Published online Aug 24, 2023. doi: 10.4291/wjgp.v14.i4.71
Peer-review started: June 1, 2023
First decision: July 19, 2023
Revised: August 2, 2023
Accepted: August 7, 2023
Article in press: August 7, 2023
Published online: August 24, 2023
Processing time: 83 Days and 19.8 Hours
The Centers for Disease Control and Prevention estimate that Clostridioides difficile (C. difficile) causes half a million infections (CDI) annually and is a major cause of total infectious disease death in the United States, causing inflammation of the colon and potentially deadly diarrhea. We recently reported the isolation of ADS024, a Bacillus velezensis (B. velezensis) strain, which demonstrated direct in vitro bactericidal activity against C. difficile, with minimal collateral impact on other members of the gut microbiota. In this study, we hypothesized that in vitro activities of ADS024 will translate in vivo to protect against CDI challenge in mouse models.
To investigate the in vivo efficacy of B. velezensis ADS024 in protecting against CDI challenge in mouse models.
To mimic disruption of the gut microbiota, the mice were exposed to vancomycin prior to dosing with ADS024. For the mouse single-dose study, the recovery of ADS024 was assessed via microbiological analysis of intestinal and fecal samples at 4 h, 8 h, and 24 h after a single oral dose of 5 × 108 colony-forming units (CFU)/mouse of freshly grown ADS024. The single-dose study in miniature swine included groups that had been pre-dosed with vancomycin and that had been exposed to a dose range of ADS024, and a group that was not pre-dosed with vancomycin and received a single dose of ADS024. The ADS024 colonies [assessed by quantitative polymerase chain reaction (qPCR) using ADS024-specific primers] were counted on agar plates. For the 28-d miniature swine study, qPCR was used to measure ADS024 levels from fecal samples after oral administration of ADS024 capsules containing 5 × 109 CFU for 28 consecutive days, followed by MiSeq compositional sequencing and bioinformatic analyses to measure the impact of ADS024 on microbiota. Two studies were performed to determine the efficacy of ADS024 in a mouse model of CDI: Study 1 to determine the effects of fresh ADS024 culture and ADS024 spore preparations on the clinical manifestations of CDI in mice, and Study 2 to compare the efficacy of single daily doses vs dosing 3 times per day with fresh ADS024. C. difficile challenge was performed 24 h after the start of ADS024 exposure. To model the human distal colon, an anerobic fecal fermentation system was used. MiSeq compositional sequencing and bioinformatic analyses were performed to measure microbiota diversity changes following ADS024 treatment. To assess the potential of ADS024 to be a source of antibiotic resistance, its susceptibility to 18 different antibiotics was tested.
In a mouse model of CDI challenge, single daily doses of ADS024 were as efficacious as multiple daily doses in protecting against subsequent challenge by C. difficile pathogen-induced disease. ADS024 showed no evidence of colonization based on the observation that the ADS024 colonies were not recovered 24 h after single doses in mice or 72 h after single doses in miniature swine. In a 28-d repeat-dose study in miniature swine, ADS024 was not detected in fecal samples using plating and qPCR methods. Phylogenetic analysis performed in the human distal colon model showed that ADS024 had a selective impact on the healthy human colonic microbiota, similarly to the in vivo studies performed in miniature swine. Safety assessments indicated that ADS024 was susceptible to all the antibiotics tested, while in silico testing revealed a low potential for off-target activity or virulence and antibiotic-resistance mechanisms.
Our findings, demonstrating in vivo efficacy of ADS024 in protecting against CDI challenge in mouse models, support the use of ADS024 in preventing recurrent CDI following standard antibiotic treatment.
Core Tip:Clostridioides difficile (C. difficile), a Gram positive pathogen associated with life-threatening gastrointestinal disease, colonizes millions of healthy people worldwide, causing disease in individuals with disrupted gut microbiomes. Here, we demonstrate in vivo efficacy of recently isolated Bacillus velezensis ADS024, without colonization, in protecting against C. difficile infection (CDI) challenge in mouse models. We also show that this novel strain has minimal effects on the gut microbiome in a human distal colon model and miniature swine. This study supports further investigation of ADS024 as a single-strain, live biotherapeutic product candidate for preventing recurrent CDI following successful standard-of-care antibiotic therapy.
- Citation: Murphy CK, O’Donnell MM, Hegarty JW, Schulz S, Hill C, Ross RP, Rea MC, Farquhar R, Chesnel L. Novel, non-colonizing, single-strain live biotherapeutic product ADS024 protects against Clostridioides difficile infection challenge in vivo. World J Gastrointest Pathophysiol 2023; 14(4): 71-85
- URL: https://www.wjgnet.com/2150-5330/full/v14/i4/71.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v14.i4.71
Clostridioides difficile (C. difficile; previously Clostridium difficile) is an opportunistic Gram positive pathogen that takes advantage of a disrupted gut microbiome to cause potentially life-threatening gastrointestinal (GI) disease[1,2]. C. difficile infects millions of people worldwide, resulting in a range of pathologies, from mild abdominal symptoms to potentially life-threatening colitis. It is the most frequently identified health care–related infection in the United States, where community-acquired infections are increasing[3]. Risk factors include prior hospitalization, residence in a nursing home care facility, advanced age, underlying disease, and alterations in the gut microbiota[3-5]. C. difficile infection (CDI) is mainly characterized by diarrhea, but the spectrum of disease can progress to colitis and toxic megacolon[6]. The pathogenicity of C. difficile is mainly mediated by 2 exotoxins, toxin A (TcdA) and toxin B (TcdB)[2]. In addition, certain strains of C. difficile may also produce a binary toxin called C. difficile transferase[2]. The clinical symptoms described above are mediated by TcdA and TcdB, which precipitate an inflammatory response and cytotoxicity in epithelial cells of the GI tract[2]. While the relative contributions of TcdA and TcdB may be specific to the host species[7,8], TcdB is the predominant mediator of CDI[7,9] and the most clinically relevant[8].
Recurrent CDI (rCDI) following initial successful antibiotic treatment remains a clinical challenge, with each episode associated with an increased risk for recurrence (25% after the first, 45% after the second, and 65% after the third and subsequent episodes), creating a cycle of infection and antibiotic use[10,11]. The causes of rCDI are primarily attributed to the failure of antibiotic-disrupted gut microbiota to restrict the germination and subsequent overgrowth of surviving spores of C. difficile and an altered immune response as a result of the action of the C. difficile toxins[10,12]. Therefore, predicting future recurrence is crucial. Machine learning is emerging as a useful method of predicting outcomes by comparing changes in fecal microbial composition and metabolome in patients with rCDI vs those without[12,13].
The gut microbiome plays a critical role in preventing CDI among its many functions. Indeed, perturbation of the microbiome through frequent antibiotic administration is a critical risk factor for subsequent recurrences, presumably as a result of the time needed to recover a diverse and protective microbiota. Even with short-term antibiotic treatment, microbiota recovery takes several months[14], and perturbations can persist up to 4 years post treatment[15,16]. In addition, the standard treatment of initial and rCDI often involves broad-spectrum antibiotics, including vancomycin[1]. Even though CDI clinical cure rates are high[17,18], patients remain at risk for recurrence[18]. Thus, degrading TcdA and TcdB, killing germinating C. difficile spores, and reducing inflammation are vital for restoring protective intestinal microbiota and successfully preventing recurrent episodes of CDI.
In a recent study, we described the isolation of ADS024, a Bacillus velezensis (B. velezensis) strain in development as a single-strain live biotherapeutic product (SS-LBP) to prevent the recurrence of CDI after clinical cure by standard antibiotic treatment[19]. LBPs are biological medicinal products containing live microorganisms as active substances. They are being studied to prevent, treat, or cure various diseases[20-24], including allergies, dental disorders, and GI, dermatologic, and gut-brain-axis–related conditions[25]. We demonstrated that ADS024 has bactericidal activity against C. difficile, with minimal impact on other common members of the gut microbiota, and produces proteases that can degrade both TcdA and TcdB[19]. The current study expands on our previous findings by demonstrating the protective effects of ADS024 in a mouse model of CDI, the minimal impact of ADS024 on the gut microbiome of miniature swine and humans, and its lack of colonization/engraftment in miniature swine and mouse models. The results of these studies support further development of ADS024 as an SS-LBP for the prevention of rCDI after clinical cure following completion of standard antibiotic treatment.
Biological samples for this work were collected using Clinical Research Ethics Committee, Cork, Ireland, approved consents for the Protocol “ControlMET” numbered APC065.
The protocol and any amendment(s) or procedures involving the care and use of animals in this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at NeoSome Life Sciences. During the study, the care and use of animals were conducted with guidance from the United States National Research Council guidelines. Veterinary care was available throughout the study for animals to be examined by the veterinary staff as warranted by clinical signs or other changes. There were no changes in the animals that required veterinary intervention to ameliorate pain or distress.
The recovery of ADS024 was assessed in intestinal and fecal samples of mice at various times after a single dose of freshly grown ADS024. Prior to dosing with ADS024, the mice were exposed to vancomycin to disrupt their microbiota in a clinically relevant manner. Further detail is provided in the Supplementary material.
The aim of this study was to investigate ADS024 fecal content from male miniature swine following a single dose of ADS024 capsules over the time course of 5 d post ADS024 dosing. Further detail is provided in the Supplementary mater
The study design was adapted from Chen et al[26] and modified to test if prior dosing with ADS024 could protect against subsequent challenge by C. difficile. Accordingly, the ADS024 treatment started 24 h prior to the CDI (spore) challenge. Upon their receipt at NeoSome Life Sciences, the mice, while not tested for C. difficile colonization/infection prior to inoculation with C. difficile, were examined by personnel to ensure acceptable health status. Veterinary care was provided by the veterinarians and staff employed by NeoSome Life Sciences. The mice were acclimated for at least 5 d prior to use. Following acclimation, the mice were infected with C. difficile strain VPI10463 (6.30 × 105 to 1.97 × 106 CFU, a higher dose compared with the 102 to 105 CFU in the paper by Chen et al[26]), and 65 and 40 mice were treated with ADS024 or saline, respectively, across the conducted studies. The mice were then monitored for survival, weight loss, and disease-related clinical observations, including wet tail, diarrhea, hunched posture, dehydration, and lethargy. The daily score of adverse health observations was calculated based on criteria specified in Supplementary Table 1. Further detail, including antibiotic pretreatment, is provided in the Supplementary material.
The micro-Matrix (Applikon Biotechnology, Delft, Netherlands) was used to model the human distal colon and examine the impact of ADS024 on gut microbial populations. The anaerobic fecal fermentation model of the distal colon has been previously described[27]. Further detail is provided in the Supplementary material.
MiSeq compositional sequencing and bioinformatic analysis: All extracted samples from the distal colon model were prepared for MiSeq DNA compositional sequencing, and 16S data were then organized by alpha diversity, beta diversity, and phylum and genus diversity. Further detail is provided in the Supplementary material.
Statistical significance determination: ADS024-treated wells were then assessed by comparing differences to wells with untreated media. Further detail is provided in the Supplementary material.
Quantitative polymerase chain reaction: Absolute quantification by quantitative polymerase chain reaction (qPCR) was performed to determine total bacterial numbers and specific populations from fecal samples. Further detail is provided in the Supplementary material.
To determine the impact of ADS024 administration on the gut composition of miniature swine, qPCR, MiSeq compositional sequencing, and bioinformatic analysis were performed in duplicate using fecal samples. Further detail is provided in the Supplementary material.
Two batches each of lyophilized drug substances (CO-33-10A2 and CO-33-12A2) and frozen glycerol stocks [research cell bank (CO-33 ART24BHI) and the master cell bank (CO-33 ART24SYD)] were tested for antimicrobial susceptibility using the reference broth microdilution method per the Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI M07 2018). Tests with metronidazole were also carried out under anaerobic conditions (CLSI M11 2018). Minimum inhibitory concentration (MIC) values obtained against ADS024 were validated by concurrently testing CLSI-recommended ATCC quality control reference strains (CLSI M100 2019). Categorical interpretations for the MIC results obtained against ADS024 used CLSI M45 (2015)[28] breakpoint criteria or European Food Safety Authority (EFSA 2012)[29] microbiological cutoff values.
Potential virulence factors and antimicrobial resistance genes of ADS024 were analyzed using IslandViewer 4. The annotated ADS024 genome was first analyzed using IslandViewer 4, and a separate web server was subsequently used to interpret the data. The output was based on mapping as detailed in Supplementary Figure 1 and stored as a Microsoft Excel spreadsheet that contained the output of the integrated IslandPath-DIMOB, SIGI-HMM, and IslandPick prediction methods.
The bioinformatics and statistical analyses relating to 16S sequencing and the micro-Matrix, as described in this manuscript, were verified by Dr. Michelle M. O’Donnell of APC Microbiome Ireland, University College Cork. The bioinformatics and statistical analyses relating to all animal-related research described in this paper were verified by Dr. Christopher Murphy of Adiso Therapeutics, Inc.
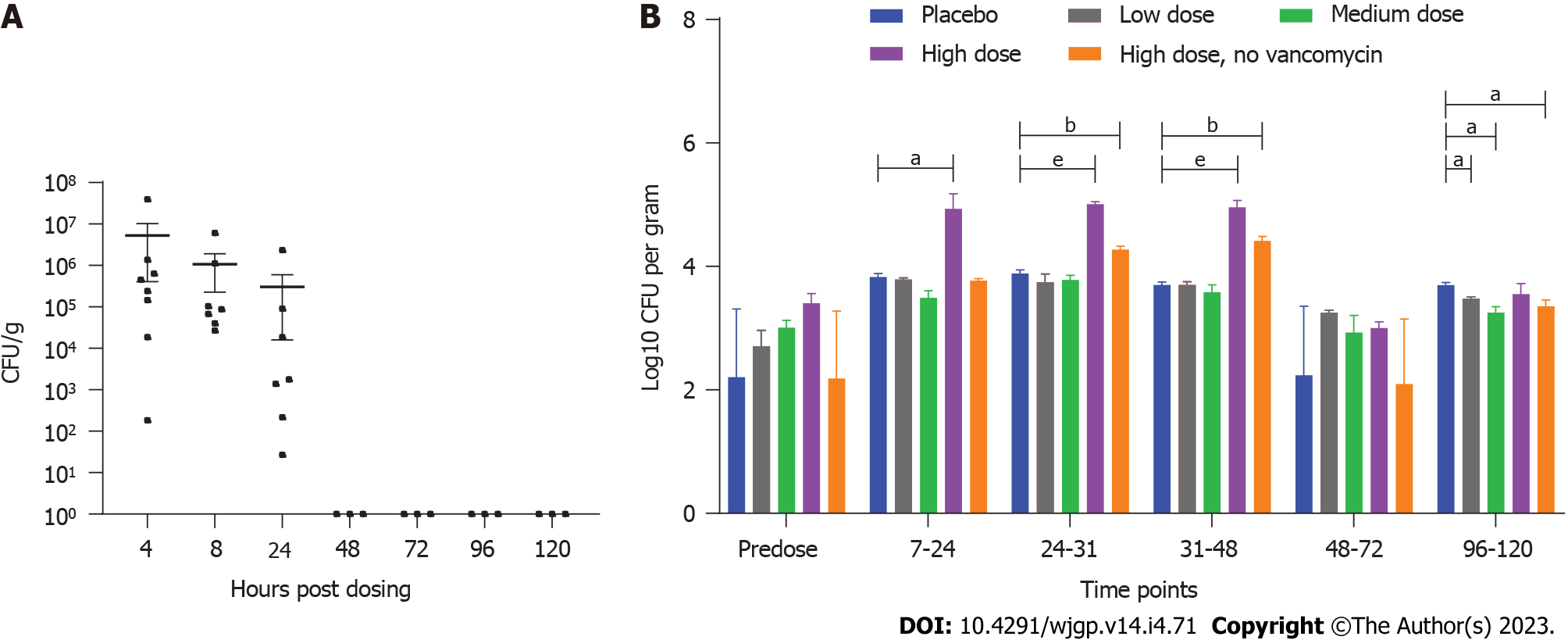
Microbiological analysis was conducted on fecal pellets and GI tissue from mice pre-dosed with vancomycin to mimic disruption of the gut microbiota. The mice were then administered a single oral dose of approximately 5 × 108 colony-forming units (CFU)/mouse of ADS024. ADS024 has a very distinctive morphology allowing for visual detection on agar plates[19]. ADS024 colonies were recovered in feces following plating on agar media at 4 h, 8 h, and 24 h post dose (Figure 1A). No ADS024 colonies were detected in feces after the 24-h post-dose time point. Only 1 GI tissue sample contained detectable amounts of ADS024, present in the upper intestine 24 h after ADS024 administration (Supple
The results from a single-dose study in minipigs (3 male miniature swine per group) were consistent with those from the mouse studies, showing a lack of detection in fecal and tissue samples past 72 h, supporting daily dosing for clinical use. The single-dose study in miniature swine included groups that had been pre-dosed with vancomycin to induce disruption of the gut microbiota, as described under Materials and Methods, and that had been exposed to a dose range of ADS024 (Groups 2, 3, and 4 received 6.2 × 107, 7.1 × 108, or 7.1 × 109 CFU, respectively), and a group that was not pre-dosed with vancomycin and received a single dose of ADS024 (Group 5 received 7.1 × 109 CFU). The control group, group 1, did not receive vancomycin or ADS024. After single oral administration of ADS024 in miniature swine, the ADS024 colonies (assessed by qPCR using ADS024-specific primers) were detected on agar plates in the ADS024-treated Groups 4 and 5, regardless of vancomycin therapy, at colony counts higher than placebo controls (which grew only Bacillus-like, but not ADS024-specific, colonies) at the 7- to 48-h time points (Figure 1B). The qPCR data (Supplementary Figure 3) confirmed the plating colony counts (Figure 1B), as no ADS024-selective agar plates were available.
ADS024 colonies were considered to have increased if the values were higher than the highest predose value of 104 CFU/g and higher than the corresponding placebo value for the given time point. The 7- to 48-h post-dose period was the Discovery Window, and this term was used for subsequent qPCR analysis. The qPCR analysis of Groups 2 to 5 showed that ADS024 was present in the Discovery Window samples at 24 h to 31 h post dose for the low-dose Group 2, 24 h to 48 h for the mid-dose Group 3, and 7 to 48 h for the high-dose Groups 4 and 5 at variable concentrations higher than pre-dose in all animals (except for the Group 5 samples) (Figure 1B).
To confirm if the toxin degradation and C. difficile–killing activities observed in our in vitro studies[19,20] translated to in vivo efficacy, we utilized a mouse model of CDI. The animal model was adapted from Chen et al[26] as described under Materials and Methods, and the C. difficile challenge was performed 24 h after the start of ADS024 exposure.
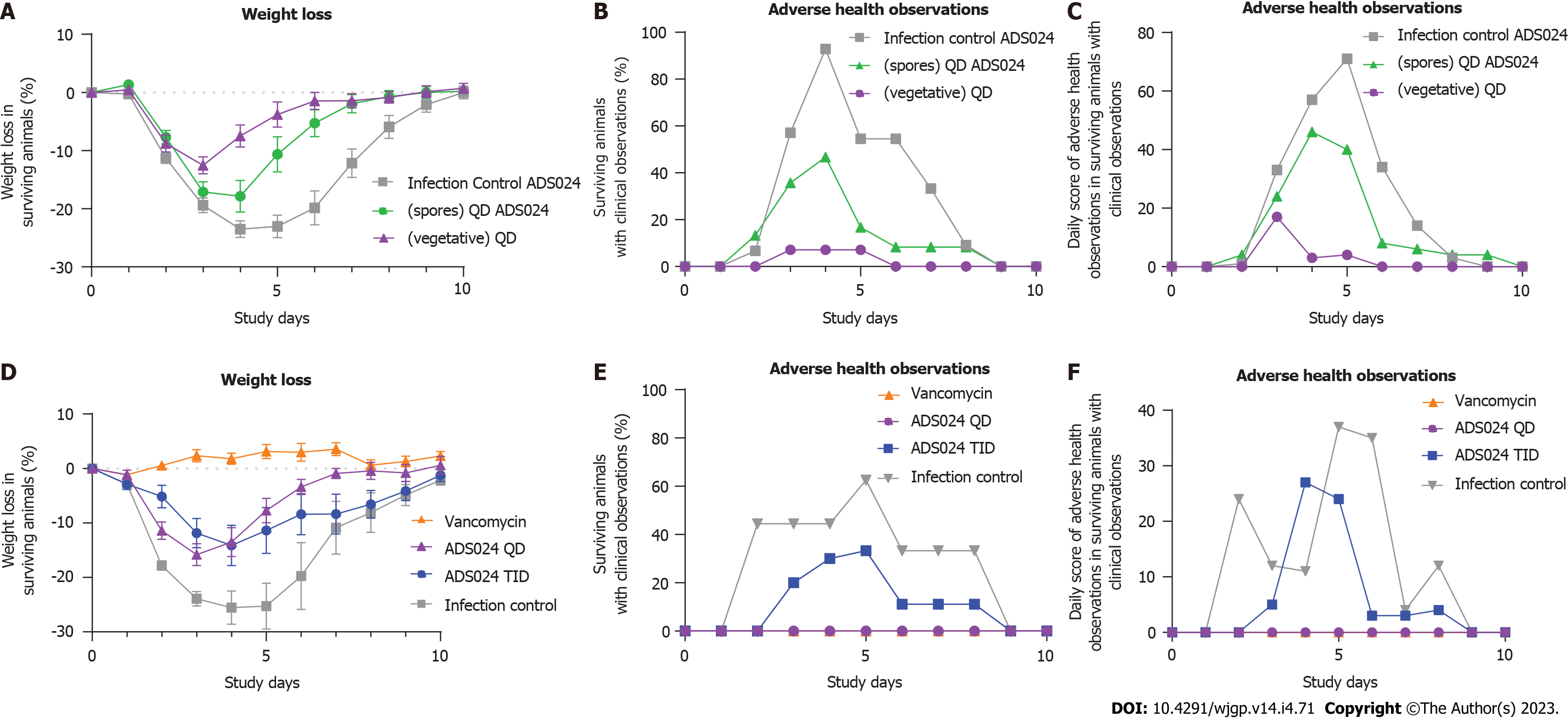
Two studies were performed to determine the efficacy of ADS024 in the mouse model of CDI. The first study (Study 1, Figure 2A-C) determined the effects of fresh ADS024 culture and ADS024 spore preparations on the clinical manifestations of CDI in mice, namely weight loss (Figure 2A) and adverse clinical features (Figure 2B and C). The second study (Study 2, Figure 2D-F) compared the efficacy of single daily doses (QD) vs dosing 3 times per day (TID) with fresh ADS024.
Study 1: ADS024 was delivered to mice (n = 15 per group) as resuspended spore suspensions [in phosphate-buffered saline (PBS)] or as a freshly prepared daily culture (ADS024 bacteria resuspended in PBS). The placebo (infection control) group demonstrated an average maximum weight loss of 24.4%, and the mice experienced adverse health effects for 33% of the days (Figure 2A-C). In comparison, mice that received ADS024 as a fresh culture suffered less weight loss compared with the infection (vehicle-dosed) controls (13.5% maximum average percentage of pre-infection weight loss; Figure 2A), and the mice experienced adverse health effects for only 3% of the days (Figure 2B), which was the lowest daily score of adverse health observations among the 3 groups (Figure 2C). The ADS024 spore preparation–treated mice exhibited a maximum weight loss of 20% on day 4, and for 19% of the days, the mice scored adverse health effects (Figure 2A-C). Therefore, mice treated with fresh ADS024 had lower morbidity and mortality than those treated with ADS024 spores or mice that received only placebo.
Study 2: Dose frequencies of QD and TID were compared in mice (n = 10 per group). The untreated (infection control) group had an average maximum weight loss of 25% on day 4 (Figure 2D), similar to observations in Study 1. In comparison, mice that received ADS024 QD had an average maximum weight loss of 16% on day 3 (from the pre-dose weight) before recovering to the group average pre-infection body weight at day 10 (Figure 2D). Mice receiving ADS024 TID demonstrated a maximum weight loss of 14% on day 4 (Figure 2D), similar to that in Study 1. The vancomycin control mice had no weight loss throughout the study (Figure 2D). The ADS024 QD and vancomycin groups did not display adverse clinical features, as demonstrated by overlapping values in Figure 2E and F. In contrast, the ADS024 TID group did display adverse clinical features (Figure 2E and F), perhaps due to the TID oral gavage–related stress. Therefore, there was no obvious benefit from repeat dosing because the efficacy in this model was similar; furthermore, there were fewer adverse health events in the QD-dosed group than in the TID-dosed group.
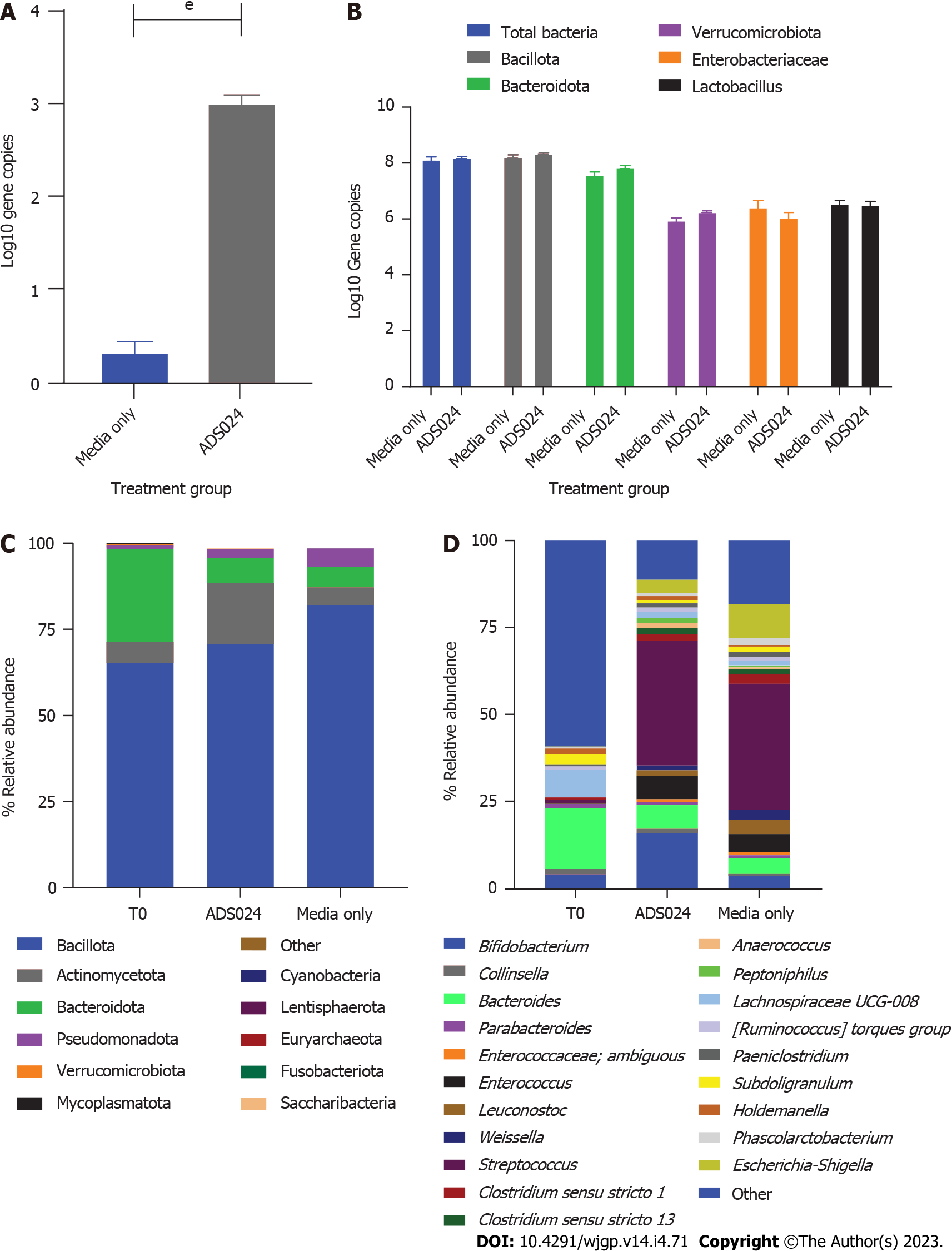
We used anaerobic fecal fermentation to model the distal colon as previously described[27]. qPCR was used to analyze the effect of ADS024 exposure on 5 different components of human colonic microbiota (Enterobacteriaceae, Bacillota, Bacteroidota, Verrucomicrobiota, and Lactobacillus) and to determine the number of ADS024 colonies in inoculated wells (Figure 3A and B). ADS024 was detectable in the ADS024-inoculated wells (Figure 3A). Still, its concentration did not increase during the experiment and had a negligible impact on other gut microbiota components after 24 h of incubation (Figure 3B).
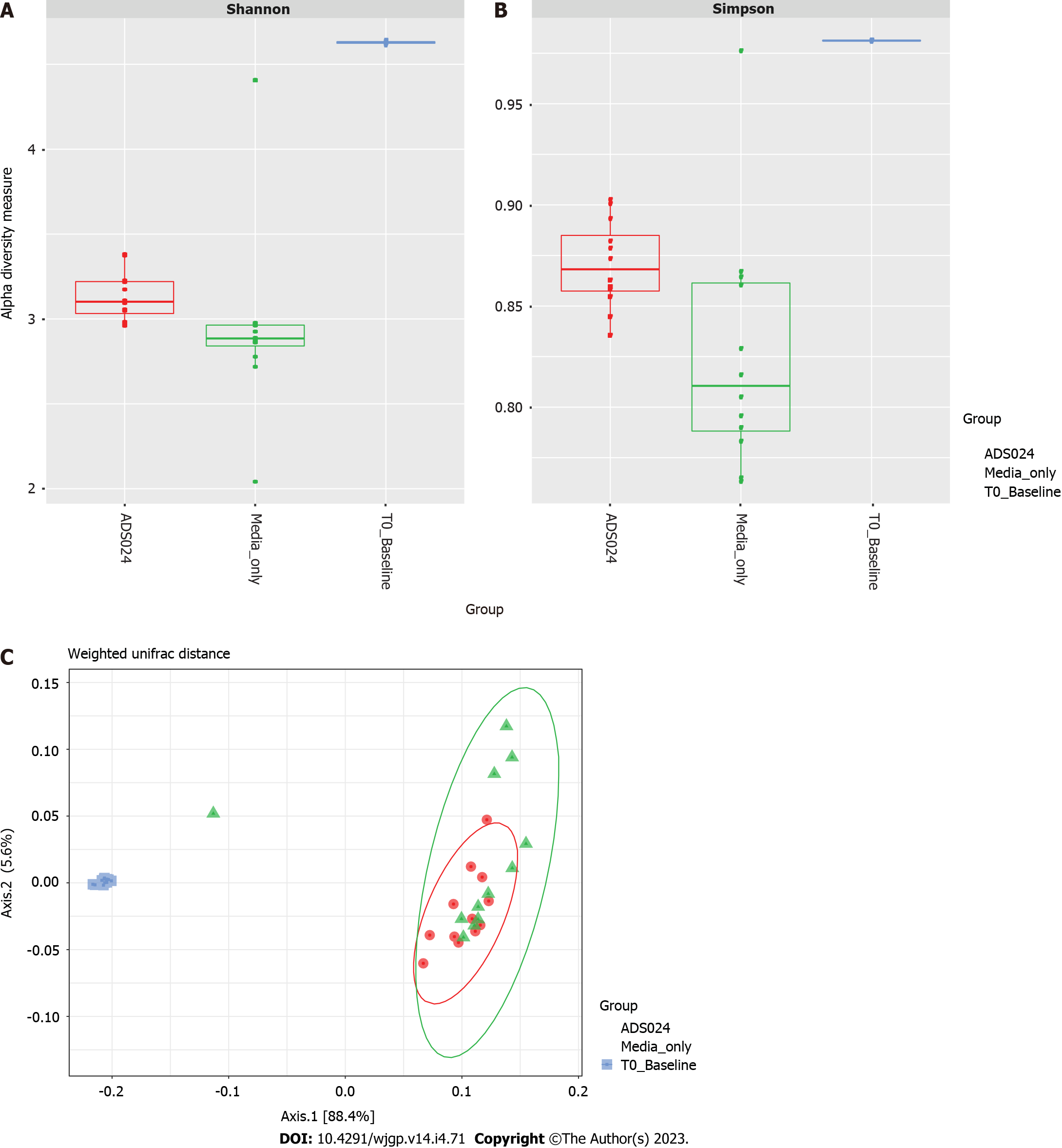
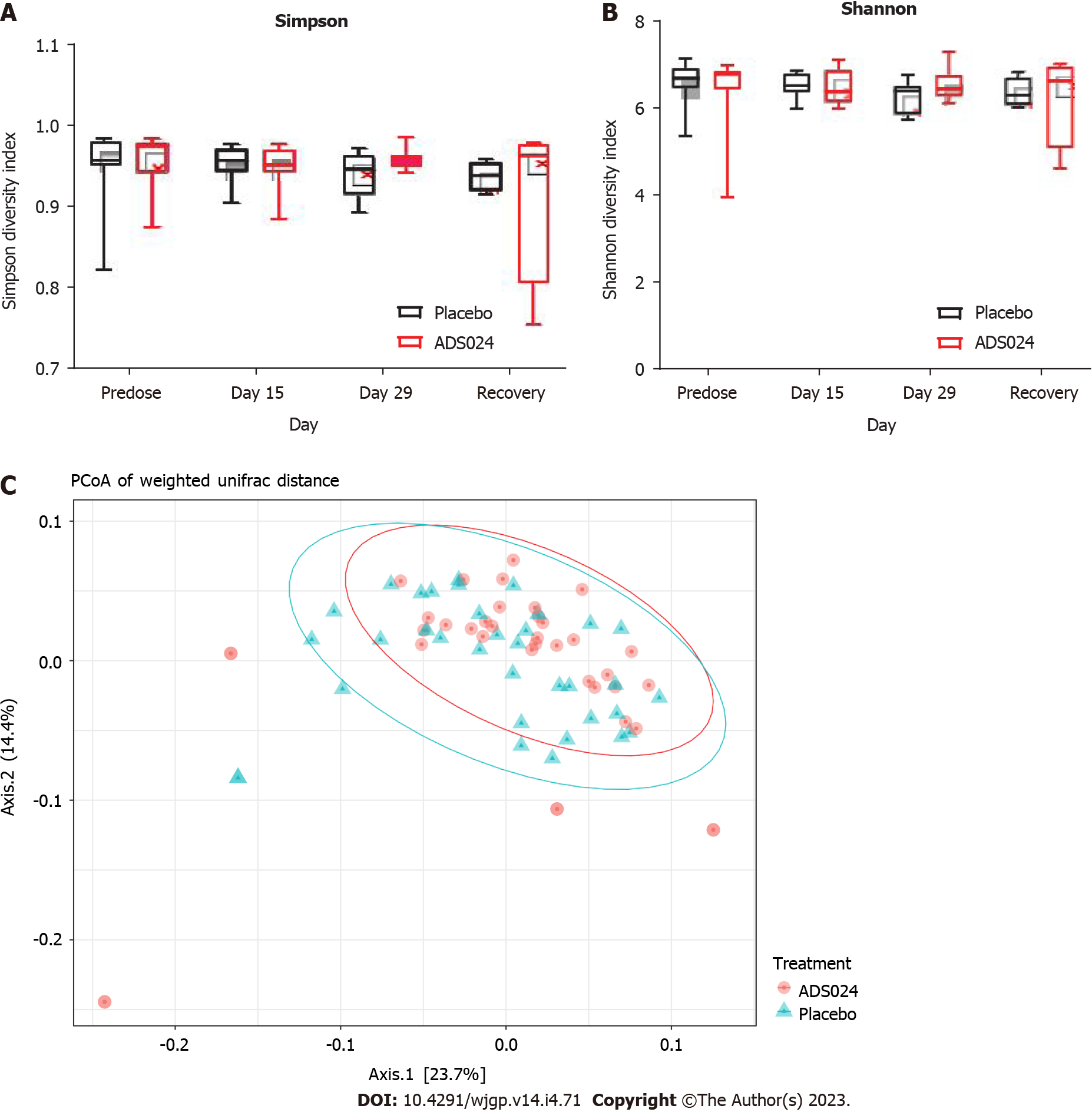
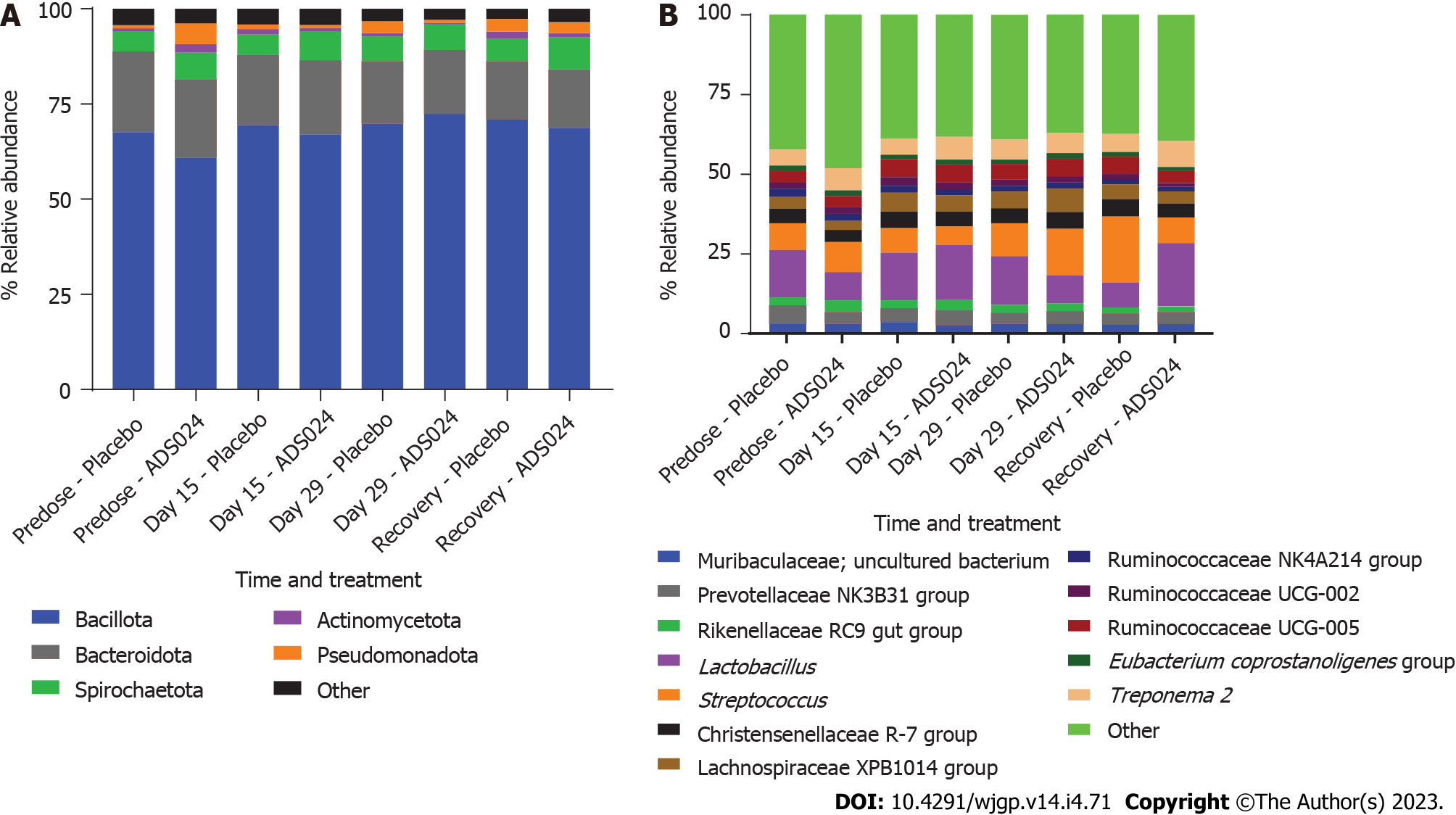
MiSeq 16S (Illumina, San Diego, CA, United States) compositional sequencing analyses at the phylum level (Figure 3C and Supplementary Table 2) revealed an increase in Actinomycetota (approximately 13%; P < 0.0001) and significant decreases in Bacillota (approximately 12%; P < 0.01), Fusobacteriota (approximately 0.001%; P < 0.05), and Verrucomicrobiota (approximately 0.4%; P < 0.05) compared with media-only treatment. Compositional analyses at the genus level (Figure 3D and Supplementary Table 3) revealed 12 genera showing significant differences after 24 h of treatment compared with the media-only controls. Among the 7 genera positively impacted by the exposure to ADS024, increases in Bifidobacterium (approximately 12%; P < 0.00003) and Bacteroides (approximately 2%; P < 0.05) were found compared with media-only treatment (Figure 3D). These results correlate with the MiSeq compositional sequencing and bioinformatic analyses that were performed to measure microbiota diversity changes following ADS024 treatment using Shannon and Simpson indexes for alpha diversity (Figure 4A and B) and UniFrac principal coordinates analysis (PCoA) for beta diversity (Figure 4C). After 24 h of incubation, ADS024 had less effect on alpha diversity than the media-only controls relative to baseline (Figure 4A and B). The microbial diversity between the media-exposed and ADS024-exposed samples was similar in beta diversity changes (Figure 4C).
ADS024 Levels from fecal samples were determined by qPCR after QD oral administration of ADS024 capsules containing 5 × 109 CFU in male and female miniature swine for 28 consecutive days as described in Materials and Methods (Supplementary Figure 4). ADS024-specific genetic material was below the limit of detection in both the placebo and ADS024-dosed groups at all assayed time points. Subsequently, MiSeq compositional sequencing and bioinformatic analyses were performed to measure the impact of ADS024 on microbiota in miniature swine fecal samples collected pre dose (day 15), on the last day of dosing (day 29), and 14 d after the last dose (recovery) using Shannon and Simpson indexes for alpha diversity (Figure 5A and B) and UniFrac PCoA for beta diversity (Figure 5C). The alpha diversity analyses indicated no significant differences in microbiota diversity between the ADS024 and placebo treatment groups at any time (Figure 5A and B). Beta diversity using PCoA plots, based on weighted UniFrac distance matrixes, indicated minor, non-significant differences between the placebo and ADS024 groups (permutational multivariate analysis of variance statistical test in R: P = 0.617), and all samples tended to cluster together regardless of treatment or time point (Figure 5C). At the phylum level, no significant differences were detected in the most abundant phyla (Bacillota, Bacteroidota, and Spirochaetota) between treatment groups across time points (Figure 6A). At the genus level, no significant differences were observed across the most abundant genera (Lactobacillus and Streptococcus) at any time point (Figure 6B).
To assess the potential of ADS024 to be a source of antibiotic resistance, its susceptibility to 18 different antibiotics was tested. The frozen glycerol stock and lyophilized forms of ADS024 samples were assessed as described under materials and methods.
Table 1 summarizes the MIC results for the 18 antimicrobials tested against one lot of ADS024 (4 lots were tested with similar results). All antimicrobial agents showed low MIC values when tested against ADS024, except for metronidazole (MIC ≥ 64 mg/L, depending on atmospheric conditions). ADS024 was susceptible to all 12 drugs tested for which interpretive criteria are available[28,29].
| Antimicrobial | MIC in mg/L (interpretation) for ADS024 |
| CO-33 ADS024 SYD | |
| Ampicillin | ≤ 0.03 (S) |
| Chloramphenicol | 2 (S) |
| Ciprofloxacin | ≤ 0.03 (S) |
| Clindamycin | 0.25 (S) |
| Erythromycin | 0.12 (S) |
| Fidaxomicin | 4 |
| Gentamicin | ≤ 0.06 (S) |
| Kanamycin | ≤ 0.50 (S) |
| Linezolid | 0.5 |
| Metronidazole (aerobic) | > 64.00 |
| Metronidazole (anaerobic) | 64 |
| Neomycin | ≤ 0.12 |
| Penicillin | 0.06 (S) |
| Quinupristin–dalfopristin | 2 |
| Rifampicin | 0.25 (S) |
| Streptomycin | 2 (S) |
| Tetracycline | 2 (S) |
| Trimethoprim | ≤ 0.25 |
| Vancomycin | ≤ 0.25 (S) |
To determine the possible presence of virulence factors or antimicrobial resistance genes, the computational genomic prediction tool IslandViewer 4 was used. Corroborating statistical analyses already published[19], no virulence factors, homologs, or operons for antibiotic resistance were identified, pointing to the low potential of ADS024 to expose the gut microbiota to virulence and antibiotic-resistance genes after oral dosing (Supplementary Figure 1).
B. velezensis ADS024, isolated from a human fecal sample, is a member of the operational group Bacillus amyloliquefaciens, consisting of the soilborne B. amyloliquefaciens and the plant-associated Bacillus siamensis and B. velezensis. Most often, B. amyloliquefaciens is used in literature to describe the operational group B. amyloliquefaciens and refers to all three closely related species[30]. In this study, we extended our in vitro characterization of ADS024 to show its protective effects against CDI challenge in mouse models. We demonstrated a lack of impact on the gut microbiota composition in human feces and miniature swine models. Our model aligned with a CDI challenge rather than a recurrent model. The animals used in our study were not subjected to an earlier round of CDI or pre-treated with antibiotics, which would damage their GI tract microbiota, the 2 conditions associated with CDI recurrence.
Previous studies have investigated the B. amyloliquefaciens operational group in a mouse model as a prophylactic treatment for C. difficile. In one such study by Geeraerts et al[21], using C57BL/6J mice and C. difficile strain VPI 10463, it was demonstrated that C. difficile toxin A and B levels were significantly lower in B. amyloliquefaciens–treated mice compared with untreated ones[21]. In addition, a significantly lower extent of colonic tissue damage was observed for B. amyloliquefaciens–treated mice compared with the untreated cohort. Consistent with these findings, ADS024 conferred protection against CDI and its clinical manifestations.
Although not directly tested in the experiments shown in this study, one possibility is that the pharmacologic effect of ADS024 was due to its bactericidal activity against C. difficile as well as its proteolytic degradation of TcdA and TcdB[19], resulting in fewer observed clinical symptoms. Furthermore, an anti-inflammatory role of Bacillus-based probiotics has been demonstrated[20,31]. To this end, the Bacillus-based probiotic, Bs 29784, decreased pro-inflammatory signals in a Caco-2 cell model for intestinal epithelia, prevented IκBα degradation, and reduced upregulation of iNOS protein levels, highlighting intestinal anti-inflammatory capabilities[31]. Similarly, ADS024 inhibited toxin B–mediated apoptosis and tissue injury in human colonic epithelial cells and colonic explants[20]. In addition, restoring the host gut microbiota to preexisting conditions plays an equally critical role in recovering from CDI and preventing recurrent infection after the completion of standard antibiotic treatment. We hypothesize that the selective impact of ADS024 on the human microbiota may therefore allow recovery of the normal host microbiota and prevent rCDI in clinical settings.
The aim of the mouse model (Figure 2) was to evaluate whether prior dosing with ADS024 could protect against subsequent challenge by C. difficile. We found that single daily (QD) doses of ADS024 were as efficacious as multiple daily (TID) doses in protecting against pathogen-induced disease (Figure 2), and, as reported in the result section of the Study 2 Mouse model of CDI, there was no significant benefit in the TID-dosed group (Figure 2D-F). While there was a slight improvement in the weight loss category in the TID-dosed group compared with the QD-dosed group (14% vs 16%), both groups showed improvement compared with the untreated group (25%) (Figure 2D). In contrast, the adverse clinical features were present only in the TID-dosed group (Figure 2E and F), possibly due to the added stress in the animals from repeated oral gavage[32], which likely negated any positive effect of ADS024. Stress would likely increase assessed disease markers if there were an infected placebo arm with the same dosing regimen.
ADS024 showed no evidence of colonization (Figure 1) based on the observation that the ADS024 colonies were not recovered 24 h after single doses in mice or 72 h after single doses in minipigs. This suggests that the ADS024-mediated protection against C. difficile pathogenesis occurred independently of the colonization of ADS024 in the animals’ GI tracts.
ADS024 was also assessed in a 28-d repeat-dose study in miniature swine (Figure 5 and 6), a species that offers a more clinically relevant model of the human GI tract[33]. ADS024 was not detected in fecal samples using plating and qPCR methods (Supplementary Figure 4), which again suggests that ADS024 did not colonize the gut following long-term treatment exposures. To achieve reproducible results with microbial therapeutics in humans, it may be advantageous to use transitory, non-colonizing strains[34], such as ADS024.
Restoring the resident host microbiota is an important factor in preventing rCDI. Thus, the effect of ADS024 on gut microbial composition was investigated using a human distal colon model (Figures 3 and 4). Ex vivo systems replicating the distal colon environment have been used to investigate the effect of antimicrobial-producing bacterial strains on the human gut microbiota[35]. To our knowledge, this is the first report examining the impact of a B. amyloliquefaciens operational group strain in a distal colon model using a standardized human fecal inoculum. Phylogenetic analysis performed in this model showed that ADS024 had a selective impact on the healthy human colonic microbiota (Figures 3 and 4). This finding is similar to the in vivo studies performed in miniature swine (Figures 5 and 6). We consider this selective impact of ADS024 on the resident commensal microbiota an important feature for its use as a therapeutic agent to prevent rCDI. Specifically, Bifidobacterium species expansion in ADS024-treated samples (Figure 3C and D) was noteworthy. The correlation between Bifidobacterium species consumption and improved human health has been well documented[35]. Various illnesses[36,37] and aging[36-38] have been associated with reduced numbers of Bifidobacterium species, which, in a healthy population, positively impact the immune system[39] and are thus important for health maintenance[36]. ADS024 appeared to have the added benefit of expanding this healthy bacterial population.
Recent research demonstrates the benefits of consuming B. velezensis isolates and their role in regulating the intestinal innate immune response and decreasing intestinal inflammation caused by pathogens in crucian carp[40]. The immunomodulatory and anti-inflammatory effects of B. velezensis and other related Bacillus species have also been studied in plants and mice[41,42], in addition to the human epithelial cells described above[31], showing their potential for treating inflammatory diseases caused by pathogens like C. difficile. By killing C. difficile and degrading its toxins[19], thereby eliminating inflammatory stimuli, ADS024 may also have offered anti-inflammatory protection, a crucial asset in tackling an inflammatory disease such as CDI.
A limitation of our studies was using the in vitro human distal colon model, which has limited physiological relevance. A batch fermentation system inevitably precludes the exchange of nutrients or waste removal. The accumulation of waste can potentially influence the bacterial microenvironment. Future assessments will include tissue histology and co-localization (C. difficile–ADS024) experiments to further characterize the mechanistic details and therapeutic effects of ADS024. Furthermore, a definitive demonstration that the direct activities of ADS024 on C. difficile are responsible for its biological activity in mice will require in vivo studies utilizing C. difficile–infected gnotobiotic mice.
Although safety in humans requires further study, our ADS024 findings were promising and support the continued development of ADS024 as an SS-LBP to prevent CDI recurrence following the completion of standard antibiotic treatment. In contrast to other LBPs that aim to restore diversity and prevent outgrowth by outcompeting the pathogen, we propose that ADS024 functioned by delivering bioactivities (described in O’Donnell et al[19,27]) directly targeting C difficile and its toxins. Fecal microbiota transplantation and most LBPs may require antibiotic administration prior to treatment to allow engraftment[43]. The lack of colonization by ADS024 contrasts with other LBPs, which depend on colonization. Given the emerging data on how microbially produced metabolites can modulate human pharmacology, the most minimally invasive, transient LBP intervention (ADS024) may be preferable. Transplanted microbiota, while effectively reducing rCDI, may cause chronic changes to the patient’s natural GI microbiome as a cost of treating CDI[43,44].
It is clear that while the burgeoning LBP field has high therapeutic potential, method optimization and the identification of selective products with fewer systemic side effects require substantially increased effort. To this end, a risk assessment should be undertaken in the early stages of LBP development[23]. The risk assessment and safety analysis of ADS024 demonstrated it to be selective, with little potential for colonizing the gut (mouse and miniature swine models), and susceptible to all the antibiotics tested for which interpretative criteria are available (Table 1). If a patient were to develop a systemic infection with ADS024 (which is not expected due to the lack of virulence factors in ADS024), a wide range of antibiotics would be available to treat the infection. In silico safety assessments indicated that ADS024 had a low potential for off-target activity or virulence and antibiotic-resistance mechanisms (Table 1; Supplementary Figure 1).
In vivo evaluation of ADS024 is consistent with the results of previous in vitro studies from our group[19,20] that showed potent C. difficile inhibition and maintenance of gut microbiota diversity. The totality of the evidence to date supports additional preclinical studies and early-phase clinical trials to evaluate the safety and efficacy of ADS024 in patients recovering from CDI.
Clostridioides difficile (C. difficile) is a Gram positive pathogen that causes C. difficile infection (CDI). It infects millions of people worldwide, causing potentially life-threatening gastrointestinal disease in individuals with disrupted gut microbiomes. Following successful antibiotic treatment, recurrent CDI (rCDI) can occur in cured patients. Therefore, development of a therapeutic product for preventing rCDI following successful standard-of-care antibiotic therapy is of vital importance.
C. difficile, a major cause of infectious disease death in the United States, causes inflammation of the colon and potentially deadly diarrhea. ADS024, a Bacillus velezensis strain, has previously demonstrated direct in vitro bactericidal activity against C. difficile, without affecting other members of the gut microbiota. In this study, we investigated the efficacy of ADS024 against CDI challenge in vivo. Following our findings, further investigation of ADS024 as a single-strain, live biotherapeutic product (SS-LBP) for prevention of rCDI following successful standard-of-care antibiotic therapy is warranted.
The objectives of the research were to investigate: (1) The in vivo efficacy of B. velezensis ADS024 in protecting against CDI challenge in mouse models; (2) the capability of ADS024 to colonize the GI tract; and (3) the impact of ADS024 on the gut microbiome, finding that it was efficacious against CDI challenge without colonization, and with minimal effects on the gut microbiome, thus supporting further development of ADS024 as an SS-LBP for prevention of rCDI.
Mouse models were used to determine: (1) The in vivo efficacy against CDI challenge; and (2) colonization status of ADS024 (in conjunction with miniature swine). Human distal colon model and miniature swine were utilized to determine the effects of ADS024 on the gut microbiome. To mimic disruption of the gut microbiota, the mice and miniature swine were exposed to vancomycin prior to dosing with ADS024. To model the human distal colon, an anerobic fecal fermentation system was used.
Single oral daily doses of ADS024, similarly to multiple doses, demonstrated efficacy in protecting against subsequent challenge by C. difficile in a mouse model of CDI challenge. ADS024 showed no colonization based on lack of recovery ADS024 colonies in fecal samples 24 h after single doses in mice, 72 h after single doses in miniature swine, or a 28-d repeat-dose study in miniature swine. Phylogenetic analysis in the human distal colon model and in vivo studies performed in miniature swine demonstrated a selective impact of ADS024 on the healthy human colonic microbiota.
In vivo efficacy of ADS024 in protecting against CDI challenge and minimal effects on the gut microbiome support development of ADS024 as an SS-LBP in preventing rCDI following standard antibiotic treatment.
In vivo investigation of ADS024 is compatible with previous in vitro studies that showed efficacy against C. difficile and maintenance of gut microbiota diversity. Altogether, findings from these studies support initiation of clinical trials to evaluate the safety and efficacy of ADS024 in patients recovering from CDI.
The authors would like to thank Lorena Puto, PhD, of Simpson Healthcare, for her assistance in medical writing and editorial support. Her participation was provided by Adiso Therapeutics, Inc.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohapatra S, India S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
| 1. | Johnson S, Lavergne V, Skinner AM, Gonzales-Luna AJ, Garey KW, Kelly CP, Wilcox MH. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin Infect Dis. 2021;73:e1029-e1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 332] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 2. | Nagy E. What do we know about the diagnostics, treatment and epidemiology of Clostridioides (Clostridium) difficile infection in Europe? J Infect Chemother. 2018;24:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2023] [Cited by in RCA: 1975] [Article Influence: 197.5] [Reference Citation Analysis (1)] |
| 4. | Morrison RH, Hall NS, Said M, Rice T, Groff H, Brodine SK, Slymen D, Lederman ER. Risk factors associated with complications and mortality in patients with Clostridium difficile infection. Clin Infect Dis. 2011;53:1173-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 430] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 6. | Guh AY, Kutty PK. Clostridioides difficile Infection. Ann Intern Med. 2018;169:ITC49-ITC64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Carter GP, Chakravorty A, Pham Nguyen TA, Mileto S, Schreiber F, Li L, Howarth P, Clare S, Cunningham B, Sambol SP, Cheknis A, Figueroa I, Johnson S, Gerding D, Rood JI, Dougan G, Lawley TD, Lyras D. Defining the Roles of TcdA and TcdB in Localized Gastrointestinal Disease, Systemic Organ Damage, and the Host Response during Clostridium difficile Infections. mBio. 2015;6:e00551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 8. | Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, Cornely OA, Rahav G, Bouza E, Lee C, Jenkin G, Jensen W, Kim YS, Yoshida J, Gabryelski L, Pedley A, Eves K, Tipping R, Guris D, Kartsonis N, Dorr MB; MODIFY I and MODIFY II Investigators. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N Engl J Med. 2017;376:305-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 624] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 9. | Steele J, Mukherjee J, Parry N, Tzipori S. Antibody against TcdB, but not TcdA, prevents development of gastrointestinal and systemic Clostridium difficile disease. J Infect Dis. 2013;207:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Tsigrelis C. Recurrent Clostridioides difficile infection: Recognition, management, prevention. Cleve Clin J Med. 2020;87:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18 Suppl 6:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 12. | Dawkins JJ, Allegretti JR, Gibson TE, McClure E, Delaney M, Bry L, Gerber GK. Gut metabolites predict Clostridioides difficile recurrence. Microbiome. 2022;10:87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Panchavati S, Zelin NS, Garikipati A, Pellegrini E, Iqbal Z, Barnes G, Hoffman J, Calvert J, Mao Q, Das R. A comparative analysis of machine learning approaches to predict C. difficile infection in hospitalized patients. Am J Infect Control. 2022;50:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 14. | Rashid MU, Zaura E, Buijs MJ, Keijser BJ, Crielaard W, Nord CE, Weintraub A. Determining the Long-term Effect of Antibiotic Administration on the Human Normal Intestinal Microbiota Using Culture and Pyrosequencing Methods. Clin Infect Dis. 2015;60 Suppl 2:S77-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5:e9836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 746] [Cited by in RCA: 822] [Article Influence: 54.8] [Reference Citation Analysis (1)] |
| 16. | Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 748] [Article Influence: 41.6] [Reference Citation Analysis (1)] |
| 17. | Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 938] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 18. | Johnson S, Louie TJ, Gerding DN, Cornely OA, Chasan-Taber S, Fitts D, Gelone SP, Broom C, Davidson DM; Polymer Alternative for CDI Treatment (PACT) investigators. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 389] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 19. | O’Donnell MM, Hegarty JW, Healy B, Schulz S, Walsh CJ, Hill C, Ross RP, Rea MC, Farquhar R, Chesnel L. Identification of ADS024, a newly characterized strain of Bacillus velezensis with direct Clostridiodes difficile killing and toxin degradation bio-activities. Sci Rep. 2022;12:9283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 20. | Xie Y, Chupina Estrada A, Nelson B, Feng H, Pothoulakis C, Chesnel L, Koon HW. ADS024, a Bacillus velezensis strain, protects human colonic epithelial cells against C. difficile toxin-mediated apoptosis. Front Microbiol. 2022;13:1072534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Geeraerts S, Ducatelle R, Haesebrouck F, Van Immerseel F. Bacillus amyloliquefaciens as prophylactic treatment for Clostridium difficile-associated disease in a mouse model. J Gastroenterol Hepatol. 2015;30:1275-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | US Food and Drug Administration, Center for Biologics Evaluation and Research. Early Clinical Trials With Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information. Guidance for Industry. FDA-2010-D-0500. [cited 1 August 2023]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/early-clinical-trials-live-biotherapeutic-products-chemistry-manufacturing-and-control-information. |
| 23. | Rouanet A, Bolca S, Bru A, Claes I, Cvejic H, Girgis H, Harper A, Lavergne SN, Mathys S, Pane M, Pot B, Shortt C, Alkema W, Bezulowsky C, Blanquet-Diot S, Chassard C, Claus SP, Hadida B, Hemmingsen C, Jeune C, Lindman B, Midzi G, Mogna L, Movitz C, Nasir N, Oberreither M, Seegers JFML, Sterkman L, Valo A, Vieville F, Cordaillat-Simmons M. Live Biotherapeutic Products, A Road Map for Safety Assessment. Front Med (Lausanne). 2020;7:237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 24. | McFarland LV. Efficacy of Single-Strain Probiotics Versus Multi-Strain Mixtures: Systematic Review of Strain and Disease Specificity. Dig Dis Sci. 2021;66:694-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 25. | Cordaillat-Simmons M, Rouanet A, Pot B. Live biotherapeutic products: the importance of a defined regulatory framework. Exp Mol Med. 2020;52:1397-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 26. | Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135:1984-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 404] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 27. | O’Donnell MM, Rea MC, Shanahan F, Ross RP. The Use of a Mini-Bioreactor Fermentation System as a Reproducible, High-Throughput ex vivo Batch Model of the Distal Colon. Front Microbiol. 2018;9:1844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Clinical and Laboratory Standards Institute. M45: Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. 3rd ed. 2016. [cited 1 August 2023]. Available from: https://clsi.org/media/1450/m45ed3_sample.pdf. |
| 29. | EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA Journal. 2012;10:2740. [RCA] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 406] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 30. | Fan B, Blom J, Klenk HP, Borriss R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis Form an "Operational Group B. amyloliquefaciens" within the B. subtilis Species Complex. Front Microbiol. 2017;8:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 217] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 31. | Rhayat L, Maresca M, Nicoletti C, Perrier J, Brinch KS, Christian S, Devillard E, Eckhardt E. Effect of Bacillus subtilis Strains on Intestinal Barrier Function and Inflammatory Response. Front Immunol. 2019;10:564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 32. | Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci. 2011;50:600-613. [PubMed] |
| 33. | Zhang Q, Widmer G, Tzipori S. A pig model of the human gastrointestinal tract. Gut Microbes. 2013;4:193-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 34. | Charbonneau MR, Isabella VM, Li N, Kurtz CB. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat Commun. 2020;11:1738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 248] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 35. | Dobson A, Crispie F, Rea MC, O’Sullivan O, Casey PG, Lawlor PG, Cotter PD, Ross P, Gardiner GE, Hill C. Fate and efficacy of lacticin 3147-producing Lactococcus lactis in the mammalian gastrointestinal tract. FEMS Microbiol Ecol. 2011;76:602-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Arboleya S, Watkins C, Stanton C, Ross RP. Gut Bifidobacteria Populations in Human Health and Aging. Front Microbiol. 2016;7:1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 438] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 37. | O’Neill I, Schofield Z, Hall LJ. Exploring the role of the microbiota member Bifidobacterium in modulating immune-linked diseases. Emerg Top Life Sci. 2017;1:333-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 38. | Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol. 2002;51:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 328] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 39. | Ménard O, Butel MJ, Gaboriau-Routhiau V, Waligora-Dupriet AJ. Gnotobiotic mouse immune response induced by Bifidobacterium sp. strains isolated from infants. Appl Environ Microbiol. 2008;74:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Zhang DX, Kang YH, Zhan S, Zhao ZL, Jin SN, Chen C, Zhang L, Shen JY, Wang CF, Wang GQ, Shan XF, Qian AD. Effect of Bacillus velezensis on Aeromonas veronii-Induced Intestinal Mucosal Barrier Function Damage and Inflammation in Crucian Carp (Carassius auratus). Front Microbiol. 2019;10:2663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Paynich ML, Jones-Burrage SE, Knight KL. Exopolysaccharide from Bacillus subtilis Induces Anti-Inflammatory M2 Macrophages That Prevent T Cell-Mediated Disease. J Immunol. 2017;198:2689-2698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 42. | Tzipilevich E, Russ D, Dangl JL, Benfey PN. Plant immune system activation is necessary for efficient root colonization by auxin-secreting beneficial bacteria. Cell Host Microbe. 2021;29:1507-1520.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 43. | Freitag TL, Hartikainen A, Jouhten H, Sahl C, Meri S, Anttila VJ, Mattila E, Arkkila P, Jalanka J, Satokari R. Minor Effect of Antibiotic Pre-treatment on the Engraftment of Donor Microbiota in Fecal Transplantation in Mice. Front Microbiol. 2019;10:2685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, Sims M, Kraft CS, Cohen SH, Berenson CS, Korman LY, Ford CB, Litcofsky KD, Lombardo MJ, Wortman JR, Wu H, Auniņš JG, McChalicher CWJ, Winkler JA, McGovern BH, Trucksis M, Henn MR, von Moltke L. SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N Engl J Med. 2022;386:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 324] [Article Influence: 108.0] [Reference Citation Analysis (0)] |