Published online Sep 22, 2022. doi: 10.4291/wjgp.v13.i5.128
Peer-review started: October 30, 2021
First decision: December 12, 2021
Revised: February 19, 2022
Accepted: July 18, 2022
Article in press: July 18, 2022
Published online: September 22, 2022
Processing time: 326 Days and 7.4 Hours
Inflammatory bowel disease (IBD) is an inflammatory disease of the gastr
Core Tip: Inflammatory bowel disease (IBD) is an inflammatory disease of the gastrointestinal tract with no known available treatment. Electrical neuromodulation is the use of electric stimulation of nerves or brain regions as a therapeutic technique. Electrical neuromodulation therapy has been studied as a possible treatment regimen for IBD. There are several forms of neuromodulation that use various types of nerves, such as sacral nerve stimulation, vagal NS (VNS), and tibial NS. As indicated by many clinical investigations, VNS as a potential therapy for IBD has a lot of promise. More research is needed to assess the possibility of VNS as a viable cure for IBD.
- Citation: Yasmin F, Sahito AM, Mir SL, Khatri G, Shaikh S, Gul A, Hassan SA, Koritala T, Surani S. Electrical neuromodulation therapy for inflammatory bowel disease. World J Gastrointest Pathophysiol 2022; 13(5): 128-142
- URL: https://www.wjgnet.com/2150-5330/full/v13/i5/128.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v13.i5.128
Inflammatory bowel disease (IBD) comprises ulcerative colitis (UC) and Crohn’s disease (CD). In these conditions, neutrophils and macrophages produce cytokines, proteolytic enzymes, and free radicals, leading to inflammation and ulceration of the intestinal lining. Both UC and CD share similar manifestations, including abdominal pain, diarrhea, weight loss, and hematochezia. Malnutrition, anemia, fatigue, fever, mouth ulcers, joint pain, and skin lesions, including erythema nodosum or pyoderma gangrenosum, are the common findings[1].
The exact etiology of IBD is unknown, but the altered immune system is suggested as a possible explanation. Risk factors include race, family history, ethnicity, cigarette smoking, and non-steroidal drugs. Colon cancer, skin infection, eye and joint infection, pharmaceutical side effects, and blood clots are all common complications of CD and UC[2].
Diagnostic procedures for IBD include blood work (for anemia and infection), endoscopic procedures (colonoscopy, flexible sigmoidoscopy, upper endoscopy, capsule endoscopy, and balloon aided enteroscopy), and imaging treatments (X-ray, computerized tomography scan, magnetic resonance imaging)[2].
The common medical treatment consists of antibiotics, corticosteroids, immune regulators, aminosalicylates, Janus kinase inhibitor (JAK), anti-tumor necrosis factor-alpha (Anti-TNF-α), anti-integrin, and anti-interleukin (IL) 12/IL23. Adverse reactions include itching, erythema, and delayed allergic reactions can be seen in patients due to these medication use[3]. Therefore, more effective, and safer therapeutic choices are needed. Nerves or brain structures electrical stimulation is being studied as an intervention in a growing number of conditions, including Parkinson’s disease, arthritis, and depressive disorders. The idea that bioelectrical neuromodulation can be used to treat gastrointestinal (GI) disorders has piqued the interest of the medical community[4].
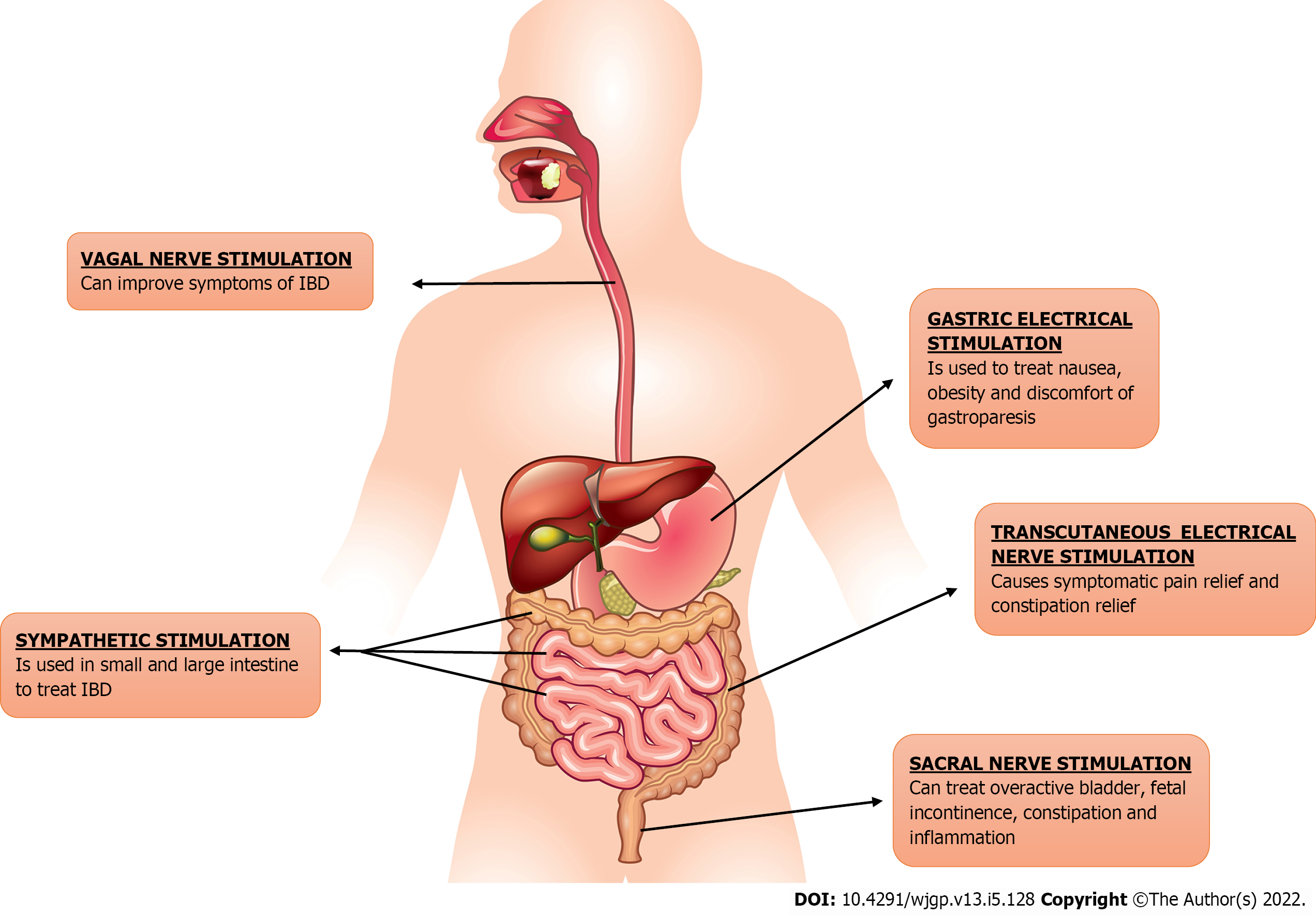
The usage of electric stimulation of nerves or brain centers as a therapeutic tool is being tested in a wide variety of conditions as Parkinson’s disease and schizophrenia. This approach is called neuromodulation or bioelectric neuromodulation, or electroceuticals[5]. GI tract is connected to the central nervous system via vagus and sacral nerve, providing disease-modifying bioelectric neuromodulation therapy opportunities[4]. Electrical neuromodulation (ENM) has been used effectively to treat variety of gastrointestinal disorders including GERD, dyspepsia, gastroparesis, fecal incontinence and constipation as shown in Figure 1. Neuromodulation may be central, as in thalamic stimulation or trans-magnetic stimulation; spinally, as in spinal cord stimulation for ache and movement in spinal cord damage; vagal as regional, as in auricular stimulation for seizures; sacral, as in stimulation for genitourinary (GU)/GI dysfunction; and peripherally, as in electrified stimulation for GU/GI dysfunction peripherally, as in electroacupuncture; and enteric, as in gastric/GI electrical stimulation (GES)[6]. Sacral nerve stimulation (SNS) is the most effective neuromodulation protocol for GI disease that is currently in use[7]. Because of the dysregulation of brain-gut interactions in IBD, ENM can be considered as a treatment option[8]. Numerous electrical neuromodulation techniques for treating IBD, i.e., we will be discussing vagus NS (VNS), SNS, and tibial NS (TNS), in this review.
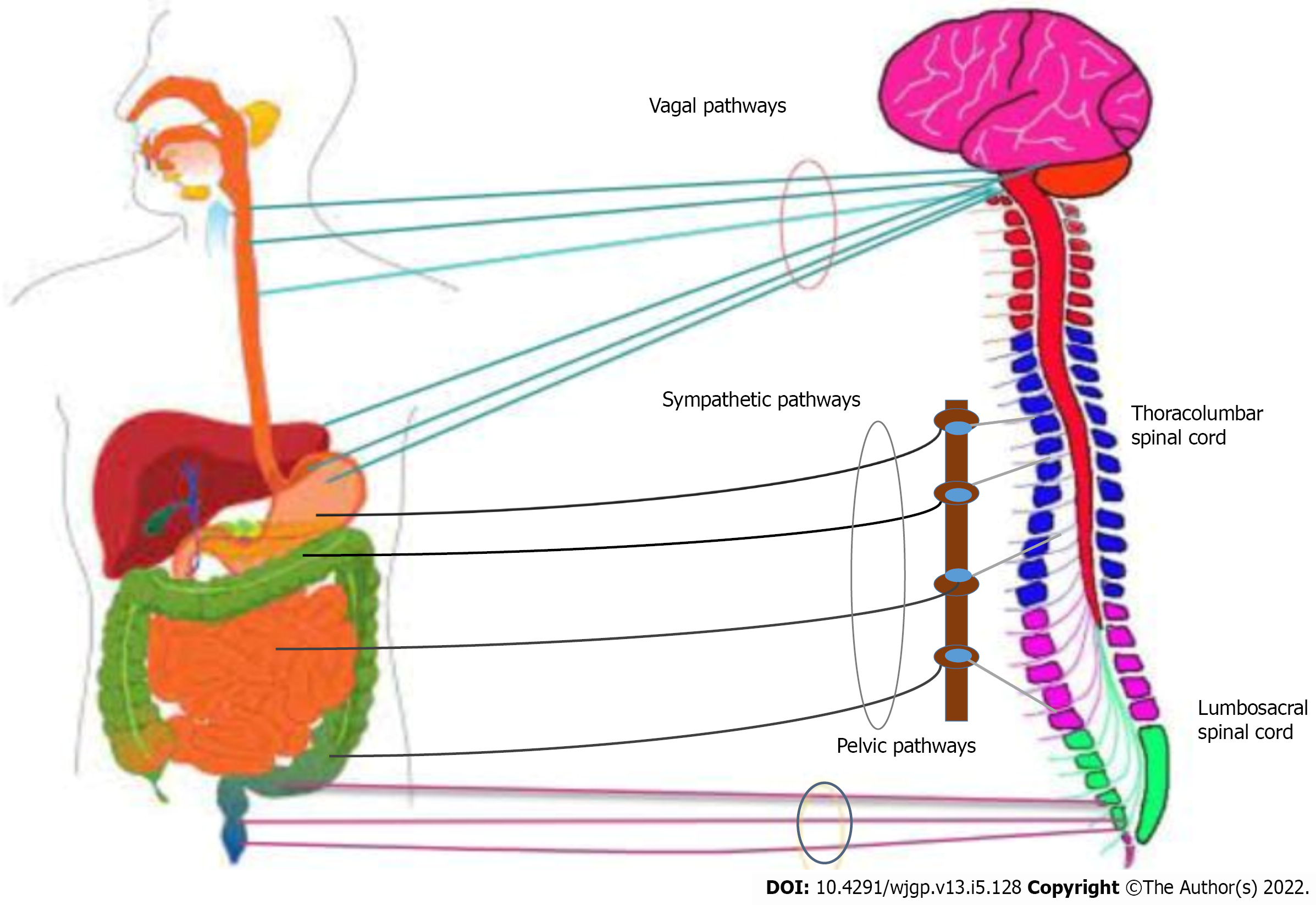
The GI tract (GIT) has intrinsic (enteric nervous system) as well as extrinsic innervation (gut-brain axis). The gut-brain axis is bidirectional in nature, mediated through hormonal, neural, metabolic, and immunological responses. It carries different sensations such as GIT pressure changes, ischemia, poisons, bacterial infection, gastric acidity, and inflammation of the brain through afferent fibers, as demonstrated in Figure 2[9]. These fibers then carry information to the brain, which sends efferent signals to the gut and associated organs, causing toxic substances to be removed, decreasing acid production, increasing satiety, and nausea, to name a few. Recently, the gut microbiota is also included in the gut-brain axis[10], which links intestinal microbiota and the brain[11].
Accurate extrinsic innervation is crucial for the proper functioning of the gut as well as for the balanced emotional and psychological responses through dual connections between brain and gut[12]. Various researches have listed the effects of the brain on the gut or vice versa, signaling, e.g., how depression and impaired brain functioning can increase an individual’s vulnerability to IBD. Whereas other experimentations have shown the prevalence of psychic and anxiety-related disorders in IBD patients, these researches show a close interplay between the gut and the brain[8].
The complex pathway connects the central nervous system (CNS), sympathetic ganglia, enteric nervous system, and gastrointestinal effector tissues. The nucleus tractus solitarius receives the communications via the vagal afferents, while the thoracolumbar spinal cord receives the input via the spinal afferents. Cervical afferents also link the esophagus to the cervical spinal cord. Intestinal-fugal neurons that amplify from the intestine to the CNS are involved in certain afferent routes. To accurately understand the specifics of the extrinsic innervation in the form of the gut-brain axis, the various pathways through which the dual interaction between the gut and the brain takes place are described in Figure 2.
Preganglionic neurons of cranial nerve corticofugal fibers protrude from the medulla’ dorsal motor nucleus of the vagus nerve and innervate the muscular and tissue layer layers of the gut, each within the lamina propria and also the muscularis externa of the viscus wall[13]. Food, antigens, potential pathogens, and symbiotic intestinal microbiota are always present in the gastrointestinal system, and some of them may present as risk factors for intestinal inflammation[14]. TNF-α, a cytokine, is released by activated macrophages, nerve fiber cells, and different tissue layer cells in response to the infective toxin and other harmful stimuli to cause inflammation[15,16]. Counter-regulatory mechanisms consist of capable immune cells and anti-inflammatory cytokines that inhibit inflammatory mediators’ transfer into the circulation. As an anti-inflammatory mechanism, there is a fine relationship between neurological and immune system processes. The dorsal vagus complicated (DVC), which has the sensory nuclei of the solitary tract (NTS), the area postema (AP), and also the dorsal motor core of the cranial nerve (DMN), responds to higher current levels of TNF-α by increasing motor levels activity within the vagus nerve[17]. Two studies have shown that electrical cranial nerve stimulation will suppress inflammation in models of inflammation[18,19]. Furthermore, due to the lack of control on immunological mediating cells, the sub-diaphragmatic vagotomy increases inflammation in the gut. The brain can monitor immunological states and detect peripheral inflammation through two mechanisms.
Stimulation of the vagus nerve is triggered directly or indirectly by cytokines discharged by nerve fiber cells, macrophages, and different vagus-associated immune cells and indirectly by chemoreceptors[20]. Visceral afferent vagus fibers within the neural structure nodosum principally end in the DVC of the medulla oblongata. DVC includes NTS, the dorsal motor nucleus of the vagus (DMV), and also the post-mammary region (AP)[21]. DMN is a critical region for the formation of preganglionic vagus efferent fibers. The majority of sensory vagal input is received by the NTS[22]. The paraventricular nucleus (PVN) of hypothalamus, receives signals from the NTS. PVN causes the production and release of corticotropin-releasing hormones (CRH), which is an important chemical on the hypothalamus-pituitary-adrenocortical (HPA) axis (described below)[23].
Circulating cytokines in the humoral route interact directly with areas of the brain involved in anti-inflammatory response. Circulatory IL-1 and TNF can move across the blood-brain barrier through a saturated transport mechanism to get into the CSF and interstitial space of the brain and spinal cord, where they can directly stimulate the brain to produce an anti-inflammatory reaction[24]. Circumventricular organs that lack regular blood-brain barrier protection uses cytokine-to-brain transmission. Postrema is the most well-known circumventricular organ[23].
Followings are a few pathways that are included in the neural control of gut inflammation.
The HPA axis is composed of three major components (the hypothalamus, the anterior and posterior pituitary gland, and the adrenal cortex). Steinlein[25] demonstrated the role of vagal afferents in the neuro-immune axis in the control of the HPA axis. According to L E Goehler and co-workers, peripheral administration of lipopolysaccharides (LPS), a pro-inflammatory cytokine that stimulates vagal afferents via IL-1 receptors, induces the production of IL-1, a pro-inflammatory cytokine[26]. The vagal nerve is susceptible to peripheral pro-inflammatory cytokines generated by macrophages and other immune cells, such as IL-1, IL-6, and TNF-α[27]. Vagal afferent receptors (IL-1 beta) convey information to the parvo-cellular zone of the paraventricular nucleus of the hypothalamus (PVH) around corticotrophin-releasing-factor (CRF)-containing neurons. These CRF neurons subsequently drive the hypophysis to release the adreno-corticotrophin hormone, which stimulates the adrenal glands to release glucocorticoids, reducing peripheral inflammation[27]. Glucocorticoids affect the inflammatory response by suppressing immune cell release of pro-inflammatory cytokines, as well as inhibiting vasodilation and vascular permeability caused by inflammation. The brain can influence the activity of functional intestinal effector cells such as immune cells, smooth muscle cells, epithelial cells, interstitial cells of Cajal, enteric neurons, and enterochromaffin cells through neuronal and hormonal communication lines[24]. These cells, on the other hand, are influenced by the gut microbiome. The internal organ microbiota encompasses a vital influence on the intestinal axis, not solely through native interaction with intestinal cells conjointly with the enteric systema nervosum, but also through direct effects on the system and metabolic processes[28]. Emerging evidence supports the function of gut bacteria in anxiety and depressive-like behavior[29].
Acetylcholine is a crucial neurochemical and neuromodulator within the brain, mediates neuronal transmission in sympathetic and parasympathetic neurons, and acts as a primary neurotransmitter in parasympathetic/pneumogastric neural structure corticoefferent neurons[23]. This neurotransmitter acts through two varieties of receptors: muscarinic (metabotropic) and nicotinic (ionotropic)[30,31]. The seven component of the nicotinic acetylcholine receptor is displayed on phagocytes[32]. TNF production from human macrophages used by endotoxins is considerably reduced by acetylcholine through a post-transcriptional mechanism and is concentration-dependent. The authors demonstrated the connection of a bungarotox-insensitive vasoconstrictor receptor in suppressing cytokine production in vitro by neurotransmitter mistreatment specific muscarinic and nicotinic agonists and antagonists[23]. Apart from TNF, acetylcholine suppresses alternative endotoxin-inducible pro-inflammatory proteins reminiscent of IL-1, IL-6, and IL-18 through a post-transcriptional mechanism. However, acetylcholine has no effect on the discharge of the anti-inflammatory cytokine IL-10 from endotoxin-stimulated macrophages[32]. Nicotinic acetylcholine receptors are a ligand-gated pentameric ion channel family. The HPA axis (afferent vagal Fibers) activates the cholinergic anti-inflammatory pathway. Proinflammatory cytokines discharged throughout the immunologic response will activate vagal receptive signals, resulting in direct or indirect activation (via the core of the neurons of the solitary tract NTS) of the vagal efferents in the DMN. As a result, the sensory vagal afferents and motor vagus efferents produce an inflammatory reflex that constantly monitors and modifies the inflammatory condition in the periphery[33]. Since the tetravalent guanyl hydrazone CNI1493 induces the activation of the vagus and, by activating the cholinergic anti-inflammatory signal pathway, confers anti-inflammatory effects in each native and general model of inflammation, it’s going to be attainable to activate the cholinergic anti-inflammatory pathway (with centrally active substances)[34].
The celiac, superior mesenteric, and inferior mesenteric ganglia contain the cell bodies of the bulk of postganglionic sympathetic neurons that innervate the gastrointestinal tract[35]. In gut noradrenaline (NA) is the primary neurotransmitter released from sympathetic postganglionic nerve terminals; however, ATP and neuropeptide Y (NPY) can also engage in sympathetic neurotransmission within the GI tract[36,37]. The vagal afferent Fibers terminate in the NTS, which ultimately activates the central autonomic network (CAN). The sympathetic outlet is operated by 5 CAN brain regions (the paraventricular nucleus of the neural structure HPV, the noradrenergic cluster A5, the area of the caudal raphe, the rostral ventrolateral medulla, and ventromedial medulla)[38]. By increasing sympathetic outflow, the vagal nerve can generate a non-direct anti-inflammatory reaction. Abe et al[39] explained the role of the C1 adrenergic cluster. They concluded that these neurons are concerned with protecting the result of stress in reperfusion injuries because of nephritic anemia via a sympathetic pathway. They conjointly mentioned how activation of vagal afferents in mice twenty-four hours before injury considerably reduced acute excretory organ inflammation and plasma levels of TNF-α[30]. Tyrosine hydroxylase is found in the lamina propria, the submucosa, the ganglia of the nerve plexus, and lymph follicles (Peyer’ plaques)[40]. Adrenergic receptors of diverse types are expressed by macrophages. In vitro, beta receptors mediate the anti-inflammatory effects of agonists on macrophages derived from the intestine[41]. Although sympathetic nerves decrease gut inflammation, persistent nerve stimulation should be avoided as it can promote stasis and aggravate bacterial growth in Crohn’s illness[4].
Through an association between the VN and the splenic nerve, the Vago-splenic pathway works collectively[42]. In general inflammatory conditions, the spleen is a crucial supply of inflammatory cytokines, and excision considerably reduces circulating TNFα levels in mouse endotoxemia[43]. Tracey et al identified the vagal splenic route, finding that VNS caused the celiac ganglion to produce acetylcholine (Ach), which subsequently adhered to the c7nAChR of the splenic neve to release norepinephrine (NE) in the spleen[24]. Following that, it binds to beta two adrenergic receptors of splenic lymphocytes, which produces acetylcholine, which will act on the α c7nAChR of splenic macrophages limiting release of TNF, resulting in an anti-inflammatory impact[44]. According to Martelli et al[45], there is also a non-nervous relationship between the vagus and splenic sympathetic nerves. In another article, Martelli et al[46] noted how the sympathetic nerve, not the vagal nerve, is the efferent mediator of the cholinergic anti-inflammatory pathway (splenic nerve).
Electrical and physiological stimulation of receptive neurons, particularly afferent nerves of the digestive tract, generates the release of transmitters at their peripheral ends, most often tachykinin and the amide (CGRP) linked to the calcitonin gene[47]. CGRP serves a number of purposes via serving as a modulator, transmitter, and hormone. CGRP-containing nerve Fibers are numerous surrounding blood vessels, particularly arterioles, suggesting that they may have a physiologic role in regulating blood flow to the gastric mucosa[48]. Capsaicin-sensitive afferent Fibers conduct protective anti-inflammatory activities in the gastrointestinal tract by releasing peptides from their peripheral ends[49-52]. Sensory inputs innervating the stomach generate CGRP, which reduces mucosal damage and improves mesenteric and mucosal blood flow in stomachic ulcer models in rats and mice[50,51]. Once administered at the time of injury, capsaicin promotes the discharge of neuropeptides and reduces the extent of ethanolin-induced gastric injury in rats[49,52]. This impact is operated by the discharge of CGRP from receptive nerve endings before their degeneration, which happens hours or days after the capsaicin injection. Numerous studies have shown that hCGRP (837), a fraction of human CGRP lacking the cyclic loop at the amino terminus of native CGRP, inhibits the action of exogenous CGRP[53,54].
VNS is a unique therapeutic method for chronic TNF-mediated inflammatory illnesses in the framework of bioelectronic medicine, with the objective of employing tiny stimulators to provide electrical nerve signals for therapeutic, rather than pharmaceutical, purposes[55-57]. VNS is already used to treat depression and epilepsy which is resistant to drugs[58]. There is currently no recognized curative medicine for IBD. Current medicines reduce disease activity, and when therapy is stopped, the condition recurs. TNF is one of the most significant cytokines in IBD, and anti-TNF medicines have transformed the therapy of the disease[59]. New compounds are available that target pro-inflammatory cytokines such as IL-12, IL-23, anti-integrin, and anti-JAK therapies[60,61]. In the case of treatment failure or an IBD consequence (perforation, abscess, stenosis), surgery is an option, although the disease reappears after the procedure. While anti-TNF medications are effective in IBD, there is a 20%-30% initial non-response rate, and the yearly chance of anti-TNF reactivity is 13% per patient year for infliximab and 20% per patient year for adalimumab[62-64]. This lack of secondary response is attributable to I the formation of autoantibodies, particularly for infliximab but also, to a lesser extent, for adalimumab, or (ii) secondary failure due to insufficient dose[65,66]. As a result of the risk of adverse effects and the requirement for ongoing therapy for these disorders, patients are increasingly hesitant to begin and maintain these treatments once they are in remission. The non-compliance rate is 30%-50%[67,68]. Therefore, targeted therapy for pro-inflammatory cytokines such as TNF-alpha and others using CAP could be extremely helpful with fewer side effects, no compliance issues, and cheaper than biologics (i.e., anti TNF-alpha). In this case, targeting the VN’s anti-inflammatory characteristics might be of interest. VNS, particularly as a non-drug therapy, has the potential to be employed as an alternative to conventional biological therapies. A number of animal and clinical research have been undertaken in recent years to investigate the efficacy of VNS in the treatment of IBD (Supplemen
Vagotomy has been found in several studies to enhance the disease activity index (DAI), gross and pro-inflammatory cytokine levels in mice[69-71]. To replicate UC, Chen and colleagues employed dextran sodium sulphate (DSS) colitis in mice. They observed that VNS eased cerebral cortical microinfarcts induced by a two-photon laser and reduced DSS colitis. This neuroprotection was linked to decreased blood-brain barrier permeability and inflammatory processes[72].
Indirect data suggests that a vagal anti-inflammatory action plays a role in IBD. Vagal activity has been demonstrated to be inversely associated with inflammatory markers in healthy and cardiac patients as evaluated by HRV spectral analysis[73]. VNS might be an attractive method for the treatment of IBD based on pre-clinical results in rats with colitis and two recent clinical pilot trials targeting two distinct categories of patients with active CD, either ignorant of anti-TNF on inclusion or resistant to biologics[74].
Miceli and Jacobson[75] published the first data on the anti-inflammatory effects of VN in digestive inflammation. Colitis in rats with 2,4,6 trinitrobenzenesulfonic acid (TNBS) improved with early treatment of anticholinesterase medications such as neostigmine, which does not cross the blood-brain barrier, or physostigmine. This impact was more pronounced with physostigmine, indicating a dominating central mechanism. In mice, vagotomy aggravated experimental colitis, indicating that NV serves a protective function[76]. It was demonstrated that in the non-vagotomized watchful rat, 3 h per day for five consecutive days, low-frequency VNS (5 Hz) led in an improvement in TNBS colitis in rats[31] VNS inhibited weight loss and inflammatory indicators.
An improvement in a multivariate measure of colitis was also observed as an anti-inflammatory impact (which includes body mass, temperature, and motor function, macroscopic area of the lesions, histological and biological parameters such as myeloperoxidase activity, cytokines, and mRNAs related to cytokines)[77]. Sun et al[32] showed that chronic VNS increased the clinical activity index, the histological scores, the biological inflammation due to myeloperoxidase activity, the iNOS, TNF, and IL-6 Levels among rats with colitis, and the inflammatory response induced by LPS in cells of the human epithelial colorectal adenocarcinoma (Caco2) by ACh in vitro. In 2000, Kevin Tracey’s team first described CAP[78,79]. They found that there is an inflammatory reflex in which proinflammatory cytokines stimulate vagus afferents, which activate vagus efferents, causing the production of these cytokines by tissue macrophages, mainly TNF, but also other pro-inflammatory cytokines such as IL-6. IL-1b, but not IL-10, an anti-inflammatory cytokine VN has anti-inflammatory effects because it inhibits pro-inflammatory cytokines.
Decreased vagus activity was observed to be related to systemic inflammatory markers in both IC and CD patients[80,81]. VNS improved several inflammatory markers in rats’ small intestines, including fecal quality, inflammatory processes, and leukocyte infiltration. Furthermore, considerable cardiac and respiratory changes happened with supra-threshold cervical VNS, while abdominal VNS caused alterations. Due to the lack of side effects and effectiveness in reducing inflammation, abdominal VNS appears to be a viable alternative to cervical VNS. This evidence supports the application of this novel peripheral nerve network for abdominal VNS as a potential therapy for IBD like CD[82]. A pilot study on VNS was carried out for the first time in patients with moderate to severe celiac disease as an alternative to drug anti-TNF therapy or in untreated patients in a translational approach from the laboratory to the bedside[56]. A VNS device and electrode were implanted in nine patients. At the time of implantation, two patients had failed immunosuppressive drugs (azathioprine), while the other seven received no treatment[56]. ENV was carried out on a continuous basis over a period of one year. In April 2012, the first patient was implanted, and then the last in March 2016.
Due to increasing condition, two patients were removed from the trial after three months of neurostimulation: The first had ileocecal resection but elected to continue neurostimulation until the end of the study due to an early good response and rejection of pharmaceutical therapy. The second patient took infliximab and azathioprine and continued to use an active VNS. Six patients were in remission owing to neurostimulation alone after one year of follow-up, while the seventh was in relapse. In April 2012, the first patient to get the implant was in remission from azathioprine in ileal CD with a history of ileocecal resection[56]. In conclusion, five out of seven patients who received the one-year VNS attained clinical improvement (CDAI 150), and all gained the CDAI70 response (CDAI decreased 70 points from baseline). Similarly, the Endoscopic CD Severity Score (CDEIS) decreased from 60% to 100% in five patients. Other than complaints caused by the output current/intensity of the device, no adverse events were observed[56]. In patients with UC decrease activity has been linked with autonomic function[83].
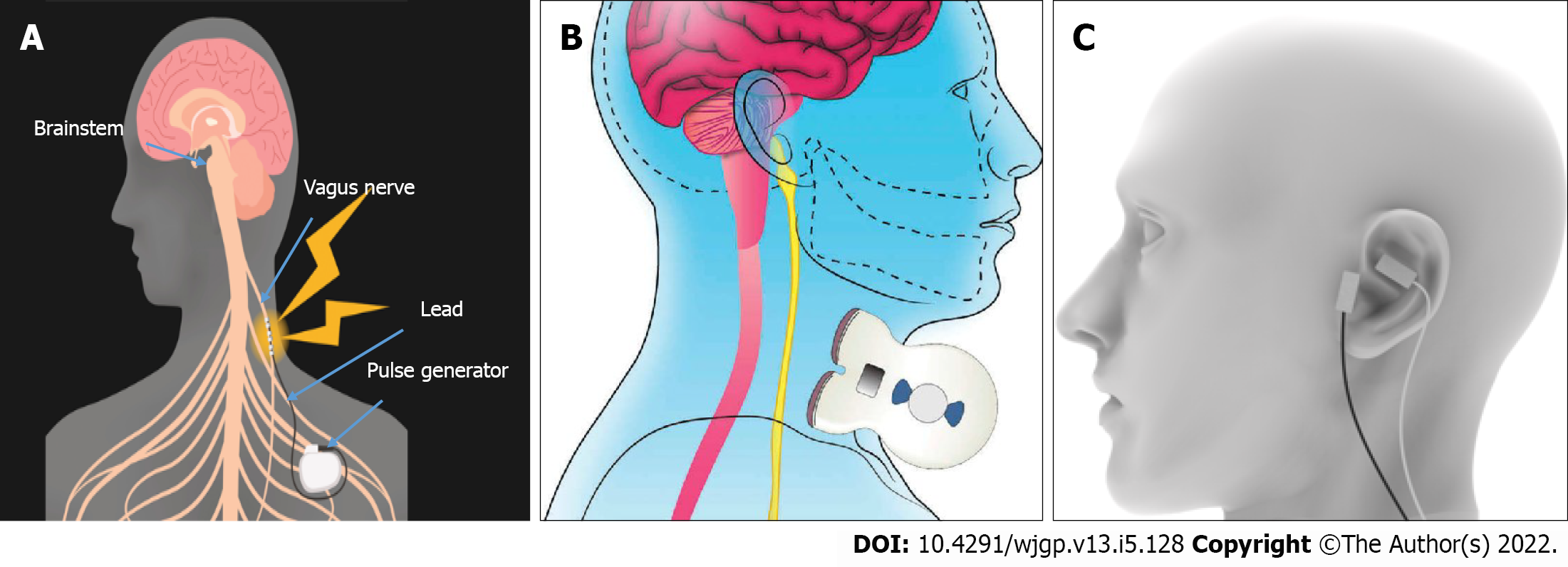
Currently, the generally used VNS therapeutic equipment is invasive and implantable. The VNS Therapy System consists of an implanted pulse generator, a bipolar VNS electrode, a small handheld device, programming software, a programming stick, and hand magnets. VNS is traditionally used to treat epilepsy and depression, as well as in the two pilot studies in patients with CD.
It is invasive, generally performed by a neurosurgeon who is experienced in the surgery, and lasts 1 h with minimal side effects. Noninvasive (n) VNS may be beneficial in certain patients who are afraid to have surgery in a vasculo-nervous location, such as the vein or the external carotid artery, which are close to the VN. Furthermore, if the device is removed, the electrode wrapped around the VN is normally kept in place, although some writers have removed it without causing significant nerve and artery damage[84]. Anesthesia is necessary for the operation, which requires two small incisions. The bipolar lead is looped around the left cervical VN and the pulse generator is positioned in the top-left chest. Physicians program the stimulator with a small handheld device, programming software, and a programming stick. After implantation, patients are given a wearable magnet to manipulate the stimulation on their own. The left vagus, which is more intimately linked to cardiovascular activities, is considered more suitable than the right cervical vagus. In the treatment of epilepsy, right-sided VNS has been observed in numerous patients[85-87]. Right-sided VNS appears to be as effective as, if not more successful than, left VNS[88]. Gadgets stimulating the VN on the cervical degree or on the auricular degree were produced (Figure 3). Certainly, the cymbal concha of the external ear is innervated by means of a sensory auricular branch of the VN that sends projection inside the NTS in cats and human beings[89-91]. These noninvasive devices have not been associated with any significant major side effects. In comparison to invasive VNS, n-VNS has the disadvantage of low compliance, which is a major concern in the treatment of IBD. Indeed, 30%-40% of IBD patients fail to take their medicine[92]. One can wonder if the same problem arises with these noninvasive devices. Furthermore, in the case of the Gamma core device, the repeatability of the placement of the discs in contact with the VN is unknown. Finally, ta-VNS was less efficient than VNS in decreasing the LPS-induced serum cytokine (TNF, IL-1, and IL-6) response in a septic shock animal[93].
An unexpected receptor mechanism underpins the anti-inflammatory effect of the Vagus nerve. In comparison to many “classical” physiological activities, which might be managed with the aid of metabotropic mAChRs, the anti-inflammatory effects of the Vagus nerve are mediated via ionotropic nicotinic acetylcholine receptors (nAChRs)[94]. The frequency of stimulation for VN activation is critical to the function of various therapies[95-100]. A couple of studies have indicated that mAChRs, especially the M1 mAChR, play a role in this regulation in endotoxemia, inflammatory bowel disorder (colitis), hemorrhagic shock, and other illnesses[101,102-104].
Increased cholinergic transmission in the brain with centrally acting acetylcholinesterase inhibitors, particularly galantamine, leads to inhibition of unusual inflammatory responses generated by vagus nerve impulses in mice models of endotoxemia, colitis, and lupus[105-107]. The most recent work, which used targeted optogenetic stimulation and sophisticated pharmacological methods, discovered that forebrain signal transduction and M1 mAChR play a unique role in the modulation of peripheral inflammatory responses in endotoxemia mice via vagus nerve transduction[108]. VNS blocks splenic TNF, which has been identified as a primary contributor to systemic TNF. It is critical to understand how the vagus nerve regulates cytokines in the spleen. The vagus nerve innervates the celiac ganglia and the superior mesenteric ganglion, which have been shown to provide neurons to the splenic nerve[101].
The sacral nerves are divided into five pairs. Each contains an afferent and efferent component, allowing for effective interaction between the lower GIT and the nervous system. The activity of the lower GIT (descending colon, rectum), sexual organs, and urinary bladder is modulated by the parasympathetic component of sacral nerves. The principal somatic nerve of the sacral plexus is the pudendal nerve (S2-S4). It is both sensory and motor. The external anal sphincter, which is under our conscious control, receives sensory and motor innervation from it. It also gives sensation to the external genitalia, the skin around the anal area, the anal canal, the perineum, and motor innervation to the external urethral sphincter.
SNS, also known as “sacral neuromodulation”, is a relatively new and promising treatment option. SNS uses an implanted device that stimulates the S3 nerve root and offers a wide range of applications in conditions such as urgency urinary incontinence, pelvic pain, detrusor stimulation with transurethral approach, FI, etc. Following are some applications of SNS in relation to IBD[3].
Experimental and clinical evidence from several studies signifies the potential of SNS as a treatment option for IBD Patients who have received SNS had less severe mucosal lesions than those who have not received SNS. SNS also improves the recovery of enema caused by trinitrobenzene sulfonic acid (TNBS enema). With elevated TNF1 and trypsin levels, SNS also increases the number of mucosal neutrophils. SNS also stopped TNBS-induced inflammatory factors, including IL-4 and IL-1, from rising. All of these variables indicate that sacral neuromodulation is beneficial in restoring the intestinal barrier following mucosa injury[109]. In IBD, SNS has a significant anti-inflammatory impact. SNS enhanced the spinal afferent-vagal efferent pathway and improved autonomic function by increased vagal efferent activity. SNS also causes anti-inflammatory effects due to the SNS-mediated release of Ach[110]. In a study using the TNBS rat model, sacral neuromodulation lowered the level of pro-inflammatory cytokines and improved colonic inflammation[111].
The inability to regulate bowel movements, which can range from modest rectum leaks to total bowel control, is known as FI. Viability of sacral neuromodulations as a treatment option for FI is tremendous. Many studies have demonstrated that FI responds positively to SNS. SNS has proven to be a reliable method for dealing with FI in children[112]. Clinical trials have also shown that SNS can help with FI[113]. Another extensive approach conveys the benefits of SNS in patients with neuropathic FI[114].
Other than SNS and VNS, other neuromodulation methods to treat IBD are TNS and Spinal Cord Stimulation (SCS). The sensory, motor and autonomic fibers in the tibial nerve make it a mixed nerve. It is caused by the L4-S3 nerves, which feed the colorectum, bladder, and pelvic floor. TNS uses electrical impulses to treat bladder and pelvic floor issues. TNS is classified into two types: Percutaneous TNS (PTNS) and transcutaneous TNS (TCTNS) (TTNS). The former makes use of a needle electrode, whilst the latter makes use of a sticky electrode[3]. PTNS is a minimally invasive method that has been demonstrated to be beneficial in treating overactive bladder, FI, and pelvic discomfort. Having few side effects is highly convenient, but it is limited by the necessity that patients visit the clinic weekly to obtain the series of treatments[115].
The actual mechanism of TNS is uncertain however it appears to involve excitation of afferent pathways to the sacral spinal cord as well as regulation of efferent nerves[116]. Retrospective research looked at 183 individuals with refractory overactive bladder (OAB) who had 30-min PTNS sessions for 12 wk during nine years. There was a significant improvement in micturition frequency, nocturia, and urge incontinence episodes in the PTNS group, with the impact obvious by week 10 of therapy. With a wide range of PTNS times, 61.5 percent of subjects self-proclaimed > 50% improvement in signs and symptoms, raising the subjective accomplishment percentages[117]. For a 12-wk treatment period, a recent randomized research of forty women with nocturia of weekly TTNS periods compared pelvic floor muscle training and behavioral therapy. Both medicines improved sleep quality by reducing the number of times people awoke to pee (45 percent reduced by 1 in both groups)[118]. A spinal cord stimulator (SCS) is surgically implanted under the skin and delivers a weak electrical current to the spinal cord. Current from a pulse generator is carried to the spinal cords’ nerve fibers by thin wires. When the SCS is activated, it stimulates the nerves in the area where a person is feeling pain. The pain signal is altered and masked by electrical impulses, prohibiting it from going to the brain[119].
For more than a half-century, spinal cord stimulation (SCS) has been used to treat chronic pain. Several studies have demonstrated that SCS can help with stomach discomfort[120]. Randomized trial has shown that SCS can lessen diarrhea and pain pain in persons with irritable bowel syndrome[121]. Although it has been quite successful, some people might experience device-related challenges such as pain at the implantation site or subsequent infections. But it doesn’t cause any serious complications like paralysis or hemorrhage in the epidural space[122,123].
The digestive system’s broad and approachable interaction with the CNS, the predominance of IBD, and the lack of effective treatment options make it an appealing target for bioelectrical neuromodulation therapy for digestive system innervation. A wide range of gastrointestinal problems has been treated with various degrees of success. This approach has been tried with different degrees of effectiveness in a range of gastrointestinal diseases. SNS for faecal incontinence has become a popular bio-electric therapy for gastrointestinal disorders. The development of bioelectrical digestive system neuromodulation medicines requires investigation. The advancement of our understanding of the multiple roles of the mixed nerve components, such as vagus nerves and sympathetic routes to the intestines, should allow us to take IBS treatment to a new level.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American College of Physicians.
Specialty type: Health care sciences and services
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soldera J, Brazil; Wen XL, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Szigethy E, McLafferty L, Goyal A. Inflammatory bowel disease. Child Adolesc Psychiatr Clin N Am. 2010;19:301-318, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Mayo Clinic gastroenterology and hepatology board review 95 ed. Oxford Medicine Online. Inflammatory bowel disease (IBD)-Symptoms and causes-Mayo Clinic. [cited 6 July 2022] . [DOI] [Full Text] |
| 3. | Cheng J, Shen H, Chowdhury R, Abdi T, Selaru F, Chen JDZ. Potential of Electrical Neuromodulation for Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:1119-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Payne SC, Furness JB, Stebbing MJ. Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms. Nat Rev Gastroenterol Hepatol. 2019;16:89-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Erratum for the Research Article "Identification of Integrator-PP2A complex (INTAC), an RNA polymerase II phosphatase" by H. Zheng, Y. Qi, S. Hu, X. Cao, C. Xu, Z. Yin, X. Chen, Y. Li, W. Liu, J. Li, J. Wang, G. Wei, K. Liang, F. X. Chen, Y. Xu. Science. 2021;371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Adams D, Stocker A, Lancaster W, Abell T. The Surgeon's Role in Gastric Electrical Stimulation Therapy for Gastroparesis. J Gastrointest Surg. 2021;25:1053-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Engel MA, Neurath MF. New pathophysiological insights and modern treatment of IBD. J Gastroenterol. 2010;45:571-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Smart NA, Fisher S, Pearson MJ. On "Physical Therapist Clinical Practice Guideline for the Management of Individuals With Heart Failure." Shoemaker MJ, Dias KJ, Lefebvre KM, Heick JD, Collins SM. Phys Ther. 2020;100:14-43. Phys Ther. 2020;100:1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Furness JB, Rivera LR, Cho HJ, Bravo DM, Callaghan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 2013;10:729-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 377] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 10. | Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 1125] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 11. | Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19:334-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 300] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 12. | Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 905] [Article Influence: 113.1] [Reference Citation Analysis (3)] |
| 13. | Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 821] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 14. | Becker C, Neurath MF, Wirtz S. The Intestinal Microbiota in Inflammatory Bowel Disease. ILAR J. 2015;56:192-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 15. | Bonaz B, Sinniger V, Pellissier S. Vagus nerve stimulation: a new promising therapeutic tool in inflammatory bowel disease. J Intern Med. 2017;282:46-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 16. | Tracey KJ. The inflammatory reflex. Nature. 2002;420:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2489] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 17. | Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 371] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | HARRIS GW. The hypothalamus and endocrine glands. Br Med Bull. 1950;6:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, Watkins LR. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 213] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol. 2016;594:5781-5790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 365] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 21. | Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125-134. [PubMed] |
| 22. | Caulfield MP, Birdsall NJM; International union of pharmacology. XVII. Classification of muscarinic acetylcholine receptors. In: Pharmacol, 1998. |
| 23. | Lindstrom J. Nicotinic acetylcholine receptors in health and disease. Mol Neurobiol. 1997;15:193-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 341] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2722] [Cited by in RCA: 2969] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 25. | Steinlein O. New functions for nicotinic acetylcholine receptors? Behav Brain Res. 1998;95:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Leonard S, Bertrand D. Neuronal nicotinic receptors: from structure to function. Nicotine Tob Res. 2001;3:203-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Tracey KJ. Hacking the inflammatory reflex. The Lancet Rheumatology. 2021;3:e237 e239. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 318] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 29. | Gacias M, Gaspari S, Santos PM, Tamburini S, Andrade M, Zhang F, Shen N, Tolstikov V, Kiebish MA, Dupree JL, Zachariou V, Clemente JC, Casaccia P. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 30. | Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1521] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 31. | Meregnani J, Clarençon D, Vivier M, Peinnequin A, Mouret C, Sinniger V, Picq C, Job A, Canini F, Jacquier-Sarlin M, Bonaz B. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci. 2011;160:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 32. | Sun P, Zhou K, Wang S, Li P, Chen S, Lin G, Zhao Y, Wang T. Involvement of MAPK/NF-κB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS One. 2013;8:e69424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 33. | Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 553] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 34. | Buller KM. Role of circumventricular organs in pro-inflammatory cytokine-induced activation of the hypothalamic-pituitary-adrenal axis. Clin Exp Pharmacol Physiol. 2001;28:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil. 2010;22:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Evans RJ, Surprenant A. Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. Br J Pharmacol. 1992;106:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 125] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Lundberg JM, Rudehill A, Sollevi A. Pharmacological characterization of neuropeptide Y and noradrenaline mechanisms in sympathetic control of pig spleen. Eur J Pharmacol. 1989;163:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989;491:274-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 394] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 39. | Abe C, Inoue T, Inglis MA, Viar KE, Huang L, Ye H, Rosin DL, Stornetta RL, Okusa MD, Guyenet PG. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat Neurosci. 2017;20:700-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 40. | Cervi AL, Lukewich MK, Lomax AE. Neural regulation of gastrointestinal inflammation: role of the sympathetic nervous system. Auton Neurosci. 2014;182:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell. 2016;164:378-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 495] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 42. | Bellinger DL, Felten SY, Lorton D, Felten DL. Origin of noradrenergic innervation of the spleen in rats. Brain Behav Immun. 1989;3:291-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623-1628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 541] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 44. | Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1134] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 45. | Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci. 2014;182:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 46. | Martelli D, Farmer DG, Yao ST. The splanchnic anti-inflammatory pathway: could it be the efferent arm of the inflammatory reflex? Exp Physiol. 2016;101:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1335] [Cited by in RCA: 1316] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 48. | Sternini C, Reeve JR Jr, Brecha N. Distribution and characterization of calcitonin gene-related peptide immunoreactivity in the digestive system of normal and capsaicin-treated rats. Gastroenterology. 1987;93:852-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 231] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Holzer P, Lippe IT. Stimulation of afferent nerve endings by intragastric capsaicin protects against ethanol-induced damage of gastric mucosa. Neuroscience. 1988;27:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 162] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Holzer P, Lippe IT. Role of calcitonin gene-related peptide in gastrointestinal blood flow. Ann N Y Acad Sci. 1992;657:228-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Jacobson ED. Vascular mediation of gastric mucosal damage and cytoprotection. Indian J Physiol Pharmacol. 1990;34:223-234. [PubMed] |
| 52. | Lambrecht N, Burchert M, Respondek M, Müller KM, Peskar BM. Role of calcitonin gene-related peptide and nitric oxide in the gastroprotective effect of capsaicin in the rat. Gastroenterology. 1993;104:1371-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Han SP, Naes L, Westfall TC. Inhibition of periarterial nerve stimulation-induced vasodilation of the mesenteric arterial bed by CGRP (8-37) and CGRP receptor desensitization. Biochem Biophys Res Commun. 1990;168:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Gardiner SM, Compton AM, Kemp PA, Bennett T, Bose C, Foulkes R, Hughes B. Antagonistic effect of human alpha-CGRP [8-37] on the in vivo regional haemodynamic actions of human alpha-CGRP. Biochem Biophys Res Commun. 1990;171:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Jo BG, Kim SH, Namgung U. Vagal afferent fibers contribute to the anti-inflammatory reactions by vagus nerve stimulation in concanavalin A model of hepatitis in rats. Mol Med. 2020;26:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 56. | Bonaz B, Sinniger V, Hoffmann D, Clarençon D, Mathieu N, Dantzer C, Vercueil L, Picq C, Trocmé C, Faure P, Cracowski JL, Pellissier S. Chronic vagus nerve stimulation in Crohn's disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. 2016;28:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 364] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 57. | Olofsson PS, Tracey KJ. Bioelectronic medicine: technology targeting molecular mechanisms for therapy. J Intern Med. 2017;282:3-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 58. | Bonaz B, Picq C, Sinniger V, Mayol JF, Clarençon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil. 2013;25:208-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 59. | Peyrin-Biroulet L. Anti-TNF therapy in inflammatory bowel diseases: a huge review. Minerva Gastroenterol Dietol. 2010;56:233-243. [PubMed] |
| 60. | Pagnini C, Pizarro TT, Cominelli F. Novel Pharmacological Therapy in Inflammatory Bowel Diseases: Beyond Anti-Tumor Necrosis Factor. Front Pharmacol. 2019;10:671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 61. | D'Amico F, Peyrin-Biroulet L, Danese S, Fiorino G. New drugs in the pipeline for the treatment of inflammatory bowel diseases: what is coming? Curr Opin Pharmacol. 2020;55:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (2)] |
| 62. | Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644-659, quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 461] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 63. | Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol. 2009;104:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 64. | Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn's disease: a systematic review. Am J Gastroenterol. 2011;106:674-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 334] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 65. | Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn's disease. Aliment Pharmacol Ther. 2011;33:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 471] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 66. | Chaparro M, Guerra I, Muñoz-Linares P, Gisbert JP. Systematic review: antibodies and anti-TNF-α levels in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:971-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 67. | Chan W, Chen A, Tiao D, Selinger C, Leong R. Medication adherence in inflammatory bowel disease. Intest Res. 2017;15:434-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 68. | Torres J, Ellul P, Langhorst J, Mikocka-Walus A, Barreiro-de Acosta M, Basnayake C, Ding NJS, Gilardi D, Katsanos K, Moser G, Opheim R, Palmela C, Pellino G, Van der Marel S, Vavricka SR. European Crohn's and Colitis Organisation Topical Review on Complementary Medicine and Psychotherapy in Inflammatory Bowel Disease. J Crohns Colitis. 2019;13:673-685e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 69. | Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 315] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 70. | Ghia JE, Blennerhassett P, El-Sharkawy RT, Collins SM. The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G711-G718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 71. | Di Giovangiulio M, Bosmans G, Meroni E, Stakenborg N, Florens M, Farro G, Gomez-Pinilla PJ, Matteoli G, Boeckxstaens GE. Vagotomy affects the development of oral tolerance and increases susceptibility to develop colitis independently of the alpha-7 nicotinic receptor. Mol Med. 2016;22:464-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Chen X, He X, Luo S, Feng Y, Liang F, Shi T, Huang R, Pei Z, Li Z. Vagus Nerve Stimulation Attenuates Cerebral Microinfarct and Colitis-induced Cerebral Microinfarct Aggravation in Mice. Front Neurol. 2018;9:798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 73. | Frasure-Smith N, Lespérance F, Irwin MR, Talajic M, Pollock BG. The relationships among heart rate variability, inflammatory markers and depression in coronary heart disease patients. Brain Behav Immun. 2009;23:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 74. | Bonaz B. Is-there a place for vagus nerve stimulation in inflammatory bowel diseases? Bioelectron Med. 2018;4:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Miceli PC, Jacobson K. Cholinergic pathways modulate experimental dinitrobenzene sulfonic acid colitis in rats. Auton Neurosci. 2003;105:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Caravaca AS, Levine YA, Drake A, Eberhardson M, Olofsson PS. Vagus Nerve Stimulation Reduces Indomethacin-Induced Small Bowel Inflammation. Front Neurosci. 2021;15:730407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 77. | Bonaz B, Sinniger V, Pellissier S. Therapeutic Potential of Vagus Nerve Stimulation for Inflammatory Bowel Diseases. Front Neurosci. 2021;15:650971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 78. | Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M, Tracey KJ. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 233] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 79. | Jarczyk J, Yard BA, Hoeger S. The Cholinergic Anti-Inflammatory Pathway as a Conceptual Framework to Treat Inflammation-Mediated Renal Injury. Kidney Blood Press Res. 2019;44:435-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 80. | Pellissier S, Dantzer C, Mondillon L, Trocme C, Gauchez AS, Ducros V, Mathieu N, Toussaint B, Fournier A, Canini F, Bonaz B. Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn's disease and irritable bowel syndrome. PLoS One. 2014;9:e105328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 81. | Gunterberg V, Simrén M, Öhman L, Friberg P, Jones MP, Van Oudenhove L, Strid H. Autonomic nervous system function predicts the inflammatory response over three years in newly diagnosed ulcerative colitis patients. Neurogastroenterol Motil. 2016;28:1655-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Payne SC, Furness JB, Burns O, Sedo A, Hyakumura T, Shepherd RK, Fallon JB. Anti-inflammatory Effects of Abdominal Vagus Nerve Stimulation on Experimental Intestinal Inflammation. Front Neurosci. 2019;13:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 83. | Maunder RG, Greenberg GR, Nolan RP, Lancee WJ, Steinhart AH, Hunter JJ. Autonomic response to standardized stress predicts subsequent disease activity in ulcerative colitis. Eur J Gastroenterol Hepatol. 2006;18:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Champeaux C, Landré E, Chassoux F, Mann MW, Devaux B, Turak B. Vagus Nerve Stimulation Removal or Replacement Involving the Lead and the Electrode: Surgical Technique, Institutional Experience and Outcome. World Neurosurg. 2017;99:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 85. | McGregor A, Wheless J, Baumgartner J, Bettis D. Right-sided vagus nerve stimulation as a treatment for refractory epilepsy in humans. Epilepsia. 2005;46:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Spuck S, Nowak G, Renneberg A, Tronnier V, Sperner J. Right-sided vagus nerve stimulation in humans: an effective therapy? Epilepsy Res. 2008;82:232-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Navas M, Navarrete EG, Pascual JM, Carrasco R, Núñez JA, Shakur SF, Pastor J, Sola RG. Treatment of refractory epilepsy in adult patients with right-sided vagus nerve stimulation. Epilepsy Res. 2010;90:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 88. | Navas M, Navarrete EG, Pascual JM, Carrasco R, Núñez JA, Shakur SF, Pastor J, Sola RG. Treatment of refractory epilepsy in adult patients with right-sided vagus nerve stimulation. Epilepsy Res. 2010;90:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Stakenborg N, Viola MF, Boeckxstaens GE. Intestinal neuro-immune interactions: focus on macrophages, mast cells and innate lymphoid cells. Curr Opin Neurobiol. 2020;62:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 90. | Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 505] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 91. | Nomura S, Mizuno N. Central distribution of primary afferent fibers in the Arnold's nerve (the auricular branch of the vagus nerve): a transganglionic HRP study in the cat. Brain Res. 1984;292:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 92. | Frangos E, Ellrich J, Komisaruk BR. Non-invasive Access to the Vagus Nerve Central Projections via Electrical Stimulation of the External Ear: fMRI Evidence in Humans. Brain Stimul. 2015;8:624-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 467] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 93. | Herman ML, Kane SV. Treatment Nonadherence in Inflammatory Bowel Disease: Identification, Scope, and Management Strategies. Inflamm Bowel Dis. 2015;21:2979-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 94. | Zhao YX, He W, Jing XH, Liu JL, Rong PJ, Ben H, Liu K, Zhu B. Transcutaneous auricular vagus nerve stimulation protects endotoxemic rat from lipopolysaccharide-induced inflammation. Evid Based Complement Alternat Med. 2012;2012:627023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 95. | Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2256] [Cited by in RCA: 2481] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 96. | Attenello F, Amar AP, Liu C, Apuzzo ML. Theoretical Basis of Vagus Nerve Stimulation. Prog Neurol Surg. 2015;29:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Zhao M, He X, Bi XY, Yu XJ, Gil Wier W, Zang WJ. Vagal stimulation triggers peripheral vascular protection through the cholinergic anti-inflammatory pathway in a rat model of myocardial ischemia/reperfusion. Basic Res Cardiol. 2013;108:345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 98. | Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, Michel K, Tracey KJ, Schemann M, Boesmans W, Vanden Berghe P, Boeckxstaens GE. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63:938-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 337] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 99. | Reyt S, Picq C, Sinniger V, Clarençon D, Bonaz B, David O. Dynamic Causal Modelling and physiological confounds: a functional MRI study of vagus nerve stimulation. Neuroimage. 2010;52:1456-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Barbella G, Cocco I, Freri E, Marotta G, Visani E, Franceschetti S, Casazza M. Transcutaneous vagal nerve stimulatio (t-VNS): An adjunctive treatment option for refractory epilepsy. Seizure. 2018;60:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 101. | Kibleur A, Pellissier S, Sinniger V, Robert J, Gronlier E, Clarençon D, Vercueil L, Hoffmann D, Bonaz B, David O. Electroencephalographic correlates of low-frequency vagus nerve stimulation therapy for Crohn's disease. Clin Neurophysiol. 2018;129:1041-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 102. | Pavlov VA, Tracey KJ. Neural circuitry and immunity. Immunol Res. 2015;63:38-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 103. | Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, Al-Abed Y, Tracey KJ. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci U S A. 2006;103:5219-5223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 104. | Munyaka P, Rabbi MF, Pavlov VA, Tracey KJ, Khafipour E, Ghia JE. Central muscarinic cholinergic activation alters interaction between splenic dendritic cell and CD4+CD25- T cells in experimental colitis. PLoS One. 2014;9:e109272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 105. | Lee ST, Chu K, Jung KH, Kang KM, Kim JH, Bahn JJ, Jeon D, Kim M, Lee SK, Roh JK. Cholinergic anti-inflammatory pathway in intracerebral hemorrhage. Brain Res. 2010;1309:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 106. | Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, Tracey KJ. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 340] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 107. | Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 2014;7:335-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 108. | Pham GS, Wang LA, Mathis KW. Pharmacological potentiation of the efferent vagus nerve attenuates blood pressure and renal injury in a murine model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2018;315:R1261-R1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 109. | Lehner KR, Silverman HA, Addorisio ME, Roy A, Al-Onaizi MA, Levine Y, Olofsson PS, Chavan SS, Gros R, Nathanson NM, Al-Abed Y, Metz CN, Prado VF, Prado MAM, Tracey KJ, Pavlov VA. Forebrain Cholinergic Signaling Regulates Innate Immune Responses and Inflammation. Front Immunol. 2019;10:585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 110. | Brégeon J, Coron E, Da Silva AC, Jaulin J, Aubert P, Chevalier J, Vergnolle N, Meurette G, Neunlist M. Sacral nerve stimulation enhances early intestinal mucosal repair following mucosal injury in a pig model. J Physiol. 2016;594:4309-4323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 111. | Tu L, Gharibani P, Zhang N, Yin J, Chen JD. Anti-inflammatory effects of sacral nerve stimulation: a novel spinal afferent and vagal efferent pathway. Am J Physiol Gastrointest Liver Physiol. 2020;318:G624-G634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 112. | Jin H, Guo J, Liu J, Lyu B, Foreman RD, Yin J, Shi Z, Chen JDZ. Anti-inflammatory effects and mechanisms of vagal nerve stimulation combined with electroacupuncture in a rodent model of TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2017;313:G192-G202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 113. | Pauwels N, Willemse C, Hellemans S, Komen N, Van den Broeck S, Roenen J, Van Aggelpoel T, De Schepper H. The role of neuromodulation in chronic functional constipation: a systematic review. Acta Gastroenterol Belg. 2021;84:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 114. | Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;CD004464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 115. | Weledji EP. A historical perspective on sacral nerve stimulation (sns) for bowel dysfunction. 2021. [cited 2021 Sep 19]. Available from: https://www.preprints.org/manuscript/202108.0404/v1. [DOI] [Full Text] |
| 116. | Gupta P, Ehlert MJ, Sirls LT, Peters KM. Percutaneous tibial nerve stimulation and sacral neuromodulation: an update. Curr Urol Rep. 2015;16:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 117. | Marti L, Galata C, Beutner U, Hetzer F, Pipitone N, Wolff K, Borovicka J, Brunner W, Sulz MC, Maurus C. Percutaneous tibial nerve stimulation (pTNS): success rate and the role of rectal capacity. Int J Colorectal Dis. 2017;32:789-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 118. | Iyer S, Laus K, Rugino A, Botros C, Lozo S, Botros SM, Goldberg R, Tomezsko J, Gafni-Kane A, Wroblewski K, Sand P. Subjective and objective responses to PTNS and predictors for success: a retrospective cohort study of percutaneous tibial nerve stimulation for overactive bladder. Int Urogynecol J. 2019;30:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 119. | Furtado-Albanezi D, Jürgensen SP, Avila MA, Correia GN, Driusso P. Effects of two nonpharmacological treatments on the sleep quality of women with nocturia: a randomized controlled clinical trial. Int Urogynecol J. 2019;30:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Spinal Cord Stimulation Cincinnati. OH Mayfield Brain & Spine. [cited 2021 Sep 19]. Available from: https://mayfieldclinic.com/pe-stim.htm. |
| 121. | Levine AB, Parrent AG, MacDougall KW. Stimulation of the Spinal Cord and Dorsal Nerve Roots for Chronic Groin, Pelvic, and Abdominal Pain. Pain Physician. 2016;19:405-412. [PubMed] |
| 122. | Lind G, Winter J, Linderoth B, Hellström PM. Therapeutic value of spinal cord stimulation in irritable bowel syndrome: a randomized crossover pilot study. Am J Physiol Regul Integr Comp Physiol. 2015;308:R887-R894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 123. | Eldabe S, Buchser E, Duarte RV. Complications of Spinal Cord Stimulation and Peripheral Nerve Stimulation Techniques: A Review of the Literature. Pain Med. 2016;17:325-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |