are warranted on the impact of aberrant Shh expression on the differentiation of recruited MSCs and their role in gastric tumorigenesis.
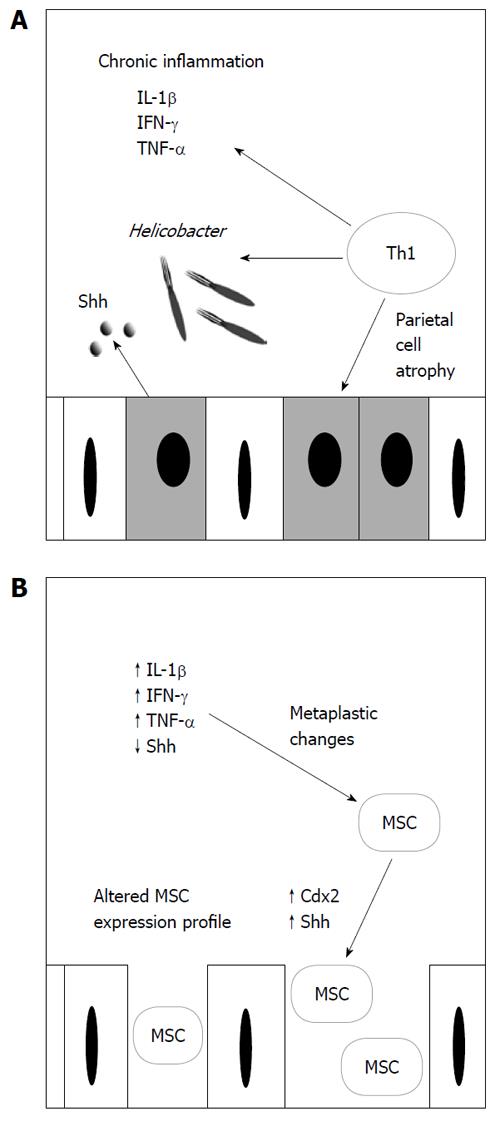
Persistent H. pylori infection remains one of the key events leading to gastric adenocarcinoma. The loss of parietal cells and secretion of inflammatory cytokines, including IL-1β and IFN-γ secreted by Th1 cells induced by persistent Helicobacter infection, causes suppression of Shh signaling (Figure 1A). When recruited MSCs repopulate the gastric epithelium, the presence of inflammatory cytokines in combination with the absence of adequate Shh expression allows for metaplastic changes, including increased expression of Cdx2, ultimately leading to dysplasia and subsequent cancer development (Figure 1B). The malignant transformation of MSCs into cancer-promoting cells may be induced by inflammatory cytokines secreted during chronic gastritis. Malignantly transformed MSCs may be the site of Shh secretion associated with gastric carcinomas. However, the in vivo malignant transformation of MSCs in response to chronic inflammation is not yet known.
Chronic inflammation is typically caused by H. pylori and is the most consistent lesion leading to gastric cancer. During the progression from inflammation to cancer, the stomach epithelium changes with evidence of the disruption of normal epithelial cell differentiation, infiltrating inflammatory cells and the recruitment of bone marrow derived MSCs. Coincident with changes in cell differentiation associated with the development of atrophic gastritis and metaplasia, is the loss of Shh. Given its predicted critical role as a regulator of gastric tissue homeostasis, the disruption of Shh expression during inflammation would be expected to result in loss of normal epithelial cell differentiation, but this has only recently been rigorously tested in vivo using the HKCre/ShhKO mouse model. Studies using HKCre/ShhKO mice reveal for the first time a direct role of Shh as a regulator of epithelial cell function and differentiation in the normal adult stomach. The dysregulation may be seen as a global increase or decrease in expression, or in altered location of expression. For example, atrophic gastritis and intestinal metaplasia are associated with the loss of Shh within the fundic mucosa[18,19], while elevated Shh and signaling is associated with gastric cancers[37,46,53]. Although the association between Shh and gastric cancer is clear, the mechanism that regulates the production of Shh protein within the tumor microenvironment and the precise role of Shh in tumor progression are still largely unknown. Understanding the role of Shh in the neoplastic transformations associated with chronic gastric inflammation would allow for the development of targeted therapeutics and preventative strategies.
Peer reviewer: Hiroyuki Matsubayashi, MD, PhD, Chief of Pancreatobiliary Endoscopy, Department of Endoscopy, Shizuoka Cancer Center, 1007 Shimonagakubo, Nagaizumi, Suntogun, Shizuoka 411-8777, Japan
S- Editor Wu X L- Editor Roemmele A E- Editor Zheng XM
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25538] [Article Influence: 1824.1] [Reference Citation Analysis (7)] |
| 2. | Takezaki T, Hide T, Takanaga H, Nakamura H, Kuratsu J, Kondo T. Essential role of the Hedgehog signaling pathway in human glioma-initiating cells. Cancer Sci. 2011;102:1306-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Wang X, Venugopal C, Manoranjan B, McFarlane N, O’Farrell E, Nolte S, Gunnarsson T, Hollenberg R, Kwiecien J, Northcott P. Sonic hedgehog regulates Bmi1 in human medulloblastoma brain tumor-initiating cells. Oncogene. 2011;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Ohta M, Tateishi K, Kanai F, Watabe H, Kondo S, Guleng B, Tanaka Y, Asaoka Y, Jazag A, Imamura J. p53-Independent negative regulation of p21/cyclin-dependent kinase-interacting protein 1 by the sonic hedgehog-glioma-associated oncogene 1 pathway in gastric carcinoma cells. Cancer Res. 2005;65:10822-10829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2958] [Cited by in RCA: 2895] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 6. | Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2382] [Cited by in RCA: 2331] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 7. | Porter JA, Ekker SC, Park WJ, von Kessler DP, Young KE, Chen CH, Ma Y, Woods AS, Cotter RJ, Koonin EV. Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell. 1996;86:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 396] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 8. | Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 605] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 9. | Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev. 1996;10:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 663] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 10. | Waghray M, Zavros Y, Saqui-Salces M, El-Zaatari M, Alamelumangapuram CB, Todisco A, Eaton KA, Merchant JL. Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. Gastroenterology. 2010;138:562-72, 572.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Ruiz i Altaba A, Sánchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 563] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 12. | Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059-3087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2300] [Cited by in RCA: 2336] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 13. | Kim TH, Kim BM, Mao J, Rowan S, Shivdasani RA. Endodermal Hedgehog signals modulate Notch pathway activity in the developing digestive tract mesenchyme. Development. 2011;138:3225-3233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Cayuso J, Ulloa F, Cox B, Briscoe J, Martí E. The Sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating Gli activity. Development. 2006;133:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Zavros Y, Waghray M, Tessier A, Bai L, Todisco A, L Gumucio D, Samuelson LC, Dlugosz A, Merchant JL. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem. 2007;282:33265-33274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763-2772. [PubMed] |
| 17. | Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology. 2010;138:550-61, 561.e1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Shiotani A, Iishi H, Uedo N, Ishiguro S, Tatsuta M, Nakae Y, Kumamoto M, Merchant JL. Evidence that loss of sonic hedgehog is an indicator of Helicobater pylori-induced atrophic gastritis progressing to gastric cancer. Am J Gastroenterol. 2005;100:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Shiotani A, Iishi H, Uedo N, Kumamoto M, Nakae Y, Ishiguro S, Tatsuta M, Graham DY. Histologic and serum risk markers for noncardia early gastric cancer. Int J Cancer. 2005;115:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Fuccio L, Eusebi LH, Bazzoli F. Gastric cancer, Helicobacter pylori infection and other risk factors. World J Gastrointest Oncol. 2010;2:342-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1907] [Article Influence: 82.9] [Reference Citation Analysis (3)] |
| 22. | D'Elios MM, Bergman MP, Azzurri A, Amedei A, Benagiano M, De Pont JJ, Cianchi F, Vandenbroucke-Grauls CM, Romagnani S, Appelmelk BJ. H(+),K(+)-atpase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology. 2001;120:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 23. | Giannakis M, Chen SL, Karam SM, Engstrand L, Gordon JI. Helicobacter pylori evolution during progression from chronic atrophic gastritis to gastric cancer and its impact on gastric stem cells. Proc Natl Acad Sci U S A. 2008;105:4358-4363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1674] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 25. | Schneider BG, Camargo MC, Ryckman KK, Sicinschi LA, Piazuelo MB, Zabaleta J, Correa P, Williams SM. Cytokine polymorphisms and gastric cancer risk: an evolving view. Cancer Biol Ther. 2008;7:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Katoh Y, Katoh M. Identification and characterization of rat Desert hedgehog and Indian hedgehog genes in silico. Int J Oncol. 2005;26:545-549. [PubMed] |
| 27. | D'Elios MM, Appelmelk BJ, Amedei A, Bergman MP, Del Prete G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol Med. 2004;10:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Zavros Y, Orr MA, Xiao C, Malinowska DH. Sonic hedgehog is associated with H+-K+-ATPase-containing membranes in gastric parietal cells and secreted with histamine stimulation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G99-G111. [PubMed] |
| 29. | Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem. 2005;280:15700-15708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Faller G, Kirchner T. Immunological and morphogenic basis of gastric mucosa atrophy and metaplasia. Virchows Arch. 2005;446:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Nishizawa T, Suzuki H, Masaoka T, Minegishi Y, Iwasahi E, Hibi T. Helicobacter pylori eradication restored sonic hedgehog expression in the stomach. Hepatogastroenterology. 2007;54:697-700. [PubMed] |
| 32. | Nishizawa T, Suzuki H, Nakagawa I, Minegishi Y, Masaoka T, Iwasaki E, Hibi T. Early Helicobacter pylori eradication restores sonic hedgehog expression in the gastric mucosa of Mongolian gerbils. Digestion. 2009;79:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Suzuki H, Minegishi Y, Nomoto Y, Ota T, Masaoka T, van den Brink GR, Hibi T. Down-regulation of a morphogen (sonic hedgehog) gradient in the gastric epithelium of Helicobacter pylori-infected Mongolian gerbils. J Pathol. 2005;206:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Shiotani A, Uedo N, Iishi H, Tatsuta M, Ishiguro S, Nakae Y, Kamada T, Haruma K, Merchant JL. Re-expression of sonic hedgehog and reduction of CDX2 after Helicobacter pylori eradication prior to incomplete intestinal metaplasia. Int J Cancer. 2007;121:1182-1189. [PubMed] |
| 35. | Mutoh H, Hayakawa H, Sashikawa M, Sakamoto H, Sugano K. Direct repression of Sonic Hedgehog expression in the stomach by Cdx2 leads to intestinal transformation. Biochem J. 2010;427:423-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Kang DH, Han ME, Song MH, Lee YS, Kim EH, Kim HJ, Kim GH, Kim DH, Yoon S, Baek SY. The role of hedgehog signaling during gastric regeneration. J Gastroenterol. 2009;44:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Kim JH, Choi YJ, Lee SH, Shin HS, Lee IO, Kim YJ, Kim H, Yang WI, Kim H, Lee YC. Effect of Helicobacter pylori infection on the sonic hedgehog signaling pathway in gastric cancer cells. Oncol Rep. 2010;23:1523-1528. [PubMed] |
| 38. | Lee KM, Lee JS, Jung HS, Park DK, Park HS, Hahm KB. Late reactivation of sonic hedgehog by Helicobacter pylori results in population of gastric epithelial cells that are resistant to apoptosis: implication for gastric carcinogenesis. Cancer Lett. 2010;287:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Fan X, Long A, Goggins M, Fan X, Keeling PW, Kelleher D. Expression of CD44 and its variants on gastric epithelial cells of patients with Helicobacter pylori colonisation. Gut. 1996;38:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 816] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 41. | Takaishi S, Okumura T, Wang TC. Gastric cancer stem cells. J Clin Oncol. 2008;26:2876-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 42. | Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 937] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 43. | Li H, Fan X, Kovi RC, Jo Y, Moquin B, Konz R, Stoicov C, Kurt-Jones E, Grossman SR, Lyle S. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Res. 2007;67:10889-10898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 44. | Li HC, Stoicov C, Rogers AB, Houghton J. Stem cells and cancer: evidence for bone marrow stem cells in epithelial cancers. World J Gastroenterol. 2006;12:363-371. [PubMed] |
| 45. | Schmuck R, Warneke V, Behrens HM, Simon E, Weichert W, Röcken C. Genotypic and phenotypic characterization of side population of gastric cancer cell lines. Am J Pathol. 2011;178:1792-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Song Z, Yue W, Wei B, Wang N, Li T, Guan L, Shi S, Zeng Q, Pei X, Chen L. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS One. 2011;6:e17687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 47. | Ghaffarzadehgan K, Jafarzadeh M, Raziee HR, Sima HR, Esmaili-Shandiz E, Hosseinnezhad H, Taghizadeh-Kermani A, Moaven O, Bahrani M. Expression of cell adhesion molecule CD44 in gastric adenocarcinoma and its prognostic importance. World J Gastroenterol. 2008;14:6376-6381. [PubMed] |
| 48. | Houghton J, Li H, Fan X, Liu Y, Liu JH, Rao VP, Poutahidis T, Taylor CL, Jackson EA, Hewes C. Mutations in bone marrow-derived stromal stem cells unmask latent malignancy. Stem Cells Dev. 2010;19:1153-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 838] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 50. | Plaisant M, Fontaine C, Cousin W, Rochet N, Dani C, Peraldi P. Activation of hedgehog signaling inhibits osteoblast differentiation of human mesenchymal stem cells. Stem Cells. 2009;27:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Cao H, Xu W, Qian H, Zhu W, Yan Y, Zhou H, Zhang X, Xu X, Li J, Chen Z. Mesenchymal stem cell-like cells derived from human gastric cancer tissues. Cancer Lett. 2009;274:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Xu X, Zhang X, Wang S, Qian H, Zhu W, Cao H, Wang M, Chen Y, Xu W. Isolation and comparison of mesenchymal stem-like cells from human gastric cancer and adjacent non-cancerous tissues. J Cancer Res Clin Oncol. 2011;137:495-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 967] [Article Influence: 44.0] [Reference Citation Analysis (0)] |