Published online Apr 22, 2024. doi: 10.4291/wjgp.v15.i1.91100
Revised: February 9, 2024
Accepted: April 1, 2024
Published online: April 22, 2024
Processing time: 119 Days and 11.2 Hours
Nonalcoholic fatty liver disease (NAFLD) includes a spectrum of conditions, progressing from mild steatosis to advanced fibrosis. Sarcopenia, characterized by decreased muscle strength and mass, shares common pathophysiological traits with NAFLD. An association exists between sarcopenia and increased NAFLD prevalence. However, data on the prevalence of sarcopenia in NAFLD and its impact on the outcomes of NAFLD remain inconsistent.
To analyze the prevalence and outcomes of sarcopenia in patients with NAFLD.
We conducted a comprehensive search for relevant studies in MEDLINE, Embase, and Scopus from their inception to June 2023. We included studies that focused on patients with NAFLD, reported the prevalence of sarcopenia as the primary outcome, and examined secondary outcomes, such as liver fibrosis and other adverse events. We also used the Newcastle-Ottawa scale for quality assessment.
Of the 29 studies included, the prevalence of sarcopenia in NAFLD varied widely (1.6% to 63.0%), with 20 studies reporting a prevalence of more than 10.0%. Substantial heterogeneity was noted in the measurement modalities for sarcopenia. Sarcopenia was associated with a higher risk of advanced fibrosis (odd ratio: 1.97, 95% confidence interval: 1.44-2.70). Increased odds were consistently observed in fibrosis assessment through biopsy, NAFLD fibrosis score/body mass index, aspartate aminotransferase to alanine aminotransferase ratio, diabetes (BARD) score, and transient elastography, whereas the fibrosis-4 score showed no such association. Sarcopenia in NAFLD was associated with a higher risk of steatohepatitis, insulin resistance, cardiovascular risks, and mortality.
This systematic review highlights the critical need for standardized diagnostic criteria and measurement methods for sarcopenia in NAFLD patients. The variability in study designs and assessment methods for sarcopenia and liver fibrosis may account for the inconsistent findings. This review demonstrates the multidimensional impact of sarcopenia on NAFLD, indicating its importance beyond liver-related events to include cardiovascular risks, mortality, and metabolic complications.
Core Tip: The prevalence of sarcopenia in nonalcoholic fatty liver disease (NAFLD) varies widely. Sarcopenia in NAFLD is consistently associated with a higher risk of advanced fibrosis. In addition to liver-related events, sarcopenia in NAFLD is associated with adverse outcomes, including an increased risk of nonalcoholic steatohepatitis, mortality, cardiovascular risks, and metabolic complications. The heterogeneity in prevalence and associations highlights the importance of accurately defining measurement modalities and cutoff criteria. Establishing consensus guidelines is crucial for advancing research and enhancing clinical management in the complex relationship between sarcopenia and NAFLD.
- Citation: Giri S, Anirvan P, Angadi S, Singh A, Lavekar A. Prevalence and outcome of sarcopenia in non-alcoholic fatty liver disease. World J Gastrointest Pathophysiol 2024; 15(1): 91100
- URL: https://www.wjgnet.com/2150-5330/full/v15/i1/91100.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v15.i1.91100
Nonalcoholic fatty liver disease (NAFLD) includes a spectrum of liver conditions, beginning with mild steatosis and potentially advancing through steatohepatitis, fibrosis, and cirrhosis[1]. Sarcopenia, prevalent in aging populations, is defined by a reduction in muscle strength and/or function, often evidenced by a decrease in muscle mass observed in cross-sectional imaging[2]. Sarcopenic obesity is characterized by the concurrent presence of sarcopenia and increased fat mass, typically measured by body mass index (BMI) or waist circumference[3]. Factors such as hyperammonemia, endotoxemia, and endocrine disturbances, including insulin resistance and decreased testosterone levels, contribute to the increased prevalence of sarcopenia in individuals with liver cirrhosis[4]. Several pathophysiological similarities exist between NAFLD and sarcopenia, including insulin resistance, myostatin and adiponectin dysregulation, hormonal imbalances, chronic inflammation, impaired glucose uptake, and myosteatosis[5,6].
Studies have reported an association of sarcopenia with a higher prevalence of NAFLD and more severe liver damage in individuals with NAFLD. An increased fat mass in patients with NAFLD is associated with a higher incidence of sarcopenic obesity. A meta-analysis of five cross-sectional studies involving 27804 patients identified an increased risk of NAFLD in individuals with sarcopenia[7]. However, data regarding the prevalence of sarcopenia among patients with NAFLD are inconsistent. Moreover, the effects of sarcopenia on the outcomes of patients with NAFLD remain unclear. Thus, this systematic review aimed to analyze the prevalence and impact of sarcopenia in individuals with NAFLD.
The current systematic review was conducted in accordance with the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[8].
We searched for relevant studies in MEDLINE, Embase, and Scopus from the inception of these databases until June 31, 2023, by using the following keywords: (NAFLD OR Fatty liver OR Steatotic liver disease OR MAFLD OR NASH) AND (Sarcopenia OR sarcopenic OR Muscle wasting). The titles and abstracts of the retrieved studies were screened by two independent reviewers, who then assessed the full texts for eligibility before inclusion. Furthermore, the bibliographies of the included studies were reviewed to identify additional relevant studies. Any disagreements between the two inde
Both prospective and retrospective studies that met the following criteria were included in this systematic review: (1) Studies including patients with NAFLD as determined by serology, ultrasonography (USG), transient elastography (TE), or magnetic resonance imaging (MRI); (2) Studies examining the prevalence of sarcopenia as the primary outcome; and (3) Studies evaluating the effect of sarcopenia on the risk of liver fibrosis or other adverse outcomes as secondary outcomes. Editorials, correspondences, case reports, case series, and review articles were excluded. Moreover, studies with insufficient or irrelevant clinical data were excluded.
Two reviewers independently extracted the data, and a third reviewer resolved any disagreements. The extracted infor
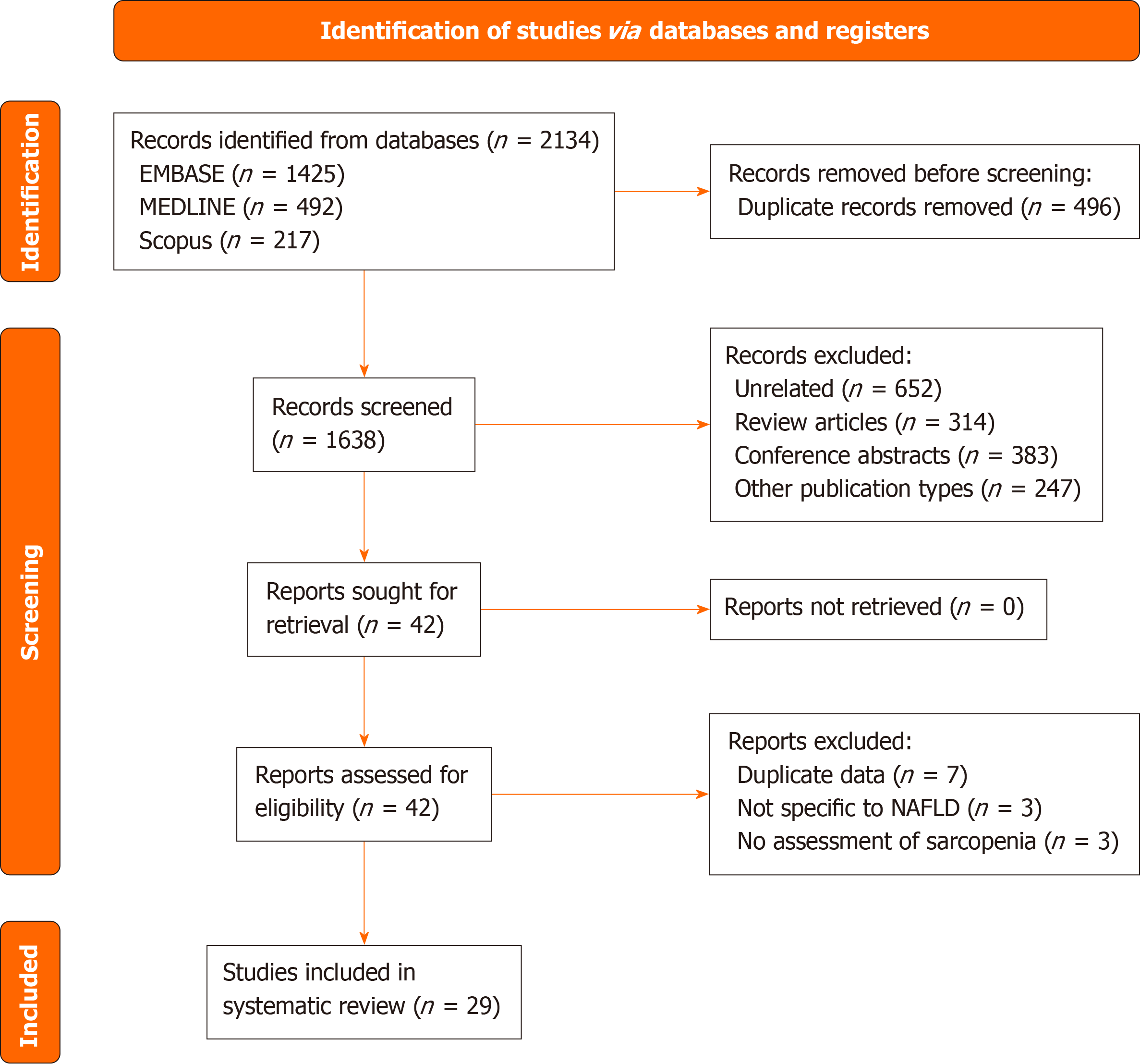
A total of 2134 records were identified using the predefined search strategy, with 29 studies ultimately included in the systematic review. Figure 1 illustrates the PRISMA flowchart detailing the study selection and inclusion process. Table 1 presents the baseline characteristics and outcomes of the included studies. The majority of the studies were from Asia, followed by North America. The mean age of participants in the included studies ranged from 41.9 to 67.8 years, and the proportion of male participants varied from 19.5% to 89.8%. Only three studies included biopsy-proven NAFLD cases[11-13], whereas the remaining studies used noninvasive methods for NAFLD diagnosis. Among the studies using noni
| Ref. | Country, study design | Population and size | Age, in years, male sex, in % | Comorbidities | Definition of NAFLD | Definition and prevalence of sarcopenia | Outcome | Study quality |
| Lee et al[10], 2016 | South Korea, retrospective | Korean National Health and Nutrition Examination Surveys 2008-2011, n = 2761 | 55.8 ± 14.3, 45% | BMI: 25.8 ± 3.1; MS: 81%; DM: 30% | NAFLD liver fat score | DEXA was used for the calculation of SI = ASM/BMI. Sarcopenia was defined using a cut-off point of SI < 0.789 in men and < 0.521 in women; n = 337 (12.2%) | Significant fibrosis was defined as FIB-4 ≥ 2.67. After adjusting for all covariates, a higher value of SI was associated with a lower risk of significant fibrosis with aOR: 0.67 (95%CI: 0.49-0.91) | Fair |
| Koo et al[11], 2017 | South Korea, prospective | Boramae NAFLD registry, n = 240 | 53.3 ± 14.3, 48.7% | BMI: 27.4 ± 3.5; DM: 39.6%; HTN: 40.4%; smoking: 22.5% | ≥ 5% macrovesicular steatosis on liver biopsy | BIA was used to calculate ASM, which was divided by weight = ASM%. ASM% < 29.0 in men or < 22.9 in women was considered as sarcopenia. n = 64 (26.7%) (21/117 in NAFLD and 43/123 in NASH) | Among patients with NAFLD, sarcopenia was associated with a higher risk of NASH (aOR: 2.59; 95%CI: 1.22-5.48). Sarcopenia was also associated with the presence of significant fibrosis (F2-F4) on liver biopsy (aOR: 2.21; 95%CI: 1.10-4.44) | Good |
| Petta et al[12], 2017 | Italy, prospective | Consecutive patients with NAFLD at a single center, n = 225 | 48.3 ± 13.4, 62.7% | BMI: 30.3 ± 5.2; DM: 45.3%; HTN: 32.9%; obesity: 71.1% | ≥ 5% macrovesicular steatosis on liver biopsy | BIA was used to calculate ASM, which was divided by weight × 100 = SMI. Sarcopenia was defined as an SMI ≤ 37 in males and ≤ 28 in females. n = 98 (43.6%) | Sarcopenia was also associated with the presence of advanced fibrosis (F3-F4) on liver biopsy (aOR: 2.36; 95%CI: 1.16-4.77). The prevalence of NASH was higher in the presence of sarcopenia (88.7% vs 76.3% in nonsarcopenic cases, P = 0.01) | Good |
| Kang et al[17], 2019 | South Korea, retrospective | Adults undergoing comprehensive health screening at a single center from 2010-2017, n = 10711 | 47.9 ± 11.6, 52.8% | BMI: 23.9 ± 2.9; MS: 12.5%; DM: 5.9%; HTN: 11.6%; obesity: 34.1% | Abdominal ultrasound1 | BIA was used to calculate ASM, which was divided by weight = ASM/BW%; ASM/BW% < 29.0 in men or < 22.9 in women was considered as sarcopenia; n = 615 (5.7%) | Advanced fibrosis was defined as NFS ≥ 0.676 and FIB-4 ≥ 2.670. Sarcopenia was also associated with the presence of advanced fibrosis (F3-F4) as defined by NFS with aOR: 2.68 (95%CI: 1.28-5.59), but not using FIB-4 (aOR: 1.58, 95%CI: 0.87-2.85) | Fair |
| Wijarnpreecha et al[18], 2019 | United States, retrospective | Analysis of the third National Health and Nutrition Examination Survey (NHANES), conducted from 1988 to 1994, n = 4188 | 45.4 ± 0.42, 50.4% | BMI: 28.9 ± 0.22; HTN: 31.6%; DM: 7.5% | Abdominal ultrasound1 | BIA was used to calculate ASM, which was divided by weight × 100 = SMI. Sarcopenia was defined as an SMI ≤ 37 in males and ≤ 28 in females; n = 2023 (48.3%) | Advanced fibrosis was defined as NFS ≥ 0.676; sarcopenia was significantly associated with advanced fibrosis (aOR: 2.39, 95%CI: 1.50-3.84) | Fair |
| Gan et al[19], 2020 | China, prospective | Lanxi cohort, a community-based prospective cohort with a focus on obesity-related diseases, n = 1088 | 55.2 ± 11.5, 32.9% | BMI: 25.9 ± 2.9; MS: 59.5%; DM: 12.9%; HTN: 48.1% | Abdominal ultrasound1 | DEXA was used for the calculation of SMI = total appendicular lean mass (ALM)/weight. The cut-off points for sarcopenia were 28.64% for men and 24.12% for women; n = 246 (22.6%) | - | Fair |
| Golabi et al[14], 2020 | United State, retrospective | Analysis of the National Health and Nutrition Examination Survey (NHANES), from 1999 to 2004, n = 1351 | 50.7 ± 0.72, 60.0% | BMI: 32.5 ± 0.32; obesity: 60.6%; HTN: 68.4%; MS: 63.9%; DM: 20.7% | Fatty liver index (FLI) ≥ 30 based on age, race/ethnicity, waist circumference, GGT, activity, fasting insulin, and fasting glucose | DEXA was used to calculate SI = ASM/BMI. Sarcopenia was defined using a cut-off point of SI < 0.789 in men and < 0.521 in women. n = 239 (17.7%) | Sarcopenia was an independent predictor of mortality in NAFLD with aHR 1.78 (95%CI: 1.16-2.73) | Fair |
| Hsieh et al[13], 2021 | Taiwan, prospective | Boramae NAFLD cohort, n = 521 | 52.0 ± 15.0, 50.9% | BMI: 27.8 ± 3.8; DM: 39.3%; HTN: 42.4% | ≥ 5% macrovesicular steatosis on liver biopsy | Cross-sectional CT images at L3 was used to calculate SMI; Sarcopenia defined by L3-SMI < 50 cm2/m2 for men and < 39 cm2/m2 for women. n = 122 (23.4%) | Sarcopenia was also associated with the presence of significant fibrosis (F2-F4) on liver biopsy (aOR: 1.72; 95%CI: 1.05-2.84) | Good |
| Kang et al[15], 2020 | South Korea, retrospective | Korean National Health and Nutrition Examination Surveys 2014-2016 with age 35-65 yr, n = 2092 | 45.6 ± 0.22, 42.4% | BMI: 23.8 ± 0.02; DM: 10.7%; HTN: 24.1%; obesity: 33.6% | HIS was calculated based on ALT, AST, BMI, DM, sex, NAFLD defined by HIS > 36 | Hand grip strength was calculated using a dynamometer, and sarcopenia was defined for individuals in the 1st quartile (Q1) of muscle strength | Advanced fibrosis was defined as either a FIB-4 score ≥ 1.30 or a BARD score ≥ 2.00. Sarcopenia was also associated with the presence of advanced fibrosis as defined by BARD with aOR: 1.68 (95%CI: 1.07-2.62), but not using FIB-4 (aOR: 1.35, 95%CI: 0.75-2.45) | Fair |
| Park et al[20], 2020 | South Korea, retrospective | Patients attending annual health examination at a single center, n = 747 | 48.9 ± 10.8, 68.1% | BMI: 24.9 ± 3.1 | Abdominal ultrasound1 | BIA was used to calculate ASM, which was divided by weight × 100 = SMI. ASM/BW% < 29.1 in men or < 23.0 in women was considered as sarcopenia. n = 66 (8.8%) | - | Fair |
| Seo et al[21], 2020 | South Korea, retrospective | Seoul Metabolic Syndrome Cohort, n = 1278 | 55.8 ± 10.8, 53.6% | BMI: 26.5 ± 3.3; DM: 100% | Abdominal ultrasound1 | BIA was used to calculate ASM, which was divided by weight = ASM/BW%. ASM/BW% < 29.0 in men or < 22.9 in women was considered as sarcopenia. n = 528 (41.3%) | - | Fair |
| Kang et al[22], 2021 | South Korea, retrospective | Patients undergoing carotid ultrasound at a single center, n = 683 | 49.1 ± 10.0, 86.1% | BMI: 26.4 ± 2.6; DM: 15.2%; obesity: 67.0%; HTN: 29.1%; MS: 43.6% | Abdominal ultrasound1 | BIA was used to calculate SI = ASM/BMI. Sarcopenia was defined using a cut-off point of SI < 0.789 in men and < 0.521 in women. n = 75 (11.0%) | Sarcopenia was an independent predictor of increased intima-media thickness (OR: 2.26, (95%CI: 1.26-4.04) and carotid plaque (OR: 2.74, 95%CI: 1.30-5.78) | Fair |
| Kim et al[23], 2021 | United States, retrospective | Analysis of the third National Health and Nutrition Examination Survey (NHANES), conducted from 1988 to 1994, n = 3773 | 45.5 ± 0.452, 50.5% | BMI: 29.0 ± 0.232; DM: 12.1%; HTN: 30.9% | Abdominal ultrasound1 | BIA was used to calculate ASM, which was divided by weight × 100 = SMI. Sarcopenia was defined as an SMI ≤ 37 in males and ≤ 28 in females. n = 1822 (48.3%) | Sarcopenia was an independent predictor of mortality in NAFLD with aHR 1.44 (95%CI: 1.16-1.80) | Fair |
| Lee et al[24], 2021 | South Korea, retrospective | Gangnam Severance Hospital Check-up (GSHC) dataset from 2016 to 2019, n = 4168 | 51.2 ± 11.5, 65.5% | BMI: 26.1 ± 3.5 | Abdominal ultrasound1 | n = 1288 (30.9%) | - | Poor |
| Lee et al[25], 2021 | South Korea, retrospective | Korean Genome and Epidemiology Study on Atherosclerosis Risk of Rural Areas in the Korean General Population data, n = 320 | 65.7 ± 7.6, 63.6% | BMI: 26.9 ± 2.9; DM: 67.9%; HTN: 60.5% | Abdominal ultrasound1 | 57 (39.6%), 107 (59.8%), and 148 (63.0%) participants had low muscle mass adjusted for height, BMI, and body weight in the NAFLD group, respectively | Appendicular muscle mass adjusted for body weight only was associated with hepatic fibrosis but not when adjusted for height and BMI | Fair |
| Linge et al[33], 2021 | United Kingdom, retrospective | Participants of United Kingdom Biobank study, aged 40-69 yr at recruitment in 2006-2010, n = 1204 | 62.9 ± 7.4, 53.5% | BMI: 30.1 ± 4.8 | MRI liver PDFF > 5% | Sarcopenia, defined as low hand grip strength [< 16/27 kg (females/males)] and low muscle quantity [MRI threshold of 3.0 and 3.6 L/m2 for thigh FFMV/height2 (females/males)]. n = 19 (1.6%) | - | Fair |
| Wang et al[26], 2021 | China, prospective | Patients attending annual health examination at a single center in 2019, n = 154 | 67.8 ± 9.3, 19.5% | BMI: 24.9 ± 2.9 | Abdominal ultrasound1 | Sarcopenia, defined as low hand grip strength (< 18 kg in women and < 26 kg in men), a gait speed < 0.8 m/s, and DEXA-based ASM/height2 < 5.4 in women and < 7.0 kg/m2 in men. n = 25 (16.2%) | - | Fair |
| Almeida et al[27], 2022 | Brazil, prospective | Consecutive patients with NAFLD at a single center, n = 57 | 52.7 ± 11.3, 24.6% | - | Abdominal ultrasound1 | Probable sarcopenia, defined as low hand grip strength [< 16/27 kg (females/males)]. n = 15 (26.3%) | - | Fair |
| Guo et al[35], 2022 | China, prospective | Patients undergoing health checkup at a single center from 2020-2021, n = 1830 | 47.4 ± 10.5, 80.2% | BMI: 27.1 ± 3.0 | Transient elastography with fat attenuation parameter > 240 dB/m | BIA was used to calculate ASM, which was divided by height × 100 = SMI. SMI gradually decreased in a stepwise manner as the severity of hepatic steatosis increased | LSM values > 7.3 kPa were classified as having liver fibrosis. Participants in the tertile 1 of SMI had significantly higher odds of liver fibrosis (aOR: 3.7, 95%CI: 2.6-5.3) compared to tertile 3 | Good |
| Seo et al[36], 2022 | South Korea, retrospective | Patients undergoing health checkup at a single center from 2017-2019, n = 3198 | 54.2 ± 9.6, 89.8% | BMI: 26.2 ± 2.9; HTN: 40.2%; DM: 20.1% | Transient elastography with controlled attenuation parameter > 248 dB/m | BIA was used to calculate ASM, which was divided by weight × 100 = SMI. ASM/BW% < 29.1 in men or < 23.0 in women was considered as sarcopenia. n = 517 (16.2%) | - | Poor |
| Song et al[37], 2023 | South Korea, retrospective | Patients undergoing health checkup at a single center from 2007-2018, n = 1180 | 53.3 ± 10.3, 71.5% | BMI: 26.7 ± 3.67; DM: 20.7% | Transient elastography with fat attenuation parameter > 260 dB/m | BIA was used to calculate ASM, which was divided by weight × 100 = SMI. ASM/BW% < 30.0 in men or < 26.8 in women was considered as sarcopenia | LSM values ≥ 7.5 kPa (≥ F2) were classified as having liver fibrosis. Sarcopenia was not a predictor of fibrosis in NAFLD with aOR: 3.80 (95%CI: 0.86-16.75) | Good |
| Zhang et al[28], 2022 | China, retrospective | T2DM patients with BMI < 25 kg/m2 were enrolled from a single center from 2017 to 2021, n = 1112 | 53.4 ± 10.7, 57.6% | BMI: 22.6; DM: 100% | Abdominal ultrasound1 | BIA was used to calculate ASM, which was divided by weight × 100 = SMI. ASM/BW% < 32.2 in men or < 25.5 in women was considered as sarcopenia. n = 290 (26.1%) | - | Fair |
| Zhu et al[29], 2023 | China, prospective | Participants of Shanghai Changfeng Study, a community-based prospective cohort study of multiple chronic diseases Jun 2009 to Dec 2012, with age > 45 yr, n = 1305 | 62.6 ± 8.9, 33.1% | BMI: 25.7 ± 3.2 | Fatty liver was diagnosed when liver fat content by ultrasound exceeded the cut-off value of 9.15% | DEXA was used to calculate SI = ASM/height2. The cut-off SI for sarcopenia were 6.88 kg/m2 in male and 5.67 kg/m2 in female. n = 260 (19.9%) | Significant fibrosis was defined as FIB-4 ≥ 2.67. The presence of sarcopenia was associated with increased risk of carotid plaque (aOR: 2.22; 95%CI: 1.23-4.02) and liver fibrosis (aOR: 2.07; 95%CI: 1.24-3.44) | Fair |
| Cho et al[30], 2023 | South Korea, retrospective | Patients with T2DM from the Seoul Metabolic Syndrome Cohort, n = 456 | 55.0 ± 9.4, 46.3% | BMI: 25.7 ± 2.8; DM: 100%; HTN: 36.0% | Abdominal ultrasound1 | BIA was used to calculate ASM, which was divided by weight = ASM/BW%; ASM/BW% < 29.0 in men or < 22.9 in women was considered as sarcopenia. n = 123 (27.0%) | Sarcopenia was an independent predictor carotid plaque progression (OR: 2.02, 95%CI: 1.32-3.08) | Good |
| Choe et al[16], 2023 | South Korea, retrospective | Korean Genome and Epidemiology Study (KoGES) Ansung-Ansan cohort, n = 1442 | 51.7 ± 8.5, 40.0% | BMI: 27.9 ± 2.5; DM: 28.4%; HTN: 34.7%; MS: 69.7% | Hepatic steatosis index (HSI) based on ALT, AST, BMI, DM, sex. NAFLD defined by HSI > 36 | - | Fibrosis was defined as FIB-4 ≥ 1.3 and APRI ≥ 0.5. In the adjusted model, low muscle mass (lowest quartile) did not contribute to progression to hepatic fibrosis (HR: 1.02, 95%CI: 0.85-1.22) | Poor |
| Chun et al[31], 2023 | South Korea, retrospective | Patients undergoing health checkup at a three center from 2014-2020, n = 23889 | 50.0 ± 11.0, 69.5% | BMI: 25.9 ± 3.3; DM: 14.4%; HTN: 37%; obesity: 56.9%; MS: 47.1% | Abdominal ultrasound1 | BIA was used to calculate ASM, which was divided by weight = ASM/BW%; ASM/BW% < 29.0 in men or < 22.9 in women was considered as sarcopenia. n = 3092 (12.9%). Sarcopenia was defined using a cut-off point of ASM/BMI = SI < 0.789 in men and < 0.521 in women. n = 1577 (6.6%) | - | Fair |
| Harring et al[38], 2023 | United States, retrospective | Analysis of the third National Health and Nutrition Examination Survey (NHANES), from 2017 to 2018, n = 1056 | 41.9 ± 0.422, 54.8% | BMI: 33.5 ± 0.372; obesity: 78.7%; DM: 18.1%; HTN: 53.9%; MS: 64.8% | Transient elastography with fat attenuation parameter > 263 dB/m | DEXA was used to calculate SI = ASM/BMI. Sarcopenia was defined using a cut-off point of SI < 0.789 in men and < 0.512 in women. n = 303 (28.7%) | - | Good |
| Lu et al[32], 2023 | China, retrospective | Patients diagnosed with obesity during health checkup at a single center from 2020-2021, n = 476 | 51.0 ± 13.7, 52.7% | BMI: 27.9 ± 3.3; obesity: 100% | Abdominal ultrasound1 | BIA was used to calculate SMI = appendicular skeletal mass/height2. Sarcopenia defined as SMI ≤ 7.0 kg/m2 for males and ≤ 5.7 kg/m2 for females; n = 261 (54.8%) | - | Fair |
| Zhou et al[34], 2023 | China, prospective | Consecutively enrolled subjects who underwent BIA at a single center, between May 2017 and July 2022, n = 1123 | 37.8 ± 10.6, 58.7% | BMI: 28.9 ± 5.1; DM: 17.6% | MRI liver PDFF > 5% | BIA was used to calculate the appendicular skeletal mass (ASM). Sarcopenia was defined as ASM/height2 or ASM/weight or ASM/BMI less than 2 SD. n = 50 (4.4%) | The MAFLD patients with lower quartiles of ASM/W had a higher risk OR for insulin resistance, both in male and female (OR: 2.14, 95%CI: 1.16-3.97), and OR: 4.26, 95%CI: 1.29, 14.02) for Q4 vs Q1 | Fair |
A total of 24 studies reported the prevalence of sarcopenia in NAFLD. The overall prevalence varied significantly, from 1.6% when determined using MRI[33] to 63.0% when assessed using DEXA[24]. Four studies reported a prevalence of less than 10.0%[17,20,33,34], 14 reported a prevalence of 10.0%-30.0%[10,11,13,14,19,22,26-31,35,38], four reported a prevalence of 30.0%-50.0%[12,14,33,38], and two reported a prevalence of more than 50.0%[24,36]. In studies using DEXA, the pre
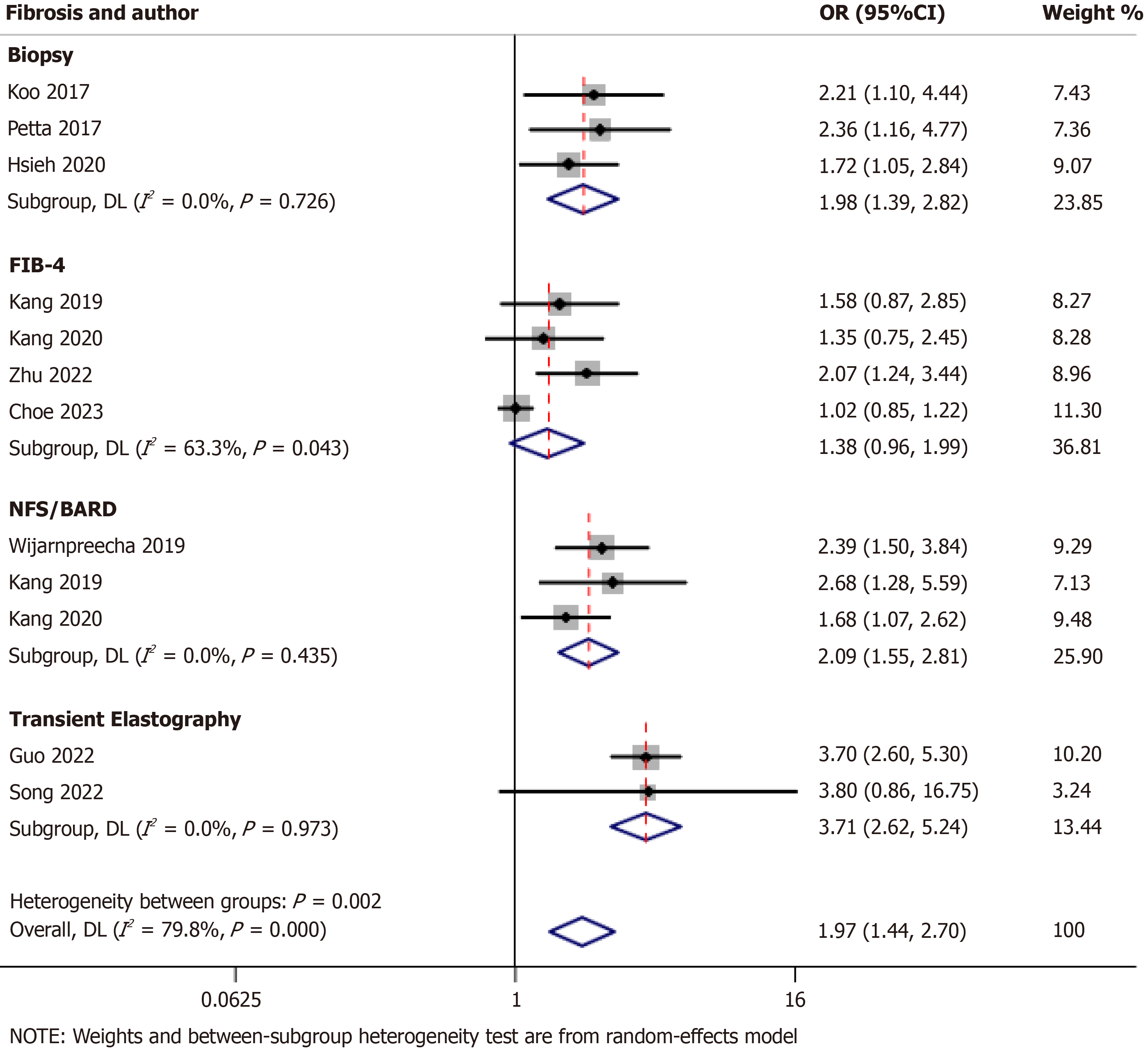
Ten studies examined the correlation between advanced fibrosis and sarcopenia in NAFLD[11-13,18,22,29,35,37]. The combined analysis of these studies revealed that sarcopenia was associated with a higher risk of advanced fibrosis, with an odds ratio (OR) of 1.97 [95% confidence interval (95%CI): 1.44-2.70; I2 = 79.8%]. When considering individual modalities for the assessment of fibrosis, including biopsy, NAFLD fibrosis scores/BMI, the ratio of aspartate aminotransferase to alanine aminotransferase, diabetes (NFS/BARD) scores, and TE, sarcopenia was consistently associated with an increased risk of advanced fibrosis with ORs of 1.98 (95%CI: 1.39-2.82; I2 = 0.0%), 2.09 (95%CI: 1.55-2.81; I2 = 0.0%), and 3.71 (95%CI: 2.62-5.24; I2 = 0.0%), respectively, indicating no heterogeneity (Figure 2). However, when using the fibrosis-4 (FIB-4) score, no association was observed between sarcopenia and advanced fibrosis with an OR of 1.38 (95%CI: 0.96-1.99; I2 = 63.3%), indicating moderate heterogeneity.
Eight studies explored the outcome of NAFLD with sarcopenia in addition to the increased risk of advanced hepatic fibrosis. Koo et al[11] reported a higher risk of nonalcoholic steatohepatitis (NASH) in NAFLD with sarcopenia (aOR: 2.59; 95%CI: 1.22-5.48). Petta et al[12] reported that the prevalence of NASH was higher in the presence of sarcopenia (88.7% vs. 76.3% in nonsarcopenic cases, P = 0.01). Two studies identified sarcopenia as a predictor of mortality in patients with NAFLD, with adjusted hazard ratios (aHRs) of 1.78 (95%CI: 1.16-2.73)[14] and 1.44 (95%CI: 1.16-1.80)[23]. Kang et al[22] reported sarcopenia as an independent predictor of increased intima-media thickness with an OR of 2.26 (95%CI: 1.26-4.04). Furthermore, Kang et al[22] and Zhu et al[29] reported that sarcopenia in NAFLD was associated with a higher risk of carotid plaque, with aORs of 2.74 (95%CI: 1.30-5.78) and 2.22 (95%CI: 1.23-4.02), respectively. However, Cho et al[30] reported higher odds of carotid plaque development with sarcopenia in those without carotid plaque at baseline (OR: 2.02, 95%CI: 1.32-3.08). Finally, Zhou et al[34] reported that NAFLD patients with sarcopenia had a higher risk of insulin resistance in both men and women (OR: 2.14, 95%CI: 1.16-3.97; OR: 4.26, 95%CI: 1.29-14.02).
An increasing number of studies have indicated the association between sarcopenia and NAFLD. However, the exact prevalence of sarcopenia in the NAFLD population remains unclear. This systematic review is the first to summarize the current evidence on the prevalence of sarcopenia in NAFLD patients. Of the 24 studies reporting the prevalence, fourteen, four, and two studies reported prevalence rates of 10%-30%, 30%-50%, and more than 50%, respectively, whereas only four studies demonstrated a prevalence rate of less than 10%. This finding indicates that a considerable number of patients with NAFLD develop sarcopenia. In addition, sarcopenia was associated with an increased risk of advanced fibrosis in NAFLD, with an OR of 1.97 (95%CI: 1.44-2.70). Furthermore, sarcopenia in patients with NAFLD was associated with increased risks of NASH, insulin resistance, carotid plaque, and mortality.
Our systematic review highlighted a considerable variation in the reported prevalence of sarcopenia among patients with NAFLD. This variation is attributed to the diagnostic modality used, from 1.6% using MRI to 63.0% with DEXA. This discrepancy is amplified by using different cutoff values and indices within the same diagnostic modality, such as the normalization of ASM to BMI, weight, or height squared. This substantial variability in sarcopenia prevalence emphasizes the need for standardized diagnostic criteria and measurement techniques for sarcopenia in NAFLD patients. The European Working Group on Sarcopenia in Older People has proposed criteria and cutoffs for the three essential components of sarcopenia: muscle mass, muscle strength, and physical performance[2]. The choice of diagnostic modality and cutoff criteria markedly affects the reported prevalence rates, highlighting the necessity for consensus guidelines to ensure consistency across studies and populations. The variation in prevalence across different studies is primarily influenced by the distribution of muscle mass index in the population and the absolute values of the cutoff points. By contrast, variations in cutoff points for gait speed and grip strength appear to have a weak impact on the prevalence rates of sarcopenia[39].
The results of this systematic review revealed a significant relationship between sarcopenia and an increased risk of advanced fibrosis in NAFLD patients despite noticeable heterogeneity across the included studies. Upon examining the various modalities used for assessing fibrosis (such as biopsy, NFS/BARD scores, TE, and FIB-4 scores), a consistent association with sarcopenia was observed for all modalities except for the FIB-4 score. The absence of an association with the FIB-4 score indicates the necessity of selecting the appropriate fibrosis assessment method when exploring the relationship between sarcopenia and advanced fibrosis in NAFLD. Additionally, a recent study examined the effectiveness of noninvasive tests for estimating fibrosis, particularly in Asian populations. A recent multicentric study highlighted that only TE and TE-based combination tests accurately predicted liver fibrosis, whereas the internationally accepted thresholds for other NITs exhibited high false-negative rates[40].
Our systematic review sheds light on the extensive range of outcomes associated with sarcopenia in NAFLD patients. Key findings included an increased risk of NASH and a higher incidence of NASH in those with sarcopenia. Additionally, our meta-analysis revealed the predictive value of sarcopenia for mortality in NAFLD, as demonstrated by two studies with aHR of 1.78 (95%CI: 1.16-2.73) and 1.44 (95%CI: 1.16-1.80[14,23]). Sarcopenia in NAFLD was also associated with cardiovascular risk factors, such as increased intima-media thickness and a higher likelihood of carotid plaque formation. Moreover, sarcopenia was associated with a higher prevalence of insulin resistance, a key player in NAFLD pathogenesis. The relationship between sarcopenia and cardiovascular risks is particularly significant, con
The strengths of our systematic review include acknowledging the significant heterogeneity in sarcopenia prevalence reports among NAFLD patients and emphasizing the necessity for standardized guidelines in this area. In addition, we examined non-liver-related events in NAFLD patients and their correlation with sarcopenia. Variability in sarcopenia and liver fibrosis assessment methods contributes to the diverse results observed in our review. The inclusion of studies with varied designs and the demographic differences among patient populations could also affect the observed prevalence of sarcopenia and its association with NAFLD outcomes. Although our review explores the association of sarcopenia with mortality and cardiovascular risks, some specific outcomes, such as carotid plaque risk and progression, were only addressed in a limited number of studies, affecting the conclusiveness of these findings. For instance, Zhang et al[28] noted a higher sarcopenia prevalence in lean versus non-lean NAFLD patients, a detail we could not further analyze due to data limitations. Moreover, the review’s focus on studies primarily from Asian populations, especially South Korea, may limit the generalizability of the findings to Western populations.
This systematic review highlights the multifaceted impact of sarcopenia on patients with NAFLD, extending beyond liver-related issues to include cardiovascular risks, mortality, and metabolic complications. The observed variations in prevalence and associations indicate the urgent need for standardized diagnostic criteria and measurement techniques. Our review offers crucial insights into the clinical implications of sarcopenia within the NAFLD context, potentially guiding future research and clinical practice.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Moriyama K, Japan S-Editor: Chen YL L-Editor: A P-Editor: Zhao YQ
| 1. | Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537-2564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1300] [Cited by in RCA: 1197] [Article Influence: 299.3] [Reference Citation Analysis (36)] |
| 2. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 7793] [Article Influence: 1298.8] [Reference Citation Analysis (1)] |
| 3. | Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, Bauer JM, Boirie Y, Cruz-Jentoft AJ, Dicker D, Frara S, Frühbeck G, Genton L, Gepner Y, Giustina A, Gonzalez MC, Han HS, Heymsfield SB, Higashiguchi T, Laviano A, Lenzi A, Nyulasi I, Parrinello E, Poggiogalle E, Prado CM, Salvador J, Rolland Y, Santini F, Serlie MJ, Shi H, Sieber CC, Siervo M, Vettor R, Villareal DT, Volkert D, Yu J, Zamboni M, Barazzoni R. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes Facts. 2022;15:321-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 404] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 4. | Dhaliwal A, Armstrong MJ. Sarcopenia in cirrhosis: A practical overview. Clin Med (Lond). 2020;20:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Li AA, Kim D, Ahmed A. Association of Sarcopenia and NAFLD: An Overview. Clin Liver Dis (Hoboken). 2020;16:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology. 2017;66:2055-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 7. | Wijarnpreecha K, Panjawatanan P, Thongprayoon C, Jaruvongvanich V, Ungprasert P. Sarcopenia and risk of nonalcoholic fatty liver disease: A meta-analysis. Saudi J Gastroenterol. 2018;24:12-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40488] [Article Influence: 10122.0] [Reference Citation Analysis (2)] |
| 9. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12656] [Article Influence: 843.7] [Reference Citation Analysis (0)] |
| 10. | Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, Lee BW, Kang ES, Cha BS, Han KH. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011). Hepatology. 2016;63:776-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 11. | Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, Lee KL, Kim W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 340] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 12. | Petta S, Ciminnisi S, Di Marco V, Cabibi D, Cammà C, Licata A, Marchesini G, Craxì A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 13. | Hsieh YC, Joo SK, Koo BK, Lin HC, Kim W. Muscle alterations are independently associated with significant fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2021;41:494-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Golabi P, Gerber L, Paik JM, Deshpande R, de Avila L, Younossi ZM. Contribution of sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Rep. 2020;2:100171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Kang S, Moon MK, Kim W, Koo BK. Association between muscle strength and advanced fibrosis in non-alcoholic fatty liver disease: a Korean nationwide survey. J Cachexia Sarcopenia Muscle. 2020;11:1232-1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Choe HJ, Lee H, Lee D, Kwak SH, Koo BK. Different effects of low muscle mass on the risk of non-alcoholic fatty liver disease and hepatic fibrosis in a prospective cohort. J Cachexia Sarcopenia Muscle. 2023;14:260-269. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Kang MK, Park JG, Lee HJ, Kim MC. Association of low skeletal muscle mass with advanced liver fibrosis in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2019;34:1633-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Wijarnpreecha K, Kim D, Raymond P, Scribani M, Ahmed A. Associations between sarcopenia and nonalcoholic fatty liver disease and advanced fibrosis in the USA. Eur J Gastroenterol Hepatol. 2019;31:1121-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Gan D, Wang L, Jia M, Ru Y, Ma Y, Zheng W, Zhao X, Yang F, Wang T, Mu Y, Zhu S. Low muscle mass and low muscle strength associate with nonalcoholic fatty liver disease. Clin Nutr. 2020;39:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Park H, Jun DW, Park HK, Park KY, Kim M, Hwang HS. A Critical Appraisal of the Definition of Sarcopenia in Patients with Non-Alcoholic Fatty Liver Disease: Pitfall of Adjusted Muscle Mass by Body Weight. Life (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Seo DH, Lee YH, Park SW, Choi YJ, Huh BW, Lee E, Huh KB, Kim SH, Cha BS. Sarcopenia is associated with non-alcoholic fatty liver disease in men with type 2 diabetes. Diabetes Metab. 2020;46:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Kang MK, Park JG. Low Skeletal Muscle Mass Is a Risk Factor for Subclinical Atherosclerosis in Patients with Nonalcoholic Fatty Liver Disease. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Kim D, Wijarnpreecha K, Sandhu KK, Cholankeril G, Ahmed A. Sarcopenia in nonalcoholic fatty liver disease and all-cause and cause-specific mortality in the United States. Liver Int. 2021;41:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Lee HJ, Chang JS, Ahn JH, Kim MY, Park KS, Ahn YS, Koh SB. Association Between Low Muscle Mass and Non-alcoholic Fatty Liver Disease Diagnosed Using Ultrasonography, Magnetic Resonance Imaging Derived Proton Density Fat Fraction, and Comprehensive NAFLD Score in Korea. J Prev Med Public Health. 2021;54:412-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 25. | Lee JH, Lee HS, Lee BK, Kwon YJ, Lee JW. Relationship between Muscle Mass and Non-Alcoholic Fatty Liver Disease. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Wang YM, Zhu KF, Zhou WJ, Zhang Q, Deng DF, Yang YC, Lu WW, Xu J, Yang YM. Sarcopenia is associated with the presence of nonalcoholic fatty liver disease in Zhejiang Province, China: a cross-sectional observational study. BMC Geriatr. 2021;21:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Almeida NS, Rocha R, de Souza CA, da Cruz ACS, Ribeiro BDR, Vieira LV, Daltro C, Silva R, Sarno M, Cotrim HP. Prevalence of sarcopenia using different methods in patients with non-alcoholic fatty liver disease. World J Hepatol. 2022;14:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 28. | Zhang X, He Z, Si Q, Hu X, Yang L, Gu X, Du L, Wang L, Pan L, Li Y, Li J, Yang B. The Association of Sarcopenia and Visceral Obesity with Lean Nonalcoholic Fatty Liver Disease in Chinese Patients with Type 2 Diabetes Mellitus. J Diabetes Res. 2022;2022:2229139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Zhu X, Huang Q, Ma S, Chen L, Wu Q, Wu L, Ma H, Li X, Li Q, Aleteng Q, Hu Y, He W, Gao J, Lin H, Tang H, Gao X, Xia M. Presence of sarcopenia identifies a special group of lean NAFLD in middle-aged and older people. Hepatol Int. 2023;17:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Cho Y, Park HS, Huh BW, Lee YH, Seo SH, Seo DH, Ahn SH, Hong S, Kim SH. Non-Alcoholic Fatty Liver Disease with Sarcopenia and Carotid Plaque Progression Risk in Patients with Type 2 Diabetes Mellitus. Diabetes Metab J. 2023;47:232-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Chun HS, Lee M, Lee HA, Lee S, Kim S, Jung YJ, Lee C, Kim H, Kim HY, Yoo K, Kim TH, Ahn SH, Kim SU. Risk Stratification for Sarcopenic Obesity in Subjects With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2023;21:2298-2307.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Lu Y, Xia Q, Wu L, Xie Z. Gender difference in association between low muscle mass and risk of non-alcoholic fatty liver disease among Chinese adults with visceral obesity. Front Nutr. 2023;10:1026054. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Linge J, Ekstedt M, Dahlqvist Leinhard O. Adverse muscle composition is linked to poor functional performance and metabolic comorbidities in NAFLD. JHEP Rep. 2021;3:100197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 34. | Zhou T, Ye J, Lin Y, Wang W, Feng S, Zhuo S, Zhong B. Impact of skeletal muscle mass evaluating methods on severity of metabolic associated fatty liver disease in non-elderly adults. Br J Nutr. 2023;130:1373-1384. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Guo W, Zhao X, Miao M, Liang X, Li X, Qin P, Lu J, Zhu W, Wu J, Zhu C, Xu N, Zhang Q. Association Between Skeletal Muscle Mass and Severity of Steatosis and Fibrosis in Non-alcoholic Fatty Liver Disease. Front Nutr. 2022;9:883015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Seo JY, Cho EJ, Kim MJ, Kwak MS, Yang JI, Chung SJ, Yim JY, Yoon JW, Chung GE. The relationship between metabolic dysfunction-associated fatty liver disease and low muscle mass in an asymptomatic Korean population. J Cachexia Sarcopenia Muscle. 2022;13:2953-2960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Song W, Yoo SH, Jang J, Baik SJ, Lee BK, Lee HW, Park JS. Association between Sarcopenic Obesity Status and Nonalcoholic Fatty Liver Disease and Fibrosis. Gut Liver. 2023;17:130-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 38. | Harring M, Golabi P, Paik JM, Shah D, Racila A, Cable R, Srishord M, Younossi ZM. Sarcopenia Among Patients With Nonalcoholic Fatty Liver Disease (NAFLD) Is Associated With Advanced Fibrosis. Clin Gastroenterol Hepatol. 2023;21:2876-2888.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 39. | Masanés F, Rojano I Luque X, Salvà A, Serra-Rexach JA, Artaza I, Formiga F, Cuesta F, López Soto A, Ruiz D, Cruz-Jentoft AJ. Cut-off Points for Muscle Mass - Not Grip Strength or Gait Speed - Determine Variations in Sarcopenia Prevalence. J Nutr Health Aging. 2017;21:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 40. | Arora U, Goyal RM, Teh KKJ, Pei Y, Goh GBB, Lin S, Kumar R, Biswas S, Vaishnav M, Swaroop S, Pathak P, Sheikh S, Bharadiya V, Elhence A, Gamanagatti S, Yadav R, Das P, Aggarwal S, Choudhary N, Anirvan P, Singh SP, De A, Duseja A, Shalimar. Poor Performance of Non-invasive Tests for Advanced Fibrosis in Nonalcoholic Fatty Liver Disease: A Multicentric Asian Study. Dig Dis Sci. 2023;68:4485-4498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep. 2016;6:33386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |