Published online May 22, 2022. doi: 10.4291/wjgp.v13.i3.96
Peer-review started: January 8, 2022
First decision: March 9, 2022
Revised: March 21, 2022
Accepted: May 14, 2022
Article in press: May 14, 2022
Published online: May 22, 2022
Processing time: 130 Days and 4.7 Hours
Vibration-controlled transient elastography (VCTE) is proposed as a second step of examination to assess liver fibrosis in patients with nonalcoholic fatty liver disease (NAFLD) after triaging by the fibrosis-4 (FIB-4) index. Recently, VCTE-based scoring systems, including FibroScan-AST (FAST), Agile 3+, and Agile 4, emerged to determine the status of NAFLD. However, the significance of these scoring systems remains unknown in narrowing the high-risk group of NAFLD patients with comorbidities, including hepatocellular carcinoma (HCC) and esophagogastric varices (EGV).
To clarify the significance of VCTE-based scoring systems to narrow the high-risk group of NAFLD patients with comorbidities.
We performed a cross-sectional study to investigate the usefulness of VCTE-based scoring systems and other fibrosis markers to narrow the high-risk group of patients with NAFLD. FIB-4 index was used for the first triage. Risk groups of FAST, Agile 3+, and Agile 4 were stratified according to the published data. Among the 191 patients with NAFLD, there were 26 (14%) and 25 patients (13%) with HCC and EGV, respectively.
When 1.3 was used as a cutoff value, the FIB-4 index narrowed the risk group to 120 patients, in which all patients with HCC and/or EGV were included. High risk group of Agile 3+ could subsequently narrow the risk group. The prevalence of HCC and EGV at this step were 33% (26/80) and 31% (25/80), respectively. In further narrowing of EGV, Agile 4 aggregated the patients with EGV into 43 patients, of whom 23 (53%) had EGV. FAST failed to narrow the risk group of patients with comorbidities. When 2.6 was used as a cutoff value of the FIB-4 index, three patients with HCC and two patients with EGV were missed at the first triage.
Agile 3+ and Agile 4 are useful to narrow the NAFLD patient group, in which patients may have HCC and/or EGV.
Core Tip: It is necessary to narrow the high-risk group of nonalcoholic fatty liver disease (NAFLD) patients with comorbidities, including hepatocellular carcinoma (HCC) and esophagogastric varices (EGV). Although the fibrosis-4 index is an excellent formula to narrow the high-risk group, there remain many patients to be ruled out. Vibration controlled transient elastography (VCTE) is proposed as a second step examination. FibroScan-AST, Agile 3+, and Agile 4 emerged as VCTE-based scoring systems to determine the status of patients with NAFLD. Here, we demonstrated that Agile 3+ and Agile 4 are good tools to narrow the high-risk group of patients with HCC and/or EGV.
- Citation: Miura K, Maeda H, Morimoto N, Watanabe S, Tsukui M, Takaoka Y, Nomoto H, Goka R, Kotani K, Yamamoto H. Utility of FibroScan-based scoring systems to narrow the risk group of nonalcoholic fatty liver disease with comorbidities. World J Gastrointest Pathophysiol 2022; 13(3): 96-106
- URL: https://www.wjgnet.com/2150-5330/full/v13/i3/96.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v13.i3.96
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide. A subset of patients with NAFLD can progress to liver cirrhosis, in which patients may have comorbidities, including hepatocellular carcinoma (HCC) and esophagogastric varices (EGV). Current studies have demonstrated that liver fibrosis is a prognostic factor of patients with NAFLD because comorbidities of NAFLD are noted in patients with liver fibrosis[1,2]. Thus, the assessment of liver fibrosis is essential to identifying patients with comorbidities.
Although liver biopsy remains the gold standard to assess liver fibrosis, it is costly and has a risk of complications, including bleeding. In addition, it is difficult to perform liver biopsy in all patients with NAFLD because the global prevalence of patients with NAFLD is approximately 25%[3]. Thus, the demand for noninvasive tests (NITs) to assess liver fibrosis is expanding. Currently, there are several markers and formulae to assess liver fibrosis using clinical parameters without liver biopsy[4]. In addition, imaging studies, including elastography and magnetic resonance imaging (MRI), are used as NITs for the assessment of liver fibrosis. Each method has both advantages and disadvantages. Among NITs, the fibrosis-4 (FIB-4) index is a widely used formula because this formula uses only 4 components, including age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet count[5], which are easily available not only for hepatologists but also for general physicians. The merits of using the FIB-4 index are high accuracy and low cost[6]. In addition, many validation studies have been performed in chronic liver diseases, including NAFLD. Furthermore, the FIB-4 index is useful for identifying NAFLD patients with extrahepatic comorbidities, including cardiovascular diseases[7]. However, elderly patients tend to show a high score. In addition, there are many patients who show an intermediate risk for liver fibrosis. As a result, the FIB-4 index is used in the first step to narrow the high-risk group of patients who may have comorbidities of NAFLD.
FibroScan, a vibration-controlled transient elastography (VCTE), is proposed as the second step of NIT that can identify such patients[8]. Liver stiffness measurement (LSM) ≥ 11.9 KPa by FibroScan is highly suspected of liver fibrosis over F4[9]. Although FibroScan shows high sensitivity and specificity in the diagnosis of liver fibrosis, some patients have unexpectedly high LSM, probably due to the presence of obesity and the examiners’ skill. Thus, a combination of LSM and laboratory data may reflect a more accurate status of patients with NAFLD. To this end, FibroScan-based scoring systems, including FibroScan-AST (FAST)[10], Agile 3+[11] and Agile 4[12], have been developed. These scoring systems use data obtained from FibroScan and some clinical parameters, including age, sex, AST, ALT, platelet count, and diabetes status. Among these scoring systems, FAST was designed to identify NAFLD patients with liver fibrosis F ≥ 2. Agile 3+ and Agile 4 were designed to identify NAFLD patients with liver fibrosis at F3-F4 and F4, respectively. Although these FibroScan-based scoring systems are correlated with liver fibrosis, little data are available on the significance of identifying NAFLD patients with comorbidities. Thus, the aim of the present cross-sectional study was to investigate the utility of these FibroScan-based scoring systems to narrow the high-risk group of NAFLD patients with comorbidities after triaging by the FIB-4 index.
We investigated 191 patients with NAFLD who visited our hospital between April 2019 and March 2022. The diagnosis of NAFLD was made as follows: Steatosis was determined by an ultrasonographic examination conducted by well-experienced gastroenterologists. Steatosis pointing out past examinations was included. Men who used alcohol > 30 g/d and women who used > 20 g/d were excluded. Patients with HBV infection (positive for HBs antigen), HCV infection (positive for HCV antibody) and other liver diseases, including autoimmune hepatitis and primary biliary cholangitis, were also excluded. In addition, we used data obtained from FibroScan as well as blood tests, including the FIB-4 index and Wisteria floribunda agglutinin-positive Mac2-binding protein glycosylation isomer (M2BPGi). Diagnosis of diabetes was defined as a fasting blood glucose ≥ 126 mg/dL, hemoglobin A1c ≥ 6.5% and/or antidiabetic drug use. All patients in the present study had FibroScan examination as well as blood tests. This study was approved by the Institutional Review Board of Jichi Medical University (20-175). The study was performed according to the ethical guidelines of the Declaration of Helsinki.
Transient elastography was performed with FibroScan (Echosens, Paris, France), using an M probe. The FIB-4 index, FAST score, Agile 3+, and Agile 4 were calculated according to published formulae using age, controlled attenuation parameter (CAP), LSM, AST, ALT, platelet count, and presence of diabetes (Supplementary Figure 1). The impact of these parameters on the scoring systems were shown in Supplementary Table 1. Blood data obtained on the same day of FibroScan examination or within 1 mo from the examination were used (Supplementary Figure 2). CAP and LSM were the mean data of 10 consecutive examinations.
Risk assessments for each formula and factor are shown in Supplementary Table 2. In addition, Baveno VI criteria[13], expanded Baveno VI criteria[14], and New NFLD-cirrhosis criteria[15] were also assessed in narrowing the risk group of patients with EGV.
The diagnosis of HCC was made by hepatologists and radiologists using contrast-enhanced computed tomography and/or contrast-enhanced MRI and/or contrast-enhanced ultrasonography. Histologically proven HCC were also added. Form 1 ≤ were defined as having EGV in patients who underwent esophagogastroduodenal endoscopy (EGD)[16,17]. Patients with histories of HCC and/or endoscopic variceal treatment were included as shown in Supplementary Figure 2. If patients did not have EGD examination within 1 year, we interviewed a history of gastrointestinal bleeding from gastrointestinal varices. If patients reported no history of variceal bleeding, the patient was defined as having no EGV.
Statistical analyses were performed using Stata 17 (STATA Corporation, College Station, United States). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. For patient background evaluation, analyses were performed by the chi-square test or Fisher’s test as appropriate. In addition, the Mann-Whitney U test was used in a comparison of two groups. In a comparison of three groups, one-way analysis of variance was used. All P values < 0.05 were considered statistically significant.
Table 1 shows the characteristics of patients. The median age was 62 years old, 81 (42.4%) were male, and 75 (39.3%) had diabetes. There were 26 patients with HCC and 25 patients with EGV. Among these patients with HCC and/or EGV, 17 had HCC alone, 16 had EGV alone, and 9 had both HCC and EGV.
| Patients (n) | 191 |
| Age (years old) | 62 (20-90) |
| Men (%) | 81 (42.4) |
| diabetes (%) | 75 (39.3) |
| HCC | 17 |
| EGV | 16 |
| Both HCC and EGV | 9 |
| AST (U/L) | 36 (13-208) |
| ALT (U/L) | 40 (10-214) |
| Platelet count (×109/L) | 207 (45-445) |
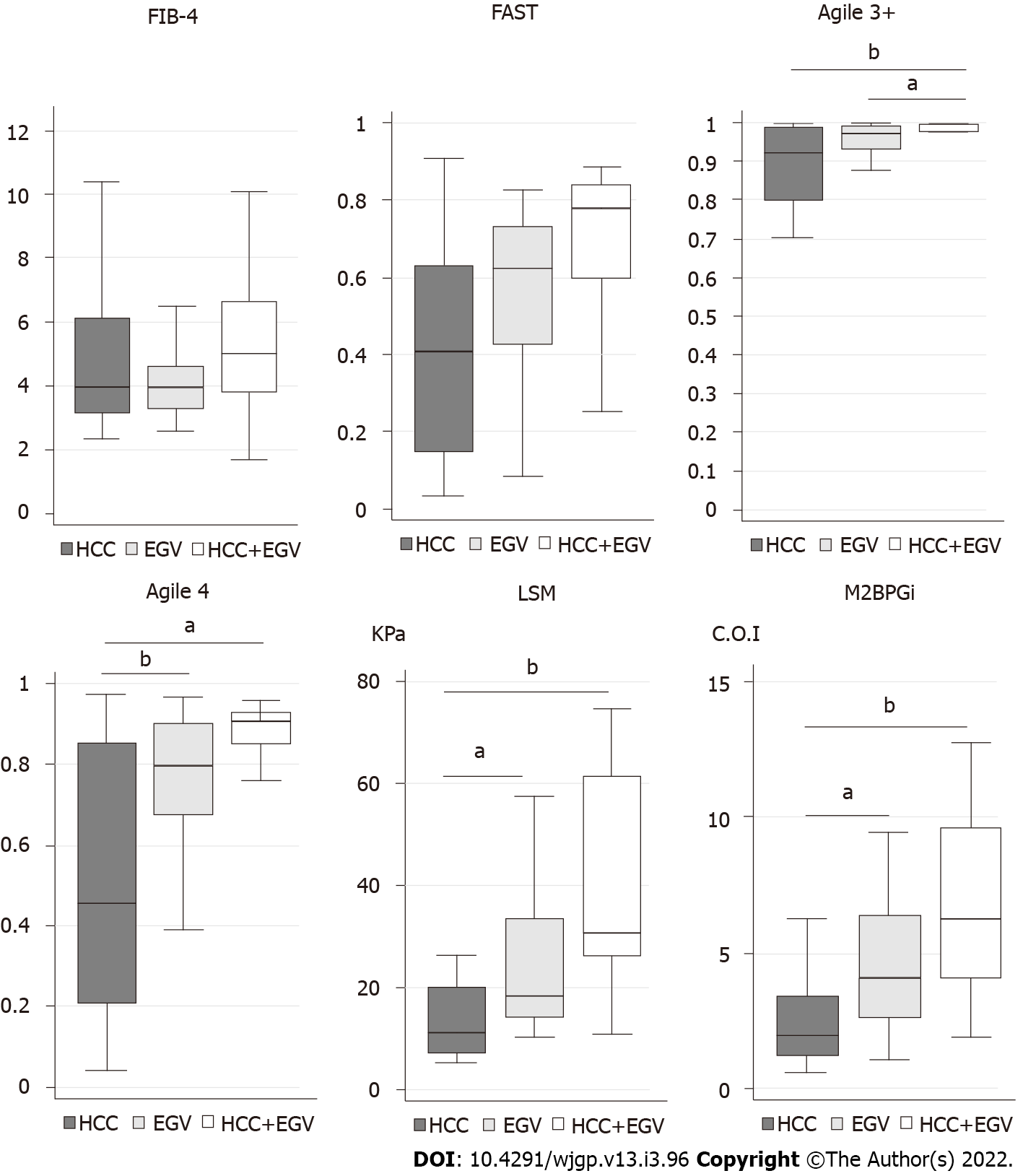
Then, we investigated the scores and values of each formula and marker in patients with HCC and/or EGV (Figure 1). In FIB-4 and FAST, the maximum and the minimum of scores were similar among patients with HCC and/or EGV. In Agile 3+, patients with HCC and/or EGV aggregated into a zone of high score. In Agile 4, LSM, and M2BPGi, the score and values tended to show a stepwise increase from HCC, EGV, and both HCC and EVG.
In a stratification of the FIB-4 index, there were 71, 51, and 69 patients in the low-, intermediate-, and high-risk groups, respectively. No patients with HCC and/or EGV were noted in the low-risk group of the FIB-4 index, while three patients with HCC and two patients with EGV were in the intermediate stage. The remining patients with HCC and/or EGV were in the high-risk group (Tables 2 and 3). Thus, the high to intermediate-risk group of FIB-4 index is suitable for the first triage.
| FIB-4 | FAST | Agile 3+ | Agile 4 | LSM | M2BPGi | |||||||
| Risk | L | I-H | L | I-H | L | I-H | L | I-H | L | I-H | L | I-H |
| n | 71 | 120 | 87 | 104 | 96 | 95 | 131 | 60 | 73 | 118 | 102 | 89 |
| HCC | 0 | 26 | 10 | 16 | 0 | 26 | 7 | 19 | 4 | 22 | 5 | 21 |
| P value | < 0.01 | 0.44 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | ||||||
| Sensitivity | 1 | 0.62 | 1 | 0.73 | 0.85 | 0.81 | ||||||
| Specificity | 0.43 | 0.53 | 0.58 | 0.79 | 0.44 | 0.62 | ||||||
| PPV | 0.22 | 0.17 | 0.27 | 0.36 | 0.19 | 0.25 | ||||||
| NPV | 1 | 0.90 | 1 | 0.95 | 0.95 | 0.95 | ||||||
| EGV | 0 | 25 | 6 | 19 | 0 | 25 | 1 | 24 | 0 | 25 | 1 | 24 |
| P value | < 0.01 | 0.02 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | ||||||
| Sensitivity | 1 | 0.76 | 1 | 0.96 | 1 | 0.96 | ||||||
| Specificity | 0.43 | 0.52 | 0.58 | 0.79 | 0.44 | 0.61 | ||||||
| PPV | 0.21 | 0.19 | 0.26 | 0.41 | 0.21 | 0.27 | ||||||
| NPV | 1 | 0.94 | 1 | 0.99 | 1 | 0.99 | ||||||
| FIB-4 | FAST | Agile 3+ | Agile 4 | LSM | M2BPGi | |||||||
| Risk | L-I | H | L-I | H | L-I | H | L-I | H | L-I | H | L-I | H |
| n | 122 | 69 | 146 | 45 | 111 | 80 | 148 | 43 | 136 | 55 | 148 | 43 |
| HCC | 3 | 23 | 18 | 8 | 0 | 26 | 12 | 14 | 10 | 16 | 11 | 15 |
| P value | < 0.01 | 0.35 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | ||||||
| Sensitivity | 0.89 | 0.31 | 1 | 0.54 | 0.62 | 0.58 | ||||||
| Specificity | 0.74 | 0.89 | 0.67 | 0.90 | 0.82 | 0.90 | ||||||
| PPV | 0.35 | 0.30 | 0.33 | 0.45 | 0.36 | 0.47 | ||||||
| NPV | 0.98 | 0.89 | 1 | 0.93 | 0.93 | 0.93 | ||||||
| EGV | 2 | 23 | 14 | 11 | 0 | 25 | 2 | 23 | 3 | 22 | 4 | 21 |
| P value | < 0.01 | 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | ||||||
| Sensitivity | 0.92 | 0.44 | 1 | 0.92 | 0.88 | 0.84 | ||||||
| Specificity | 0.74 | 0.88 | 0.67 | 0.89 | 0.82 | 0.89 | ||||||
| PPV | 0.34 | 0.36 | 0.31 | 0.56 | 0.42 | 0.54 | ||||||
| NPV | 0.98 | 0.91 | 1 | 0.99 | 0.98 | 0.97 | ||||||
Then, we investigated the prevalence of patients with HCC and/or EGV (Tables 2 and 3). When the patients were divided into two groups, including low-risk and high to intermediate-risk, there were no patients with HCC and/or EGV in the low-risk group of Agile 3+ (Table 2). In addition, Agile 3+ was the only examination that included all patients with HCC and/or EGV in the high-risk group (Table 3). As a result, Agile 3+ showed extremely high sensitivity and NPV. In contrast, there were patients with HCC in the low-risk group of FAST, Agile 4, LSM, and M2BPGi and patients with EGV in the low-risk group of FAST, Agile 4, and M2BPGi (Table 2), suggesting that FAST, Agile 4, LSM, and M2BPGi are unsuitable for screening of patients with HCC and/or EGV. Thus, Agile 3+ is a good tool to narrow the high-risk group of patients with HCC and/or EGV.
Although the Agile 3+ could narrow the patients with EGV, we further attempted to narrow the patients with EGV. Patients with EGV tended to have a more advanced stage of fibrosis based on Agile 4, LSM, and M2BPGi (Figure 1). Although there were no patients with EGV in the low-risk group of LSM, the PPV was 21% (Table 2). In contrast, the high-risk groups of Agile 4 and M2BPGi missed one patient with EGV, their PPVs were higher than that of LSM. In addition, the PPV of the high-risk group of Agile 4 was 56%, the highest among tests (Table 3). Despite the high-risk group of Agile 4 missed two patients with EGV, Agile 4 is a potential tool to narrow the risk group of patients with EGV.
Baveno VI criteria, expanded Baveno VI criteria, and new NAFLD-cirrhosis criteria, using LSM and platelet count, are simple tools to rule out patients with varices needing treatment. There were 13 (52%), 17 (68%), and 19 patients (76%) with EGV who were defined as “rule out “of the Baveno VI criteria, expanded Baveno VI criteria, and new NAFLD-cirrhosis criteria, respectively (Table 4). Thus, it was difficult to narrow the patients with EGV using a combination of LSM and platelet count.
| Baveno VI | Exp. Baveno VI | New NASH C.C | ||||
| LSM | platelet | LSM | platelet | LSM | platelet | |
| < 20 | 150 < | < 25 | 110 < | < 30 | 110 < | |
| EGV/rule in (n) | 12/26 | 8/13 | 6/9 | |||
| EGV/rule out (n) | 13/165 | 17/178 | 19/182 | |||
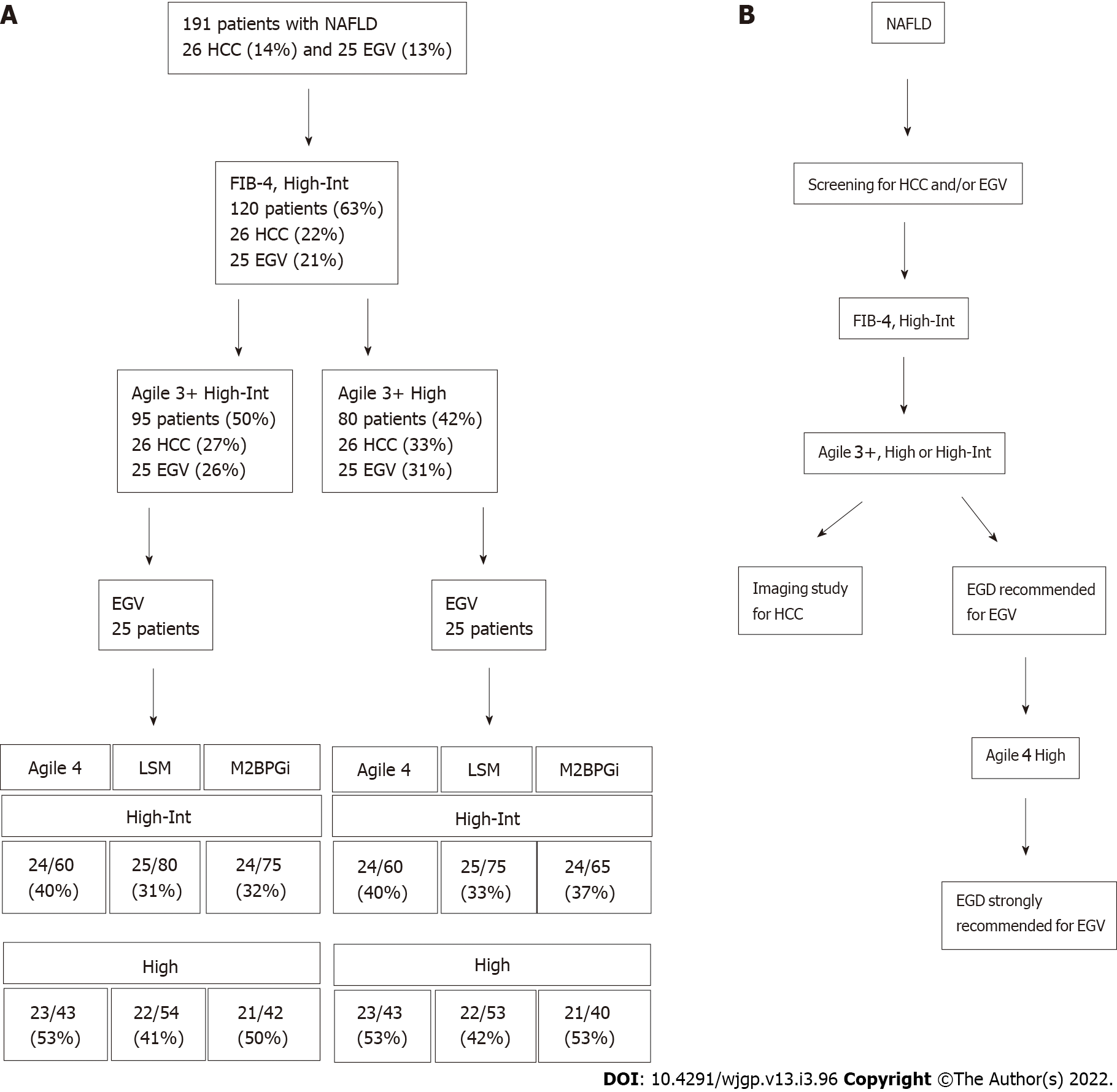
We applied our patient group to determine whether VCTE-based scoring systems and other fibrosis markers can narrow the risk group of patients with HCC and/or EGV after triaging by the FIB-4 index (Figure 2A). There were 26 patients with HCC (14%) and 25 patients with EGV (13%) among 191 patients. At the first triage using the FIB-4 index at 1.3 (high to intermediate-risk group), we could narrow the risk group to 120 patients, in whom all patients with HCC and/or EGV were included. In the first step, the prevalence of HCC and EGV was 22% (26/120) and 21% (25/120), respectively. Then, we narrowed the patients using Agile 3+ at the second step, in which all patients with HCC and/or EGV were included. When the high to intermediate-risk group of Agile 3+ was used, the prevalence of HCC was 27% (26/95) and 26% (25/95), respectively. When the high-risk group of Agile 3+ was used, the prevalence of HCC was 33% (26/80) and 31% (25/80), respectively. Because the low-risk group of Agile 4, LSM, and M2BPGi included patents with HCC, further narrowing was difficult without missing patients with HCC.
Then, we attempted to narrow the patients with EGV. The high to intermediate and high-risk of Agile 3+ groups subsequently narrowed the patients with EGV. Although the high to intermediate-risk group of LSM successfully narrowed the risk group without missing patients with EGV, the prevalence was a small increase, up to 33% (25/75). In contrast, high-risk group of Agile 4 could concentrated the patients with EGV. Although the high-risk group of Agile 4 missed two patients (8%), the prevalence of patients with EGV increased to 53% (23/43). Thus, Agile 4 is a good tool to further narrow the risk group of patients with EGV.
Based on our results, sorting patients using the FIB-4 index, Agile 3+, and Agile 4 is a potential screening method to narrow the high-risk group of NAFLD patients with comorbidities (Figure 2B).
The requirement for NITs to narrow the risk group of patients with comorbidities is expanding because a quarter of people in the world have NAFLD, a risk factor for HCC and/or EGV. The FIB-4 index, which is simple and inexpensive, was used in the first triage to narrow the high-risk group of NAFLD patients with comorbidities. However, there remain many patients even after triage. In the present study, we demonstrated that Agile 3+ and Agile 4, VCTE-based scoring systems, were good tools for further narrowing the high-risk group of patients with HCC and/or EGV at the second and third steps, respectively.
Agile 3+, developed by Yonoussi’s group, was suitable to narrow the risk group of patients with HCC and/or EGV in the present study. Agile 3+ has been designed to optimize PPV and reduce cases of intermediate stage (Gray zone) among patients with advanced liver fibrosis[11]. Our data demonstrated that Agile 3+ had high sensitivity and high NPV for HCC and EGV. Although the number of patients in the high-risk group of Agile 3+ was larger than that of other scoring systems and fibrosis markers, Agile 3+ did not miss the patients with HCC and/or EGV, which is contrast to other tools, including FAST, Agile 4, LSM, and M2BPGi. Indeed, all patients with HCC and/or EGV were included in the high-risk group of Agile 3+, suggesting that Agile 3+ is useful for screening patients with HCC and/or EGV. Because the background liver of NAFLD patients with HCC is often characterized by less fibrosis[18], fibrosis markers sometimes fail to identify patients with HCC. Some patients with HCC were included in the low-risk group of Agile 4, LSM, and M2BPGi. The Agile 3+ scoring system includes age, AST, ALT, platelet count, LSM, sex, and diabetes. Because old age and diabetic individuals are prone to HCC[19], it is reasonable to include these variables in the scoring system to find HCC.
Agile 4, also developed by Yonoussi’s group, was suitable to narrow the high-risk group of patients with EGV. Agile 4 was designed to identify patients with NASH cirrhosis. Agile 4 showed high specificity and high PPV for EGV. There were 23 (92%) and 24 patients (96%) with EGV in the high- and high to intermediate-risk groups, respectively. We also applied our patient group to the Baveno VI criteria, expanded Baveno VI criteria, and New NAFLD-cirrhosis criteria, which are combinations of LSM and platelet count. However, more than half of the patients were included in the rule-out group. In the Asian cohort, the Baveno VI criteria performed better than the expanded Baveno VI criteria[20], suggesting that Asian people may have EGV at lower LSM and higher platelet counts than people in the USA and Europe. Although it remains unknown why the Baveno VI criteria and its derivatives did not work in the present study, further studies are required. As a result, Agile 4 can be used at the third step to identify patients with EGV.
FAST failed to narrow the high-risk group of patients with HCC and/or EGV. FAST showed low sensitivity to identify such patients. In addition, there were 10 (38%) with HCC and 6 patients (23%) with EGV in the low-risk (rule out) group, respectively. FAST, designed for identifying patients with NAFLD activity score ≥ 4 and fibrosis stage (F ≥ 2), is calculated using LSM, CAP, and AST. However, the FAST score did not include risk factors for HCC, including age, sex, and diabetes. The association between the grade of CAP, fat content in the liver, and HCC remains unknown. Izumi et al[21] reported that CAP was significantly lower in the HCC group than in the non-HCC group in patients with NAFLD. Indeed, our data revealed that CAP tended to be low in patients with HCC (data not shown). Thus, FAST is unlikely suitable for the screening of patients with HCC and/or EGV. However, patients with high FAST scores should be followed up because these patients have a risk of progressive NASH in the future.
There are a couple of limitations in the present study. Our study is a single-center study, and the number of patients examined was small. Thus, the bias of NAFLD population is noted. In a previous study, the proportions of patients in the low- and high-risk FIB-4 index groups were 58.3% and 10.2%, respectively, among patients with biopsy-proven NAFLD[22]. The proportions in the present study showed small size of the low-risk group (37.2%) but large size of the high-risk group (36.1%). In addition, a total of 42 patients (22.0%) had HCC and/or EGV among patients with NAFLD. Because our hospital is a referral center, patients with comorbidities were aggregated into our hospital. In addition, the present study counted patients with histories of HCC and/or EGV, suggesting that scores of FIB-4 and Agile 3+ may be higher than those when comorbidities first developed. Thus, prospective study will clarify the significance of Agiles for finding patients with HCC and/or EGV. At least, the stream from FIB-4 index to Agiles worked in narrowing the high-risk patients with HCC and/or EGV in the present study.
In conclusion, Agile 3+ and Agile 4 can narrow the high-risk group of patients who may have HCC and/or EGV after triaging by the FIB-4 index. Because Agile 3+ and Agile 4 share common parameters, including LSM and clinical data, they have a potential use in screening for such patients.
It is necessary to narrow the high-risk group of nonalcoholic fatty liver disease (NAFLD) patients with comorbidities, including hepatocellular carcinoma (HCC) and esophagogastric varices (EGV).
Although the fibrosis-4 index is an excellent formula to narrow the high-risk group, there remain many patients to be ruled out.
This study aimed to assess the utility of VCTE-based scoring systems to narrow the risk group of nonalcoholic fatty liver disease with comorbidities.
We performed a cross-sectional study to investigate the usefulness of VCTE-based scoring systems and other fibrosis markers to narrow the high-risk group of patients with NAFLD.
The high-risk group of Agile 3+ could narrow the patients with HCC and/or EGV without missing one patient. The high-risk group of Agile 4 showed a high PPV for patients with EGV.
The brand new VCTE-based scoring systems, Agile 3+ and Agile 4, are useful to narrow the NAFLD patient group, in which patients may have HCC and/or EGV.
Agile 3+ and Agile 4 will be used for screening of NAFLD patients with HCC and/or EGV.
We thank Eri Noguchi (Jichi Medical University) for her excellent assistance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Japanese Society of Gastroenterology, No. 030879.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Cao X, China; Portius D, Germany S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Ioannou GN. Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol. 2021;75:1476-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 209] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 2. | Magaz M, Baiges A, Hernández-Gea V. Precision medicine in variceal bleeding: Are we there yet? J Hepatol. 2020;72:774-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7477] [Article Influence: 830.8] [Reference Citation Analysis (0)] |
| 4. | Eguchi Y, Wong G, Akhtar O, Sumida Y. Non-invasive diagnosis of non-alcoholic steatohepatitis and advanced fibrosis in Japan: A targeted literature review. Hepatol Res. 2020;50:645-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3525] [Article Influence: 185.5] [Reference Citation Analysis (0)] |
| 6. | Sumida Y, Yoneda M, Tokushige K, Kawanaka M, Fujii H, Imajo K, Takahashi H, Eguchi Y, Ono M, Nozaki Y, Hyogo H, Koseki M, Yoshida Y, Kawaguchi T, Kamada Y, Okanoue T, Nakajima A; Japan Study Group Of Nafld Jsg-Nafld. FIB-4 First in the Diagnostic Algorithm of Metabolic-Dysfunction-Associated Fatty Liver Disease in the Era of the Global Metabodemic. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Sato Y, Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Misaka T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Saitoh SI, Takeishi Y. Liver stiffness assessed by Fibrosis-4 index predicts mortality in patients with heart failure. Open Heart. 2017;4:e000598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Paternostro R, Reiberger T, Bucsics T. Elastography-based screening for esophageal varices in patients with advanced chronic liver disease. World J Gastroenterol. 2019;25:308-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, Le MD, Hooker J, Tu X, Bettencourt R, Yin M, Sirlin CB, Ehman RL, Nakajima A, Loomba R. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin Gastroenterol Hepatol. 2019;17:630-637.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 299] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 10. | Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, Yilmaz Y, Czernichow S, Zheng MH, Wong VW, Allison M, Tsochatzis E, Anstee QM, Sheridan DA, Eddowes PJ, Guha IN, Cobbold JF, Paradis V, Bedossa P, Miette V, Fournier-Poizat C, Sandrin L, Harrison SA. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 534] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 11. | Younoussi ZM, Harrison SA, Newsome PN, Chan WK, Yilmaz Y, de Ledinghen V, Costentin C, Zheng MH, Wong V, Elkhashab M, Huss R, Myers RP, Foucquier J, Labourdette A, Destro M, Fourier CD, Miette V, Sandrin L, Boursier J, Sanyal A. Development and validation of Agile 3+: novel FibroScan based score for the diagnosis of advanced fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2021;75 Supple 2:S 257. |
| 12. | Younoussi ZM, Harrison SA, Newsome PN, Chan WK, Yilmaz Y, Zheng MH, Wong V, Elkhashab M, Kersey K, Myers RP, Foucquier J, Labourdette A, Destro M, Fourier CD, Miette V, Sandrin L, Sanyal A, and NASH Clinical Research Network, NIDDK, NIH. Improving diagnosis of cirrhosis in patient with NAFLD by combining liver stiffness measurement by vibration-controlled transient elastography and routine biomarkers: A global derivation and validation study. AASLD 2020 Late-breaking Abstracts LB12. |
| 13. | Kamath PS, Mookerjee RP. Individualized care for portal hypertension: Not quite yet. J Hepatol. 2015;63:543-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Augustin S, Pons M, Maurice JB, Bureau C, Stefanescu H, Ney M, Blasco H, Procopet B, Tsochatzis E, Westbrook RH, Bosch J, Berzigotti A, Abraldes JG, Genescà J. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017;66:1980-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 15. | Petta S, Sebastiani G, Bugianesi E, Viganò M, Wong VW, Berzigotti A, Fracanzani AL, Anstee QM, Marra F, Barbara M, Calvaruso V, Cammà C, Di Marco V, Craxì A, de Ledinghen V. Non-invasive prediction of esophageal varices by stiffness and platelet in non-alcoholic fatty liver disease cirrhosis. J Hepatol. 2018;69:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 16. | Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, Kobayashi M. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc. 1981;27:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 551] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Hashizume M, Kitano S, Yamaga H, Koyanagi N, Sugimachi K. Endoscopic classification of gastric varices. Gastrointest Endosc. 1990;36:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 142] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Kawada N, Imanaka K, Kawaguchi T, Tamai C, Ishihara R, Matsunaga T, Gotoh K, Yamada T, Tomita Y. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44:1190-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1184] [Article Influence: 296.0] [Reference Citation Analysis (0)] |
| 20. | Sharma S, Agarwal S, Gunjan D, Kaushal K, Anand A, Saraya A. Deciding Among Noninvasive Tools for Predicting Varices Needing Treatment in Chronic Liver Disease: An Analysis of Asian Cohort. Am J Gastroenterol. 2020;115:1650-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Izumi T, Sho T, Morikawa K, Shigesawa T, Suzuki K, Nakamura A, Ohara M, Kawagishi N, Umemura M, Shimazaki T, Kimura M, Nakai M, Suda G, Natsuizaka M, Ogawa K, Kudo Y, Nishida M, Ono K, Baba M, Furuya K, Sakamoto N. Assessing the risk of hepatocellular carcinoma by combining liver stiffness and the controlled attenuation parameter. Hepatol Res. 2019;49:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, Fujita K, Chayama K, Saibara T, Kawada N, Fujimoto K, Kohgo Y, Yoshikawa T, Okanoue T; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD). Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |