Published online Mar 22, 2022. doi: 10.4291/wjgp.v13.i2.50
Peer-review started: March 17, 2021
First decision: May 1, 2021
Revised: May 6, 2021
Accepted: January 25, 2022
Article in press: January 25, 2022
Published online: March 22, 2022
Processing time: 362 Days and 3.8 Hours
Hepatitis E virus (HEV) is a small non-enveloped single stranded RNA virus whose genotypes 3 and 4 have been associated with zoonotic transmission in industrialized countries. HEV infection is considered the main cause of acute hepatitis worldwide. In some cases, transfusion of blood components or organ transplantation have been reported as the source of infection. We have conducted a literature review on the risk of transmission through cell and tissue allografts. Although no case was found, measures to control this risk should be taken when donor profile (based upon geographical and behavioural data) recommended it. Issues to be considered in donor screening and tissue processing to assess and to reduce the risk of HEV transmission are approached.
Core Tip: This manuscript provide a novel perspective of the mode of transmission of hepatitis E virus (HEV). HEV is mainly transmitted via fecal-oral route, but in recent years other transmission routes have been reported, including blood-borne transmission. The processing of tissue allografts in duly accredited tissue banks provides safe and efficient products.
- Citation: Villalba R, Mirabet V. Risk assessment of hepatitis E transmission through tissue allografts. World J Gastrointest Pathophysiol 2022; 13(2): 50-58
- URL: https://www.wjgnet.com/2150-5330/full/v13/i2/50.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v13.i2.50
There are several types of human tissues which are commonly used as allografts: Bone, tendon, cartilage, skin, cornea, amniotic membrane, stem cells, heart valve, blood vessel, etc. Almost all surgical disciplines benefit of its availability. Thus, millions of human tissue transplants are performed worldwide every year[1].
One of the drawbacks of these procedures is the potential for donor to recipient disease transmission. Although the real incidence of tissue allograft transmitted infection is unknown, some articles have published cases of viral, bacterial and fungal infections transmitted by tissues[2-5]. Regarding the different infectious agents, hepatotropic viruses have represented traditionally the real workhorse in maintaining the safety of tissues used for transplantation.
Hepatitis B virus (HBV) and hepatitis C virus (HCV) can cause acute and chronic hepatitis and potentially lead to the development of cirrhosis, liver cancer and death. In the European Union, estimated 4.7 million people have a chronic HBV infection, and 3.9 million people have chronic hepatitis C. Many of these infections may go undiagnosed as chronic infection is often asymptomatic and a hypothetical tissue donor could be a potential transmitter of the disease[6].
Risk factors for HBV and HCV infection are now clearly established[7-11]. In recent decades, various factors have contributed towards changes in HBV and HCV epidemiology, including improvements in donor tissue safety. A rigorous evaluation of clinical, behavioral, and personal risks is now performed as it may completely exclude a donor[12,13]. In addition to this, all potential tissue donors must be tested for both serological anti-HBc, HBsAg anti-HCV and for HCV-HBV by nucleic acid testing. Based on both criteria, the risk of HCV and HCV transmission is currently very low established in 1 in 34000 for HBV and 1 in 42000 for HCV[14].
Hepatitis E virus (HEV) infection is one of the main causes of acute hepatitis in both developed and developing countries. This infectious disease has a high prevalence and incidence in Europe and has a greater clinical impact in vulnerable populations, such as immunosuppressed patients, pregnant women, and patients with underlying liver disease[10,15,16].
To date, there are no specific recommendations for the screening of this disease in blood, tissue, or organ donors, which may cause this route to be an important source of disease transmission.
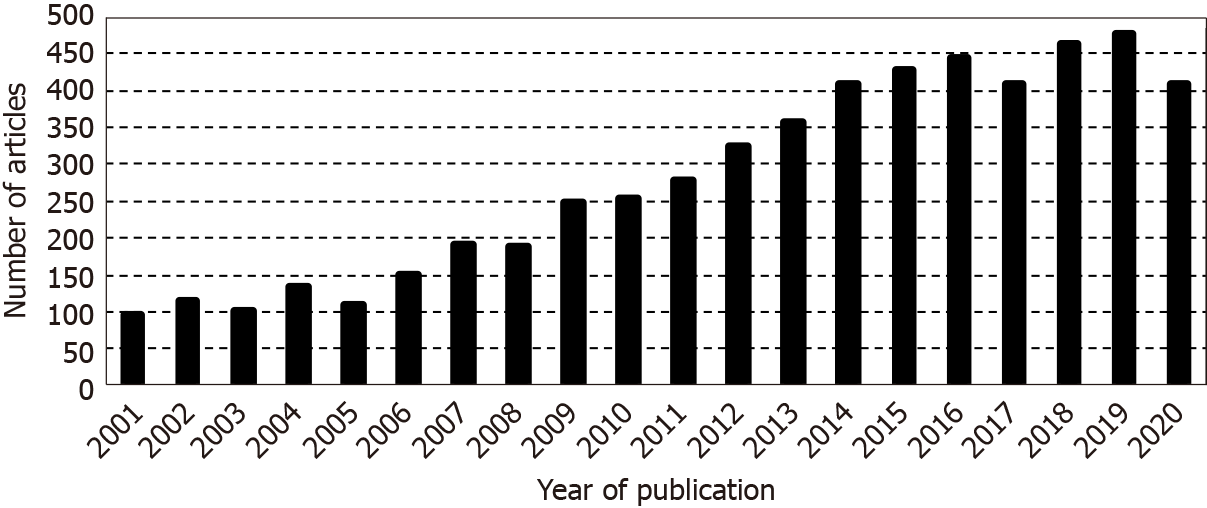
A search using the following search string: ‘hepatitis E virus [Title/Abstract] OR HEV [Title/Abstract] NOT high endothelial venules [Title/Abstract]’ was conducted. Applying these criteria on PubMed database (for articles published in last 20 years) 5485 records were recovered (Figure 1). This search was developed on 5th December 2020. Six hundred forty-three (11.7%) of them corresponded to reviews and 0.6% to systematic reviews (the first being published in 2009). When the search was restricted (using the Boolean operator AND) to the articles involving the word ‘allograft’, only 19 (0.3%) complied to the new condition. Seventy nine percent (15/19) of these last articles dealt only on organ transplantation, 2 on the transfusion of blood components (specially in relation to hematopoietic transplantation) and the other 2 were discarded because the reason for their recovery was the use of the acronym HEV (without description) to refer to high endothelial venules. Thus, to the best of our knowledge, the present paper is the first cross reference between HEV and tissue allografts.
HEV is a small non-enveloped positive-sense, single-stranded RNA virus, encased within an icosahedral capsid of between 27 and 34 nm in size belonging to the family Hepeviridae within the genus Orthohepevirus. Seven different genotypes have been described for the HEV. Five of them (1-4 and 7) can infect humans and the other two (5, 6) are found only in animals (boar). Genotypes 1 and 2 (HEV-1, HEV-2) have been found only in humans while genotypes 3 and 4 circulate in several animals (including pigs, rabbit, cattle, sheep, horse, boar, deer, and shellfish) and genotype 7 in camel. Genotypes 1 and 2 are directly transmitted fecal-orally, or indirectly, mainly via contaminated water. Genotypes 3 and 4 (HEV-3, HEV-4) are zoonotic infections with an animal reservoir, being indirectly transmitted through food (when consumed raw or undercooked) or by direct contact with infected animals. Thus, professionals who work in contact with animals or their wastes and carcasses (farmers, veterinarians, workers attending animals, slaughterers, traders, and suppliers) could be in higher risk of HEV infection[14,15-18]. In an effort to avoid inconsistencies when the HEV subtypes are named, Smith et al[19] have proposed standardization for the assignation of HEV sequences to each subtype. Likewise, the World Health Organization promoted the development of international standards for diagnostic assays[15,20].
Additionally to the host and mode of transmission, HEV genotypes also vary in geographical distribution. Genotype 1 is prevalent in Africa and Asia, whereas HEV-2 can be found in México and West Africa. Thus, HEV-1 and HEV-2 are responsible for HEV outbreaks in developing countries, with limited sanitary conditions, due to contaminated drinking water. Genotypes 3 and 4 are associated with zoonotic transmission as autochthonous (locally acquired) infection in industrialized countries[21].
Clinical symptoms of HEV infection do not differ from other pathogens causing hepatitis. Therefore, diagnosis is performed by HEV RNA detection using real-time reverse transcription polymerase chain reaction with primers detecting all 4 genotypes affecting humans. Additionally, detection of HEV immunoglobulin (Ig) M and IgG antibodies is performed by enzyme linked immunosorbent assay. These data characterize HEV infection as acute (demonstration of specific IgM, rising levels of IgG, or detection of HEV RNA), passed, or chronic (positive results for HEV-RNA for more than 6 mo)[14,22]. Likewise, HEV antigen detection assay has been found to be used when HEV RNA testing is not available or time is limited[16]. Although HEV Ag shows low sensitivity with viral loads lower than 1000 copies/mL, it has shown good correlation with HEV-RNA, being useful in diagnosing infection in immunosuppressed patients[23,24]. Another issue to be considered, when Epstein-Barr virus and cytomegalovirus infection are present, is the risk of false positive results from anti-HEV IgM assays[25].
HEV transmission between persons by direct contact has proven very inefficient probably due to the high infective dose required[26]. Although it is associated with low mortality rates (< 1%) in the general population, this risk increases (approximately 20%) during pregnancy[14].
Although hepatitis viruses have been suggested to play a role of in the development of autoimmune hepatitis (AIH)[27], a large multicentre study did not find differences in the prevalence of anti-HEV IgG between AIH and healthy patients[28]. Additionally, they did not identify chronically HEV-infected patients within the AIH cohort.
HEV virions, as those of HAV (both hepatotropic virus but phylogenetically unrelated), are known to be non-enveloped in feces, but they circulate in the blood
The main risk factors on HEV infection to be considered for donor screening can be summarized in: Areas with limited access to essential services as water, sanitation, and health care facilities; Consumption of undercooked or raw foodstuffs from animals; Middle-aged and elderly men.
The severity of the consequences increases when these factors occur together with others related to the recipients, as pregnant women (because fulminant hepatitis occurs more frequently during pregnancy) or immunocompromised patients (as solid organ transplant recipients or patients receiving hematopoietic progenitor cell transplantation).
HEV is considered to be the most common cause of acute hepatitis worldwide[30]. Its infection typically follows a fairly routine clinical course with an incubation period of 2 wk to 6 wk, followed by a detectable viraemia in serum along to symptoms such as abdominal pain, vomiting, jaundice, etc. Usually, the disease course is self-limiting. As said before, some individual profiles can lead to a more severe hepatic complication.
Whereas HEV-3 infection in healthy humans is mostly asymptomatic, HEV 3 can induce chronic infection in immunocompromised individuals and acute on chronic liver failure in patients with underlying liver diseases. Recent data suggest that the number of reported cases of HEV infections in Europe increased significantly during recent years[31].
Although HEV is not routinely screened during blood donation in most countries, there have been prospective studies that have been conducted searching for markers of HEV infection in serum samples from potential blood donors to assess the local risk for transfusion related HEV[30,32]. The prevalence of detectable anti-HEV IgG positivity among blood donors varies among countries (Table 1). Nevertheless, data can also vary among geographical regions of the same country[40]. Moreover, differences can also be observed depending on the type of diagnostic assay used for the seroprevalence assessment[38,55].
| Country | IgG positive rate (%) | Ref. |
| Argentina | 11.3 | Di Lello et al[33] |
| Austria | 13.5 | Fischer et al[34] |
| Bolivia | 16.2 | Konomi et al[35] |
| Brazil | 7 | Tengan et al[36] |
| China | 30 | Zhang et al[37] |
| Croatia | 20.2 | Miletić et al[38] |
| England | 10 | Beale et al[39] |
| France | 22.4 | Mansuy et al[40] |
| India | 17.7 | Tripathy et al[41] |
| Iran | 8.1 | Hesamizadeh et al[42] |
| Italy | 8.7 | Spada et al[43] |
| New Zealand | 9.7 | Hewitt et al[44] |
| Norway | 14 | Lange et al[45] |
| Poland | 43.5 | Grabarczyk et al[46] |
| Scotland | 9.3 | Thom et al[47] |
| Serbia | 15 | Petrović et al[48] |
| South Africa | 42.8 | Maponga et al[49] |
| Switzerland | 20.4 | Niederhauser et al[50] |
| Thailand | 29.7 | Jupattanasin et al[51] |
| The Netherlands | 24 | Alberts et al[52] |
| Uruguay | 10 | Bangueses et al[53] |
| United States | 9.5 | Stramer et al[54] |
A few cases of HEV infection have been reported to be transmitted by blood transfusion[56]. Since the first reported case of transmission human to human in Japan, some other cases have been reported in many countries[31]. In all of these, the HEV genomic sequence from blood donor and patient matched identically, confirming that the origin of the HEV infection was from the blood and had been transmitted to the patient by transfusion.
There are few data regarding the prevalence of HEV in organ transplant patients. HEV transmission through solid organ transplant have been reported after liver, heart, lung and kidney transplantation[57-60], although to date the risk of HEV infection transmitted by transplantation is unknown.
We did not find data regarding HEV transmission by tissue allografts.
Damaged or absent tissues can be replaced by biological (autografts and allografts) or artificial substitutes. Nowadays, tissue banks offer great availability of different kind of human tissues to be used as allografts, with high standards of safety and efficiency. Therefore, studies analyzing the prevalence of HEV among tissue donors would be needed, in addition to other studies carried out in tissue recipients that could reveal its potential infectivity.
The drawbacks of these studies must be taken into account since many recipients of bone, valves or skin are also recipients of blood components. It is therefore important in a risk assessment procedure to know the degree of imputability that human tissues could have at the implants for HEV transmission. Additionally, these studies could also provide data to evaluate the probability of transmission. The Netherlands provided a definition for both transfusion-associated hepatitis E and transplant-associated infection (Euro CDC). Based in that criteria, tissue transplant-associated HEV infection can be defined as “an acute hepatitis E within 6-8 wk after tissue transplantation (detected by HEV-RNA), where the donor was HEV-RNA positive and at least HEV ORF1/ORF2 hypervariable regions of donor and recipient strains are identical by sequencing”.
It would be important to know the possible medium-long-term side effects for HEV regardless of the implant results. These studies could be obtained by the knowledge about their severity, in order to complete the risk assessment.
There are tissues which can be sterilized since cell viability is not relevant for their clinical efficiency or their biomechanical properties are not significantly altered by the procedure. Likewise, the avascular character of some tissues (as cornea) carries lower risk than vascularized ones (as heart valves).
As very simple forms of life (small size and absence of free water) viruses can be preserved by freezing, not requiring controlled cooling or use of cryoprotectants, as glycerol, dimethyl sulphoxide or polyethylene glycol (the only presence of albumin in the storage solution could be effective for virus cryoprotection). Although virus infectivity can be compromised with long term storage at -20 °C, temperatures ≤ -80 °C allow virus to survive. Additionally, virus can survive to several cycles of freezing/thawing[61]. Conversely, the process of drying and storing at room temperature (conditions associated to lyophilization), could lead to the collapse of the lipid membrane[62].
The storage in liquid nitrogen vs. vapour nitrogen has been related to higher risk of cross-contamination due to faulty seal, leak, or breakage of the containers (bags, cryovials, straws), by acting the liquid environment as vehicle for infectious agent diffusion[63,64].
It is mandatory for tissue banks that provide sterile tissue allograft to follow several steps as donor screening, microbiological testing, aseptic harvesting and processing, disinfection, and, finally, terminal sterilization. According with the standards of the International Atomic Energy Agency (IAEA)[65], sterilization is defined as a validated process to destroy, inactivate, or reduce microorganisms to a sterility assurance level (SAL) of 10-6. Achieving this SAL by a validated process allows labelling of terminally sterilized allografts as sterile[66]. Validation refers to establishing documentary evidence that provides a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and quality attributes, and shall include the following elements[65]: (1) Qualification of the tissue allografts and their packaging for sterilization; (2) Qualification of the irradiation facility; (3) Process qualification using a specified tissue allograft or simulated products in qualified equipment; (4) A certification procedure to review and approve documentation of (1)-(3); and (5) Activities performed to support maintenance of validation.
A validated procedure for the sterilization of tissue allografts must demonstrate efficacy against all classes of microorganisms, throughout the tissue volume and, additionally, must not adversely affect the biological and biomechanical properties which are critical for its clinical use. The inclusion of a terminal inactivation step provides safety against not usually tested viruses in donor screening, such as HEV.
Both enveloped and non-enveloped viruses containing either DNA or RNA have been inactivated by low dose gamma irradiation of musculoskeletal tissues[67]. Both directly (by ionizing radiation) and indirectly (due to aqueous free radicals as intermediaries in the transfer of radiation energy to biological molecules) effects are involved in the inactivation of allografts bioburden[68].
Ethylene oxide inactivates all classes of microorganisms by alkylation of nucleic acids and proteins. However, concerns regarding its potential toxicity have led to a decrease of its use[69].
HEV retained infectivity at temperatures up to 60 °C[70], and heating for 1 min at 70 °C yielded a log reduction of 0.48, which was increased up to 3.67 at 95 °C[71]. Thus, virus heat inactivation at 71 °C for, at least, 20 min has been suggested[72]. Using a Lobator sd-2 system (telos, Marburg, Germany) validated to achieve a temperature of 82.5 °C the centre of femoral heads with a diameter of ≤ 56 mm, Pruss et al[73] obtained a titre reduction (4 Log10 steps) of clinically relevant viruses.
Pruss et al[74] showed the treatment of spongiosa blocks with the peracetic acid-ethanol procedure as a methodology to sterilize bones (maximum thickness ≤ 15 mm). In this study, very slow inactivation kinetics for hepatitis A virus was observed. Thus, while a general reduction of virus titres by more than 4 log10 was determined, only HAV showed a reduction below that threshold (2.87), with residual infectivity.
Current evidence does not recommend to date the universal screening with HEV in tissue donors, although it could be advisable to include the revision of medical-social history about risk practices and in those cases be able to selectively screen for HEV.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Geramizadeh B, Gong N S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | World Health Organization. Ethics, access and safety in tissue and organ transplantation: Issues of global concern Madrid, Spain, 6-9 October 2003: Report. [cited 16 Jan 2020]. In: World Health Organization [Internet]. Available from: https://apps.who.int/iris/handle/10665/42886. |
| 2. | Eastlund T. Bacterial infection transmitted by human tissue allograft transplantation. Cell Tissue Bank. 2006;7:147-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noël L, Strong DM. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation Project NOTIFY. Int Orthop. 2012;36:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Jashari R, Goffin Y, Vanderkelen A, Van Hoeck B, du Verger A, Fan Y, Holovska V, Brahy O. European homograft bank: twenty years of cardiovascular tissue banking and collaboration with transplant coordination in Europe. Transplant Proc. 2010;42:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Wang D, Xie W, Chen T, Dong C, Zhao C, Tan H, Tian H, Xie Q. Evaluation of the Potential Risk of Hepatitis B Virus Transmission in Skin Allografting. Transplant Proc. 2015;47:1993-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Hofstraat SHI, Falla AM, Duffell EF, Hahné SJM, Amato-Gauci AJ, Veldhuijzen IK, Tavoschi L. Current prevalence of chronic hepatitis B and C virus infection in the general population, blood donors and pregnant women in the EU/EEA: a systematic review. Epidemiol Infect. 2017;145:2873-2885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Thursz M, Fontanet A. HCV transmission in industrialized countries and resource-constrained areas. Nat Rev Gastroenterol Hepatol. 2014;11:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 9. | Solves P, Mirabet V, Alvarez M. Hepatitis B transmission by cell and tissue allografts: how safe is safe enough? World J Gastroenterol. 2014;20:7434-7441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Zampino R, Boemio A, Sagnelli C, Alessio L, Adinolfi LE, Sagnelli E, Coppola N. Hepatitis B virus burden in developing countries. World J Gastroenterol. 2015;21:11941-11953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 219] [Cited by in RCA: 197] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 11. | Society for Maternal-Fetal Medicine (SMFM), Dionne-Odom J, Tita AT, Silverman NS. #38: Hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 12. | Fishman JA, Greenwald MA, Grossi PA. Transmission of infection with human allografts: essential considerations in donor screening. Clin Infect Dis. 2012;55:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Zou S, Dodd RY, Stramer SL, Strong DM; Tissue Safety Study Group. Probability of viremia with HBV, HCV, HIV, and HTLV among tissue donors in the United States. N Engl J Med. 2004;351:751-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8:698-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 483] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 15. | Adlhoch C, Manďáková Z, Ethelberg S, Epštein J, Rimhanen-Finne R, Figoni J, Baylis SA, Faber M, Mellou K, Murphy N, O'Gorman J, Tosti ME, Ciccaglione AR, Hofhuis A, Zaaijer H, Lange H, de Sousa R, Avellón A, Sundqvist L, Said B, Ijaz S. Standardising surveillance of hepatitis E virus infection in the EU/EEA: A review of national practices and suggestions for the way forward. J Clin Virol. 2019;120:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | van der Eijk AA, Pas SD, Cornelissen JJ, de Man RA. Hepatitis E virus infection in hematopoietic stem cell transplant recipients. Curr Opin Infect Dis. 2014;27:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Kasem A, Azeem K, Vlčková J, Zatloukalová S, Štěpánek L, Kyselý Z, Kollárová H. Epidemiology of hepatitis E virus infection. Epidemiol Mikrobiol Imunol. 2019;68:176-182. [PubMed] |
| 18. | Donnelly MC, Scobie L, Crossan CL, Dalton H, Hayes PC, Simpson KJ. Review article: hepatitis E-a concise review of virology, epidemiology, clinical presentation and therapy. Aliment Pharmacol Ther. 2017;46:126-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Smith DB, Simmonds P, Izopet J, Oliveira-Filho EF, Ulrich RG, Johne R, Koenig M, Jameel S, Harrison TJ, Meng XJ, Okamoto H, Van der Poel WHM, Purdy MA. Proposed reference sequences for hepatitis E virus subtypes. J Gen Virol. 2016;97:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 324] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 20. | Baylis SA, Blümel J, Mizusawa S, Matsubayashi K, Sakata H, Okada Y, Nübling CM, Hanschmann KM; HEV Collaborative Study Group. World Health Organization International Standard to harmonize assays for detection of hepatitis E virus RNA. Emerg Infect Dis. 2013;19:729-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | van der Eijk AA, Pas SD, de Man RA. Hepatitis E virus: A potential threat for patients with liver disease and liver transplantation. Best Pract Res Clin Gastroenterol. 2017;31:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Pas SD, de Man RA, Mulders C, Balk AH, van Hal PT, Weimar W, Koopmans MP, Osterhaus AD, van der Eijk AA. Hepatitis E virus infection among solid organ transplant recipients, the Netherlands. Emerg Infect Dis. 2012;18:869-872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Wen GP, Tang ZM, Yang F, Zhang K, Ji WF, Cai W, Huang SJ, Wu T, Zhang J, Zheng ZZ, Xia NS. A valuable antigen detection method for diagnosis of acute hepatitis E. J Clin Microbiol. 2015;53:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Zhang F, Li X, Li Z, Harrison TJ, Chong H, Qiao S, Huang W, Zhang H, Zhuang H, Wang Y. Detection of HEV antigen as a novel marker for the diagnosis of hepatitis E. J Med Virol. 2006;78:1441-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Fogeda M, de Ory F, Avellón A, Echevarría JM. Differential diagnosis of hepatitis E virus, cytomegalovirus and Epstein-Barr virus infection in patients with suspected hepatitis E. J Clin Virol. 2009;45:259-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Mirazo S, Ramos N, Mainardi V, Gerona S, Arbiza J. Transmission, diagnosis, and management of hepatitis E: an update. Hepat Med. 2014;6:45-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Iakimchuk KS, Malinnikova EIu, Poleshchuk VF, Mikhaĭlov MI. [Role of hepatitis A and E viruses in the development of autoimmune diseases]. Vopr Virusol. 2011;56:27-29. [PubMed] |
| 28. | van Gerven NM, van der Eijk AA, Pas SD, Zaaijer HL, de Boer YS, Witte BI, van Nieuwkerk CM, Mulder CJ, Bouma G, de Man RA; Dutch Autoimmune Hepatitis Study Group. Seroprevalence of Hepatitis E Virus in Autoimmune Hepatitis Patients in the Netherlands. J Gastrointestin Liver Dis. 2016;25:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Yin X, Li X, Feng Z. Role of Envelopment in the HEV Life Cycle. Viruses. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Bi H, Yang R, Wu C, Xia J. Hepatitis E virus and blood transfusion safety. Epidemiol Infect. 2020;148:e158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Denner J, Pischke S, Steinmann E, Blümel J, Glebe D. Why all blood donations should be tested for hepatitis E virus (HEV). BMC Infect Dis. 2019;19:541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Boland F, Martinez A, Pomeroy L, O'Flaherty N. Blood Donor Screening for Hepatitis E Virus in the European Union. Transfus Med Hemother. 2019;46:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 33. | Di Lello FA, Blejer J, Alter A, Bartoli S, Vargas F, Ruiz R, Galli C, Blanco S, Carrizo LH, Gallego S, Fernández R, Martínez AP, Flichman DM. Seroprevalence of hepatitis E virus in Argentinean blood donors. Eur J Gastroenterol Hepatol. 2021;33:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Fischer C, Hofmann M, Danzer M, Hofer K, Kaar J, Gabriel C. Seroprevalence and Incidence of hepatitis E in blood donors in Upper Austria. PLoS One. 2015;10:e0119576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Konomi N, Miyoshi C, La Fuente Zerain C, Li TC, Arakawa Y, Abe K. Epidemiology of hepatitis B, C, E, and G virus infections and molecular analysis of hepatitis G virus isolates in Bolivia. J Clin Microbiol. 1999;37:3291-3295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Tengan FM, Figueiredo GM, Nunes AKS, Manchiero C, Dantas BP, Magri MC, Prata TVG, Nascimento M, Mazza CC, Abdala E, Barone AA, Bernardo WM. Seroprevalence of hepatitis E in adults in Brazil: a systematic review and meta-analysis. Infect Dis Poverty. 2019;8:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Zhang L, Jiao S, Yang Z, Xu L, Liu L, Feng Q, Zhang X, Hou Y, He S, Saldanha J, Wang S, Wang B. Prevalence of hepatitis E virus infection among blood donors in mainland China: a meta-analysis. Transfusion. 2017;57:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Miletić M, Vuk T, Hećimović A, Stojić Vidović M, Jemeršić L, Jukić I. Estimation of the hepatitis E assay-dependent seroprevalence among Croatian blood donors. Transfus Clin Biol. 2019;26:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Beale MA, Tettmar K, Szypulska R, Tedder RS, Ijaz S. Is there evidence of recent hepatitis E virus infection in English and North Welsh blood donors? Vox Sang. 2011;100:340-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Mansuy JM, Gallian P, Dimeglio C, Saune K, Arnaud C, Pelletier B, Morel P, Legrand D, Tiberghien P, Izopet J. A nationwide survey of hepatitis E viral infection in French blood donors. Hepatology. 2016;63:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 41. | Tripathy AS, Puranik S, Sharma M, Chakraborty S, Devakate UR. Hepatitis E virus seroprevalence among blood donors in Pune, India. J Med Virol. 2019;91:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Hesamizadeh K, Sharafi H, Keyvani H, Alavian SM, Najafi-Tireh Shabankareh A, Sharifi Olyaie R, Keshvari M. Hepatitis A Virus and Hepatitis E Virus Seroprevalence Among Blood Donors in Tehran, Iran. Hepat Mon. 2016;16:e32215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Spada E, Pupella S, Pisani G, Bruni R, Chionne P, Madonna E, Villano U, Simeoni M, Fabi S, Marano G, Marcantonio C, Pezzotti P, Ciccaglione AR, Liumbruno GM. A nationwide retrospective study on prevalence of hepatitis E virus infection in Italian blood donors. Blood Transfus. 2018;16:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 44. | Hewitt J, Harte D, Sutherland M, Croucher D, Fouche L, Flanagan P, Williamson D. Prevalence of hepatitis E virus antibodies and infection in New Zealand blood donors. N Z Med J. 2018;131:38-43. [PubMed] |
| 45. | Lange H, Øverbø J, Borgen K, Dudman S, Hoddevik G, Urdahl AM, Vold L, Sjurseth SK. Hepatitis E in Norway: seroprevalence in humans and swine. Epidemiol Infect. 2017;145:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 46. | Grabarczyk P, Sulkowska E, Gdowska J, Kopacz A, Liszewski G, Kubicka-Russel D, Baylis SA, Corman VM, Noceń E, Piotrowski D, Antoniewicz-Papis J, Łętowska M. Molecular and serological infection marker screening in blood donors indicates high endemicity of hepatitis E virus in Poland. Transfusion. 2018;58:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Thom K, Gilhooly P, McGowan K, Malloy K, Jarvis LM, Crossan C, Scobie L, Blatchford O, Smith-Palmer A, Donnelly MC, Davidson JS, Johannessen I, Simpson KJ, Dalton HR, Petrik J. Hepatitis E virus (HEV) in Scotland: evidence of recent increase in viral circulation in humans. Euro Surveill. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 48. | Petrović T, Lupulović D, Jiménez de Oya N, Vojvodić S, Blázquez AB, Escribano-Romero E, Martín-Acebes MA, Potkonjak A, Milošević V, Lazić S, Saiz JC. Prevalence of hepatitis E virus (HEV) antibodies in Serbian blood donors. J Infect Dev Ctries. 2014;8:1322-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Maponga TG, Lopes T, Cable R, Pistorius C, Preiser W, Andersson MI. Prevalence and risks of hepatitis E virus infection in blood donors from the Western Cape, South Africa. Vox Sang. 2020;115:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Niederhauser C, Widmer N, Hotz M, Tinguely C, Fontana S, Allemann G, Borri M, Infanti L, Sarraj A, Sigle J, Stalder M, Thierbach J, Waldvogel S, Wiengand T, Züger M, Gowland P. Current hepatitis E virus seroprevalence in Swiss blood donors and apparent decline from 1997 to 2016. Euro Surveill. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Jupattanasin S, Chainuvati S, Chotiyaputta W, Chanmanee T, Supapueng O, Charoonruangrit U, Oota S, Louisirirotchanakul S. A Nationwide Survey of the Seroprevalence of Hepatitis E Virus Infections Among Blood Donors in Thailand. Viral Immunol. 2019;32:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Alberts CJ, Schim van der Loeff MF, Sadik S, Zuure FR, Beune EJAJ, Prins M, Snijder MB, Bruisten SM. Hepatitis E virus seroprevalence and determinants in various study populations in the Netherlands. PLoS One. 2018;13:e0208522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Bangueses F, Abin-Carriquiry JA, Cancela F, Curbelo J, Mirazo S. Serological and molecular prevalence of hepatitis E virus among blood donors from Uruguay. J Med Virol. 2021;93:4010-4014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Stramer SL, Moritz ED, Foster GA, Ong E, Linnen JM, Hogema BM, Mak M, Chia CP, Dodd RY. Hepatitis E virus: seroprevalence and frequency of viral RNA detection among US blood donors. Transfusion. 2016;56:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 55. | Al-Absi ES, Al-Sadeq DW, Younis MH, Yassine HM, Abdalla OM, Mesleh AG, Hadwan TA, Amimo JO, Thalib L, Nasrallah GK. Performance evaluation of five commercial assays in assessing seroprevalence of HEV antibodies among blood donors. J Med Microbiol. 2018;67:1302-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Gallian P, Pouchol E, Djoudi R, Lhomme S, Mouna L, Gross S, Bierling P, Assal A, Kamar N, Mallet V, Roque-Afonso AM, Izopet J, Tiberghien P. Transfusion-Transmitted Hepatitis E Virus Infection in France. Transfus Med Rev. 2019;33:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 57. | Wang Y, Chen G, Pan Q, Zhao J. Chronic Hepatitis E in a Renal Transplant Recipient: The First Report of Genotype 4 Hepatitis E Virus Caused Chronic Infection in Organ Recipient. Gastroenterology. 2018;154:1199-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 58. | Schlosser B, Stein A, Neuhaus R, Pahl S, Ramez B, Krüger DH, Berg T, Hofmann J. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J Hepatol. 2012;56:500-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 59. | Sridhar S, Cheng VCC, Wong SC, Yip CCY, Wu S, Lo AWI, Leung KH, Mak WWN, Cai J, Li X, Chan JFW, Lau SKP, Woo PCY, Lai WM, Kwan TH, Au TWK, Lo CM, Wong SCY, Yuen KY. Donor-Derived Genotype 4 Hepatitis E Virus Infection, Hong Kong, China, 2018. Emerg Infect Dis. 2019;25:425-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 60. | Pourbaix A, Ouali N, Soussan P, Roque Afonso AM, Péraldi MN, Rondeau E, Peltier J. Evidence of hepatitis E virus transmission by renal graft. Transpl Infect Dis. 2017;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Tedeschi R, De Paoli P. Collection and preservation of frozen microorganisms. Methods Mol Biol. 2011;675:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Salvucci JT. Bone tissue, lyophilized and stored at room temperature for 15 days or more, is not capable of transmitting HIV, HCV or HBV. Cell Tissue Bank. 2011;12:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Hawkins AE, Zuckerman MA, Briggs M, Gilson RJ, Goldstone AH, Brink NS, Tedder RS. Hepatitis B nucleotide sequence analysis: linking an outbreak of acute hepatitis B to contamination of a cryopreservation tank. J Virol Methods. 1996;60:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Mirabet V, Alvarez M, Solves P, Ocete D, Gimeno C. Use of liquid nitrogen during storage in a cell and tissue bank: contamination risk and effect on the detectability of potential viral contaminants. Cryobiology. 2012;64:121-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Association for the Advancement of Medical Instrumentation. ST67:2011/(R) 2017. Sterilization of health care products. Requirements and guidance for selecting a sterility assurance level (SAL)for products labelled ‘sterile’. [cited 20 Dec 2020]. In: Association for the Advancement of Medical Instrumentation [Internet]. Available from: https://www.aami.org. |
| 66. | International Atomic Energy Agency. Radiation sterilization of tissue allografts: requirements for validation and routine control. A code of practice. [cited 20 Dec 2020]. In: International Atomic Energy Agency [Internet]. Available from: https://www-pub.iaea.org/MTCD/Publications/PDF/Pub1307_web.pdf. |
| 67. | Moore MA. Inactivation of enveloped and non-enveloped viruses on seeded human tissues by gamma irradiation. Cell Tissue Bank. 2012;13:401-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Singh R, Singh D, Singh A. Radiation sterilization of tissue allografts: A review. World J Radiol. 2016;8:355-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 119] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (3)] |
| 69. | Bienek C, MacKay L, Scott G, Jones A, Lomas R, Kearney JN, Galea G. Development of a bacteriophage model system to investigate virus inactivation methods used in the treatment of bone allografts. Cell Tissue Bank. 2007;8:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Emerson SU, Arankalle VA, Purcell RH. Thermal stability of hepatitis E virus. J Infect Dis. 2005;192:930-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 71. | Schielke A, Filter M, Appel B, Johne R. Thermal stability of hepatitis E virus assessed by a molecular biological approach. Virol J. 2011;8:487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 72. | Barnaud E, Rogée S, Garry P, Rose N, Pavio N. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl Environ Microbiol. 2012;78:5153-5159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 73. | Pruss A, Kao M, von Garrel T, Frommelt L, Gürtler L, Benedix F, Pauli G. Virus inactivation in bone tissue transplants (femoral heads) by moist heat with the 'Marburg bone bank system'. Biologicals. 2003;31:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Pruss A, Göbel UB, Pauli G, Kao M, Seibold M, Mönig HJ, Hansen A, von Versen R. Peracetic acid-ethanol treatment of allogeneic avital bone tissue transplants--a reliable sterilization method. Ann Transplant. 2003;8:34-42. [PubMed] |