Published online Apr 22, 2024. doi: 10.4291/wjgp.v15.i1.90893
Peer-review started: December 16, 2023
First decision: January 31, 2024
Revised: February 13, 2024
Accepted: March 26, 2024
Article in press: March 26, 2024
Published online: April 22, 2024
Processing time: 124 Days and 10.7 Hours
Alpha-fetoprotein (AFP), a commonly used biomarker for hepatocellular car
To evaluate the diagnostic performance of des-gamma-carboxy-prothrombin (DCP) alone and in combination with AFP.
In this study, 202 patients with radiologically proven HCC were enrolled, and their DCP and AFP levels were evaluated for their diagnostic performance.
The mean age of the enrolled patients was 58.5 years; 72.0% were male. DCP was elevated in 86.6% (n = 175) of all patients, 100.0% (n = 74) of patients with portal vein thrombus, and 87.4% (n = 111) of patients with multicentric HCC. AFP was elevated in 64.3% (n = 130) of all the patients, 74% (n = 55) of the patients with portal vein thrombus, and 71.6% (n = 91) of the patients with multicentric HCC (P = 0.030, 0.001, and 0.015, respectively). In tumors less than 2 cm in size (n = 46), DCP was increased in 32 (69.5%) patients, and AFP was increased in 25 (54.3%) patients (P = 0.801). There was good pairing between DCP and AFP for HCCs of 2 cm size or larger (P < 0.001); however, the pairing among tumors < 2 cm size was not significant (P = 0.210). In 69 of the patients (34.1%), only one of the tumor markers was positive; DCP was elevated alone in 57/202 (28.2%) of all patients, and AFP alone was elevated in 12/202 (5.9%) of the patients. The areas under receiver operating characteristic curves (AUROC) for tumors > 2 cm was 0.74 for DCP and 0.59 for AFP; combining both markers resulted in an AUROC of 0.73. For tumors < 2 cm, the AUROC was 0.25 for DCP and 0.40 for AFP.
DCP, as an individual marker, had a better diagnostic performance in many cases of HCC. Hence, DCP may replace AFP as the primary HCC biomarker.
Core Tip: In this prospective study, the performance of des-gamma-carboxy prothrombin (DCP) relative to alpha-fetoprotein (AFP) was assessed in 202 patients diagnosed with hepatocellular carcinoma (HCC). DCP, when used as a standalone marker, exhibited superior diagnostic performance compared to AFP. Combining both tumor markers increased the overall detection rate of HCC, particularly in tumors less than 2 cm in length. Nevertheless, it is recommended that, if a single tumor marker is used, DCP is preferred. The role of DCP as a screening biomarker should be incorporated into the HCC guidelines.
- Citation: Qadeer MA, Abbas Z, Amjad S, Shahid B, Altaf A, Siyal M. Des-gamma-carboxy prothrombin and alpha-fetoprotein levels as biomarkers for hepatocellular carcinoma and their correlation with radiological characteristics. World J Gastrointest Pathophysiol 2024; 15(1): 90893
- URL: https://www.wjgnet.com/2150-5330/full/v15/i1/90893.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v15.i1.90893
Hepatocellular carcinoma (HCC), the predominant liver malignancy, is the sixth most commonly diagnosed cancer[1]. Radiological modalities have been at the forefront of screening and diagnosing HCC, but tumor markers have also contributed to its early detection[2]. Early detection of HCC not only dictates treatment modality but also influences expected survival[3]. Tumor markers such as alpha-fetoprotein (AFP) and the protein induced by vitamin K absence-II (PIVKA-II) also known as des-gamma-carboxy-prothrombin (DCP), if elevated beyond certain limits, can influence the HCC recurrence rate after liver transplantation[4].
Various studies have noted the inadequacy of AFP for screening for HCC, with almost 40% of tumors flagged as non-AFP-producing[5]. PIVKA II/DCP, an abnormal prothrombin precursor, has also been described as a potential screening tool for liver cancer[6]. A large body of evidence supports that it has better sensitivity than AFP, with reported sensitivities reaching up to 84%[7-9]. DCP has been shown to be effective not only for detecting HCC but also for predicting its radiological and histological characteristics[9]. However, despite this supportive literature, conflicting data have also been published that have limited the use of DCP in clinical practice. Hence, we evaluated the diagnostic performance of DCP alone and in combination with AFP and analyzed its correlation with radiographic parameters such as size, lobe involvement, and vascular invasion. The diagnostic performance of DCP for AFP-negative HCC was also analyzed.
A total of 202 patients with radiologically confirmed HCC were included in this prospective study at Dr. Ziauddin University Hospital from January 2019 to March 2022. This study was approved by Dr. Ziauddin University’s ethical review board. Informed consent was obtained from all the participants following the ethical standards of the 1964 Helsinki Declaration.
HCC was diagnosed based on the Liver Imaging Reporting and Data System v2017. Liver lesions falling within the LIRAD-V were considered acceptable for inclusion in this study. Patients aged less than 18 years with a history of HCC treatment, use of vitamin K antagonists, or obstructive jaundice were excluded.
The ARCHITECT PIVKA-II assay3C10 (Abbott Laboratories, IL, United States) using chemiluminescent technology was used for the quantification of PIVKA-II. DCP was considered normal if the value fell below 46 mAU/mL. The AFP concentration was analyzed using an ARCHITECT AFP 3P36 (Abbott Laboratories, IL, United States) kit, which uses a two-step immunoassay for quantitative measurement. AFP was considered normal if the value was less than 8.78 ng/mL.
SPSS version 26 was used for the statistical analysis. For all dichotomous variables, the data are summarized as per
A total of 202 HCC patients with a mean age of 58.5 years ± 10.3 years were enrolled. Seventy-two percent (n = 146) of the enrolled patients were male. The main causes of HCC were hepatitis C virus (HCV) in 51.0% (n = 103) and hepatitis B virus (HBV) in 25% (n = 52), while non-alcoholic fatty liver disease (NAFLD) was diagnosed in 13.9% (n = 28) of patients. A decompensated CLD was found in 77% (n = 157) of the patients. A total of 36.6% (n = 74) of the included patients had portal vein thrombosis. A total of 22.8% (n = 46) of the HCCs were less than 2 cm in length. HCC was classified as multicentric in 62.9% (n = 127) of patients. Satellite lesions were identified in 15.3% (n = 31) of the patients. A total of 63.9% (n = 129) of the HCCs involved one lobe, while 36.1% (n = 73) had bilobar involvement (Table 1).
| Variables | Values |
| Age (yr) | 58.5 ± 10.3 |
| Gender: Males | 146 (72.3) |
| Etiology | |
| HCV | 103 (51.0) |
| HBV | 52 (25.7) |
| Alcohol | 8 (4.0) |
| NAFLD | 28 (13.9) |
| Autoimmune liver disease | 7 (3.5) |
| Cryptogenic | 9 (4.5) |
| Lab parameters | |
| Hemoglobin (g/dL) | 11.3 ± 2.1 |
| White dell count (× 109/L) | 8.3 ± 5.5 |
| Platelets (× 109/L) | 148.0 ± 102.0 |
| Total bilirubin (mg/dL) | 3.3 ± 5.6 |
| Alanine aminotransferase (IU/L) | 63.9 ± 51.4 |
| Aspartate aminotransferase (IU/L) | 113.0 ± 206.0 |
| Alkaline phosphatase (IU/L) | 203.0 ± 227.0 |
| International normalization ratio | 1.3 ± 0.4 |
| Tumor parameters | |
| HCC size 2 cm or more | 156 (77.2) |
| HCC less than 2 cm | 46 (22.8) |
| Portal vein thrombus | 74 (36.6) |
| Unilobed | 129 (63.9) |
| Bilobed | 73 (36.1) |
| Satellite lesions | 31 (15.3) |
| Multicentric tumor | 127 (62.9) |
| Des-gamma carboxyprothrombin (mAU/L) | 669.4 (16.7-300000.0) |
| Alpha-fetoprotein (ng/mL) | 32 (1-20000) |
A total of 86.6% (n = 175) of the total HCC patients enrolled had elevated DCP levels (> 45 mAU/L), while 64.3% (n = 130) of the total patients had elevated AFP levels (> 10 ng/mL; P = 0.03). For small HCCs, less than 2 cm in size (n = 46), the DCP was increased in 69.5% (n = 32) and the AFP was increased in 54.3% (n = 25; P = 0.801). Among a total of 127 patients with multicentric HCC, 87.4% (n = 111) had increased DCP, while 71.6% (n = 91) had increased AFP (P = 0.015). Radiographic evidence of satellite lesions was observed in 31 patients, of which DCP and AFP were elevated in 87% (n = 27) and 54.8% (n = 17; P = 0.835), respectively. Thrombi in the portal vein were observed in 74 patients; all had increased DCP, and 74.3% (n = 55) had increased AFP (P < 0.001).
In patients with HCC caused by hepatitis C (n = 103), the DCP concentration increased by 92.2% (n = 95), while the AFP concentration increased by 66.9% (n = 69; P = 0.009). Of the 52 HCC patients with underlying hepatitis B etiology, 76.9% (n = 40) had a positive DCP result, and 67.3% (n = 35) had a positive AFP result (P = 0.095). Among the 28 patients with NAFLD and HCC, DCP was elevated in 82.1% (n = 23), and AFP was elevated in 57.1% (n = 16; P = 0.887).
Among the 202 patients, 58.4% had elevated both tumor marker levels (n = 118). In 34.1% (n = 69) of the patients, one of the tumor markers was positive, and in 7.4% of the patients, both tumor markers were negative (n = 15). In the group in which one of the tumor biomarker markers was positive, DCP alone was elevated in 57 (28.2% of all patients), whereas AFP alone was elevated in 12 (5.9%) patients. There was a strong pairing between DCP and AFP levels for HCCs of all sizes (P < 0.001) and for HCCs of 2 cm or larger (P < 0.001), but the pairing was weaker for smaller HCCs (P = 0.210).
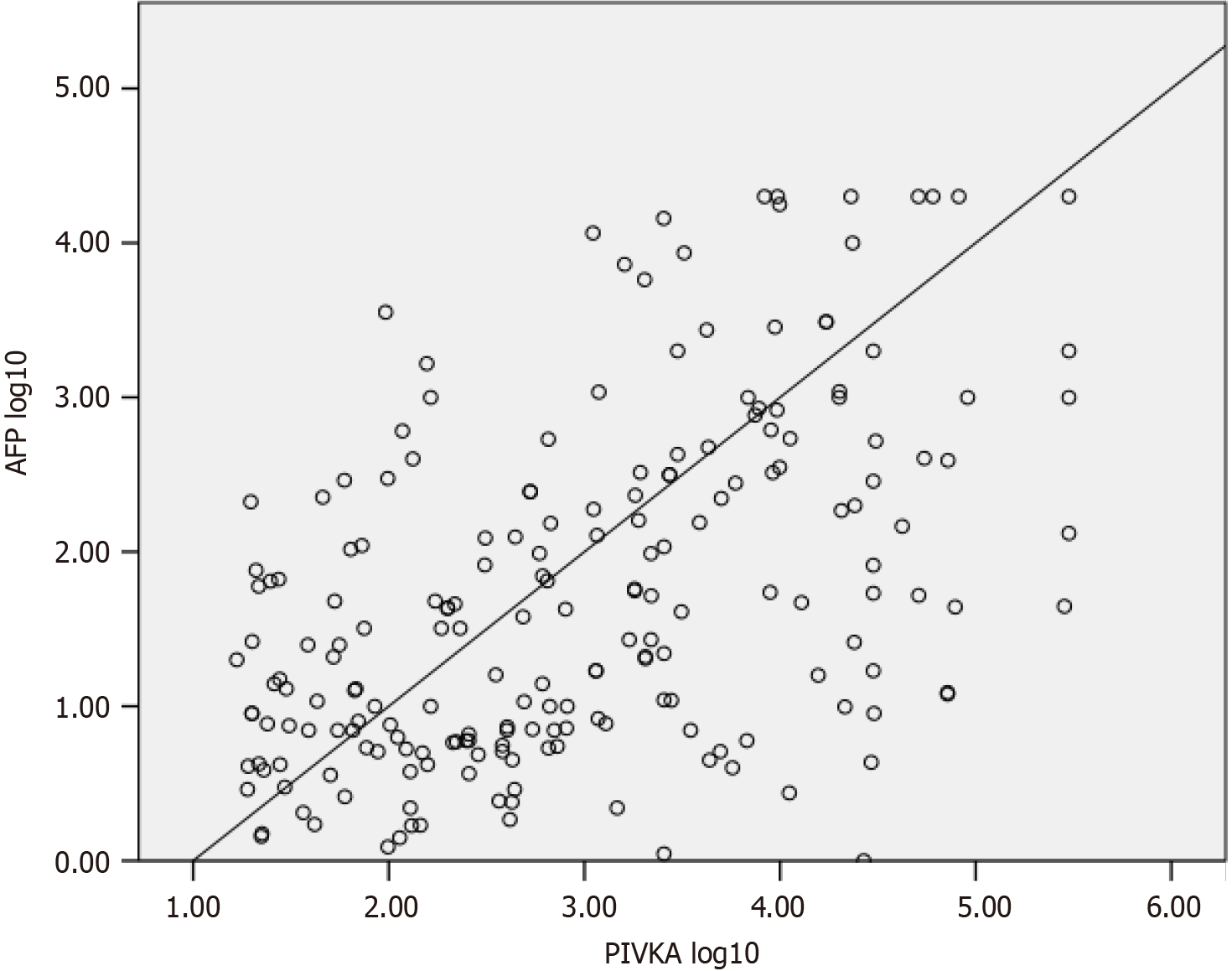
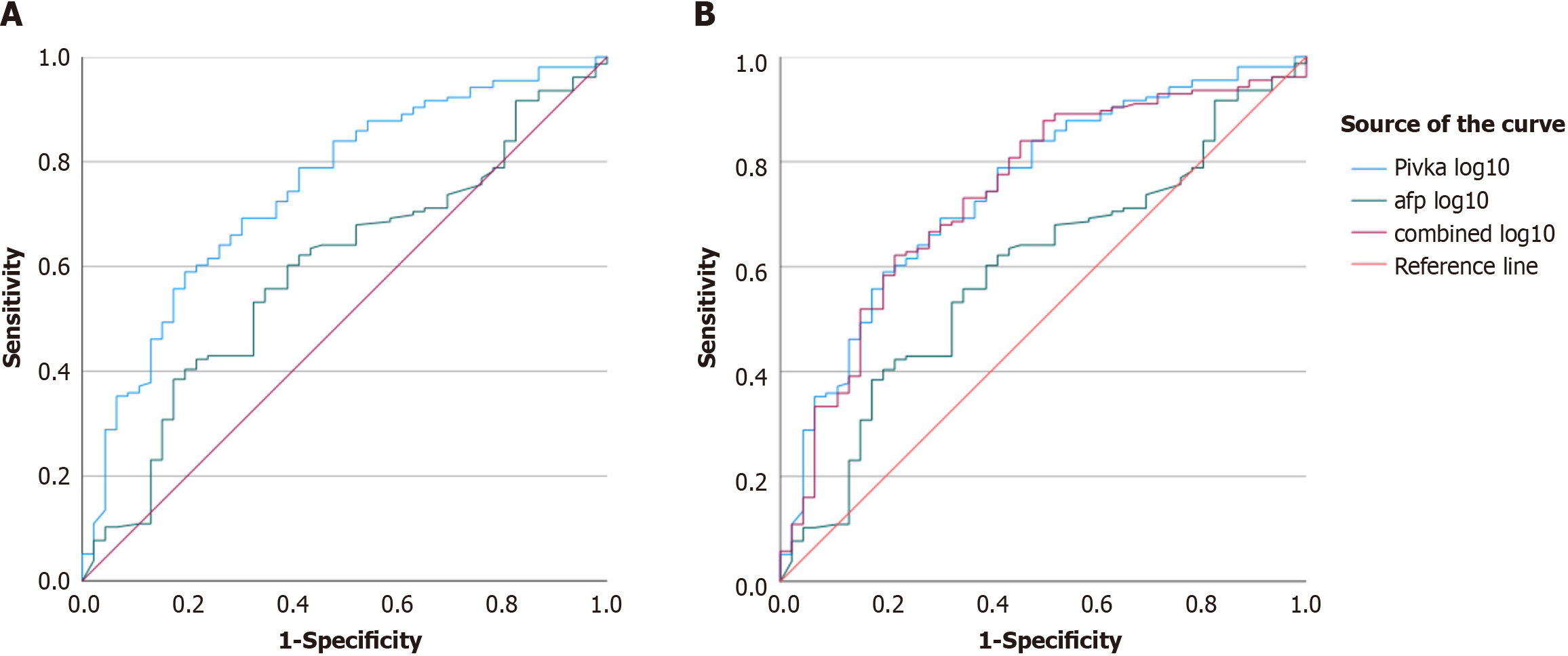
There was a correlation between the DCP and AFP according to Spearman’s correlation test (P < 0.001; Figure 1). ROC plots were drawn to analyze the magnitude of the increase in DCP and AFP levels. For tumors larger than 2 cm in size, the log10 values of DCP exceeded the log10 values of AFP, with areas under the curve of 0.74 and 0.59, respectively (Figure 2). Combining the values of two markers to detect HCC did not improve diagnostic ability, with an AUROC of 0.739. For tumors less than 2 cm in length, the area under the curve for the log value of DCP was 0.250 vs 0.409 for AFP. Therefore, DCP elevation may be modest in smaller localized tumors, although it crossed the positivity threshold in more patients than did AFP elevation.
HCC, a leading liver cancer, not only has a considerable mortality rate but also imposes an enormous economic burden[1,10]. The false-negative rate of AFP, the most widely used tumor marker for HCC, is 30%-40%, motivating researchers to discover a more potent tumor marker with better diagnostic performance[11-13].
In this study, we examined the performance of DCP compared to AFP and correlated the values with radiological features. Our study showed that DCP performed better as a single marker than AFP for detecting HCC, but the com
Consistent with previous data from Pakistan, HCV appears to be a major cause of HCC development[16]. A subgroup analysis showed that DCP performed better in the HCV group. Similar findings were made in a Chinese study by Liu et al[17]. A statistically significant difference was not found between the two tumor markers in the HBV group in our study, possibly due to the small sample size, but DCP was still able to outcompete AFP in terms of diagnostic performance because of its detectability in a larger number of HCC patients. Several studies conducted to date on patients with HBV have shown that DCP alone and in combination with AFP yield better results than AFP alone[7,18].
It has been reported that DCP, when elevated, acts as a predictor of microvascular invasion, even in the absence of radiological evidence[9,19]. Although we did not assess microvascular invasion histologically, we did evaluate the diagnostic ability of these two markers for tumor portal vein thrombosis (PVT). Interestingly, our findings showed that DCP was elevated in all subjects with portal vein thrombus. Similar results were reported by Xu et al[20]. In their study, all 65 participants with PVT had elevated DCP levels.
Since patients with small HCCs (< 2 cm) have a good prognosis, it is important to detect them early in the disease course[3]. Interestingly, although a greater percentage of small HCC lesions exceeded the positivity threshold for DCP, the log values of AFP were much greater. The McNemar test did not reveal good agreement between the two markers, underscoring the value of testing both tumor markers in patients with small HCC. Data from other studies also have similar conclusions regarding sensitivity[21].
The GALAD scoring system, which consists of these two tumor markers in addition to sex, age, and AFP-L3, has been proposed and validated for determining the risk of HCC[22,23]. We did not test for AFP-L3. Recent studies have shown that sex, age, AFP, and DCP combination, the “GAAD” score, can be used to predict the presence of HCC effectively when the value is greater than 2.57[24]. AFP and DCP (PIVKA II) assays were performed on the Elecsys platform with an AFP cutoff of 20 ng/mL and a PIVKA II cutoff of 28.4 ng/mL. We used a cutoff AFP of 10 ng/mL to increase the sen
This study has several notable strengths that are worth acknowledging. First, our research, which focused on the effectiveness of DCP and AFP as biomarkers for HCC, is relevant and significant, given the increasing incidence of this cancer globally. Second, we employed rigorous statistical analyses, such as McNemar’s test and AUROC analysis, to evaluate the correlation between DCP and AFP levels in patients with HCC. Third, the study illustrates the complementarity of DCP and AFP as biomarkers for diagnosing HCC in patients with a tumor diameter of less than 2 cm. Fourth, the study contributes to the mounting evidence supporting the use of DCP and AFP as biomarkers for HCC, which could result in better patient outcomes through earlier detection and treatment. Finally, the study offers valuable insights into the potential use of DCP as a biomarker for patients with HCC with portal vein thrombosis, which may guide future research in this field.
The current study has some limitations that must be considered when interpreting the results. First, the sample size was relatively small, and the study was conducted in a single center, which may limit the generalizability of the findings to other populations. Second, the cross-sectional design of the study makes it difficult to establish a causal relationship between DCP and AFP levels and the development of HCC. Additionally, the lack of a control group limits the ability to compare the results to individuals without HCC or other liver diseases. Finally, due to the small sample size, there was limited statistical power to obtain statistically significant differences in DCP and AFP levels for tumors smaller than 2 cm, which may limit the generalizability of the results to individuals with early-stage HCC. Overall, these limitations must be taken into consideration when interpreting the findings of this study.
According to the results of our study, DCP was found to be a better biomarker than AFP for HCC detection, especially in patients with portal vein thrombosis. DCP, as an individual marker, performed better in many categories of HCC. Hence, DCP may replace AFP as the primary HCC biomarker. The findings also suggest that DCP and AFP may have complementary roles in the diagnosis of small HCC, and the combination of both markers could be considered for early de
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nasrallah O, Syria S-Editor: Chen YL L-Editor: A P-Editor: Zhao YQ
| 1. | Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2020. [cited 10 July 2023]. Available from: https://gco.iarc.fr/today. |
| 2. | Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA, Mendiratta-Lala M, Brown DB, Rilling WS, Goyal L, Wei AC, Taddei TH. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 736] [Article Influence: 368.0] [Reference Citation Analysis (23)] |
| 3. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2593] [Article Influence: 864.3] [Reference Citation Analysis (59)] |
| 4. | Kim SH, Moon DB, Kim WJ, Kang WH, Kwon JH, Jwa EK, Cho HD, Ha SM, Chung YK, Lee SG. Preoperative prognostic values of α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) in patients with hepatocellular carcinoma for living donor liver transplantation. Hepatobiliary Surg Nutr. 2016;5:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (25)] |
| 5. | Chi X, Jiang L, Yuan Y, Huang X, Yang X, Hochwald S, Liu J, Huang H. A comparison of clinical pathologic characteristics between alpha-fetoprotein negative and positive hepatocellular carcinoma patients from Eastern and Southern China. BMC Gastroenterol. 2022;22:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (23)] |
| 6. | Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 421] [Article Influence: 10.3] [Reference Citation Analysis (16)] |
| 7. | Seo SI, Kim HS, Kim WJ, Shin WG, Kim DJ, Kim KH, Jang MK, Lee JH, Kim JS, Kim HY, Lee MS, Park CK. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2015;21:3928-3935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (16)] |
| 8. | Zakhary NI, Khodeer SM, Shafik HE, Abdel Malak CA. Impact of PIVKA-II in diagnosis of hepatocellular carcinoma. J Adv Res. 2013;4:539-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (23)] |
| 9. | Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, Bedossa P, Paradis V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (4)] |
| 10. | Likhitsup A, Parikh ND. Economic Implications of Hepatocellular Carcinoma Surveillance and Treatment: A Guide for Clinicians. Pharmacoeconomics. 2020;38:5-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (21)] |
| 11. | Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 515] [Article Influence: 21.5] [Reference Citation Analysis (4)] |
| 12. | Nikolova D. Alpha Fetoprotein and Hepatocellular Carcinoma: An Opinion. BJSTR. 2019;12. [DOI] [Full Text] |
| 13. | Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 295] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 14. | Xing H, Zheng YJ, Han J, Zhang H, Li ZL, Lau WY, Shen F, Yang T. Protein induced by vitamin K absence or antagonist-II versus alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: A systematic review with meta-analysis. Hepatobiliary Pancreat Dis Int. 2018;17:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Hafeez Bhatti AB, Dar FS, Waheed A, Shafique K, Sultan F, Shah NH. Hepatocellular Carcinoma in Pakistan: National Trends and Global Perspective. Gastroenterol Res Pract. 2016;2016:5942306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Parkash O, Hamid S. Next big threat for Pakistan Hepatocellular Carcinoma (HCC). J Pak Med Assoc. 2016;66:735-739. [PubMed] |
| 17. | Liu S, Sun L, Yao L, Zhu H, Diao Y, Wang M, Xing H, Lau WY, Guan M, Pawlik TM, Shen F, Xu M, Tong X, Yang T. Diagnostic Performance of AFP, AFP-L3, or PIVKA-II for Hepatitis C Virus-Associated Hepatocellular Carcinoma: A Multicenter Analysis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Perne MG, Sitar-Tăut AV, Alexescu TG, Ciumărnean L, Milaciu MV, Coste SC, Vlad CV, Cozma A, Sitar-Tăut DA, Orăşan OH, Crăciun A. Diagnostic Performance of Extrahepatic Protein Induced by Vitamin K Absence in the Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Masuda T, Beppu T, Okabe H, Nitta H, Imai K, Hayashi H, Chikamoto A, Yamamoto K, Ikeshima S, Kuramoto M, Shimada S, Baba H. Predictive factors of pathological vascular invasion in hepatocellular carcinoma within 3 cm and three nodules without radiological vascular invasion. Hepatol Res. 2016;46:985-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Xu F, Zhang L, He W, Song D, Ji X, Shao J. The Diagnostic Value of Serum PIVKA-II Alone or in Combination with AFP in Chinese Hepatocellular Carcinoma Patients. Dis Markers. 2021;2021:8868370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Saitta C, Raffa G, Alibrandi A, Brancatelli S, Lombardo D, Tripodi G, Raimondo G, Pollicino T. PIVKA-II is a useful tool for diagnostic characterization of ultrasound-detected liver nodules in cirrhotic patients. Medicine (Baltimore). 2017;96:e7266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, Morse J, Hull D, Patman G, Kagebayashi C, Hussain S, Graham J, Reeves H, Satomura S. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 23. | Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, Satomura S, Schweitzer N, Vogel A, Manns MP, Benckert J, Berg T, Ebker M, Best J, Dechêne A, Gerken G, Schlaak JF, Weinmann A, Wörns MA, Galle P, Yeo W, Mo F, Chan SL, Reeves H, Cox T, Johnson P. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin Gastroenterol Hepatol. 2016;14:875-886.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 225] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 24. | Piratvisuth T, Hou J, Tanwandee T, Berg T, Vogel A, Trojan J, De Toni EN, Kudo M, Eiblmaier A, Klein HG, Hegel JK, Madin K, Kroeniger K, Sharma A, Chan HLY. Development and clinical validation of a novel algorithmic score (GAAD) for detecting HCC in prospective cohort studies. Hepatol Commun. 2023;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |