Published online Nov 22, 2021. doi: 10.4291/wjgp.v12.i6.115
Peer-review started: April 16, 2021
First decision: June 23, 2021
Revised: July 8, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: November 22, 2021
Processing time: 213 Days and 16.1 Hours
Acute pancreatitis (AP) is an inflammatory disease, which presents with epigastric pain and is clinically diagnosed by amylase and lipase three times the upper limit of normal. The 2012 Atlanta classification stratifies the severity of AP as one of three risk categories namely, mild AP (MAP), moderately severe AP (MSAP), and severe AP (SAP). Challenges in stratifying AP upon diagnosis suggest that a better understanding of the underlying complex pathophysiology may be beneficial.
To identify the role of the chemokine receptor 8 (CCR8), expressed by T-helper type-2 Lymphocytes and peritoneal macrophages, and its possible association to Interleukin (IL)-6 and AP stratification.
This study was a prospective case-control study. A total of 40 patients were recruited from the Chris Hani Baragwanath Academic Hospital and the Charlotte Maxeke Johannesburg Academic Hospital. Bioassays were performed on 29 patients (14 MAP, 11 MSAP, and 4 SAP) and 6 healthy controls as part of a preliminary study. A total of 12 mL of blood samples were collected at Day (D) 1, 3, 5, and 7 post epigastric pain. Using multiplex immunoassay panels, real-time polymerase chain reaction (qRT-PCR) arrays, and multicolour flow cytometry analysis, immune response-related proteins, genes, and cells were profiled respectively. GraphPad Prism™ software and fold change (FC) analysis was used to determine differences between the groups. P<0.05 was considered significant.
The concentration of IL-6 was significantly different at D3 post epigastric pain in both the MAP group and MSAP group with P = 0.001 and P = 0.013 respectively, in a multiplex assay. When a FC of 2 was applied to identify differentially expressed genes using RT2 Profiler, CCR8 was shown to increase steadily with disease severity from MAP (1.33), MSAP (38.28) to SAP (1172.45) median FC. Further verification studies using RT-PCR showed fold change increases of CCR8 in MSAP and SAP ranging from 1000 to 1000000 times when represented as Log10, compared to healthy control respectively at D3. The findings also showed differing lymphocyte and monocyte cell frequency between the groups. With monocyte population frequency as high as 70% in MSAP at D3.
The higher levels of CCR8 and IL-6 in the severe patients and immune cell differences compared to MAP and controls provide an avenue for exploring AP stratification to improve management.
Core Tip: Chemokine receptor 8 (CCR8) is a chemokine receptor that is highly expressed on monocytes and cells of T helper type-2 (Th2) lineage including innate lymphoid cells group 2 and 3 (ILC2 and 3). This study shows possible linkages between increasing CCR8 expression and severity in mainly moderately severe acute pancreatitis (MSAP) patients when compared to mild acute pancreatitis (MAP). Differing lymphocyte and monocyte cell frequencies suggest that in MAP, interleukin (IL)-6 was highly expressed in lymphocytes, and in the severe patients [MSAP and severe acute pancreatitis (SAP)] were highly expressed by monocytes. The findings open doors for future work, which could include an in-depth look at IL-6 producing cells such as Th2 Lymphocytes, monocytes, and innate ILC2 to determine cell-associated cytokine as a novel approach in prognosticating AP disease severity.
- Citation: Nalisa M, Nweke EE, Smith MD, Omoshoro-Jones J, Devar JW, Metzger R, Augustine TN, Fru PN. Chemokine receptor 8 expression may be linked to disease severity and elevated interleukin 6 secretion in acute pancreatitis. World J Gastrointest Pathophysiol 2021; 12(6): 115-133
- URL: https://www.wjgnet.com/2150-5330/full/v12/i6/115.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v12.i6.115
Acute pancreatitis (AP) is an inflammatory disease that presents with epigastric pain and is clinically diagnosed by amylase and lipase levels three times the upper limit of normal[1]. The disease is localized to the pancreas and is triggered by the premature release of digestive enzymes resulting from damaged pancreatic acinar cells[2-3]. Through activation of the immune system, patients develop a systemic inflammatory response syndrome (SIRS) and subsequently, single or multiple organ failure leading to high mortality[4]. This disease is one of the most common cause of hospital admissions and has an annual incidence of 80 in 100000 people worldwide[5-7].
The severity of acute pancreatitis is classified as mild, moderate, or severe[1]. Mild AP (MAP) presents with no organ failure and no local complications. Moderately severe acute pancreatitis (MSAP) only differs from severe AP (SAP) in that the patients have transient organ failure (OF) within 48 h and possibly pancreatic necrosis[1,8-9]. If OF persists for more than 48 h the patient is classified as severe[1]. MSAP is further defined by specified local complications or exacerbation of the co-morbid disease. Local complications include pancreatic fluid collections, pancreatic and peripancreatic necrosis (sterile or infected), pseudocyst, and walled-off necrosis (sterile or infected)[1]. Due to the complications and subsequent high mortality observed with increasing disease severity, the need arises for early stratification of the disease through the understanding of the pathogenesis of the disease and its systemic inflammatory response[4,7,10].
It is generally accepted that the premature release or activation by trypsin of proenzymes (including trypsinogen) is the initial trigger of pancreatitis[11]. Under normal conditions, trypsin and other proteolytic enzymes are blocked from activation by serine protease inhibitor, Kazal type 1, which is secreted by acinar cells[11]. AP is characterized by the activation of trypsin and other events such as obstruction and passage of gallstones in the bile duct (in the case of acute biliary pancreatitis), which in turn blocks the transport of trypsin to the small intestine[7,12]. This leads to premature activation of lipase and elastase causing intracellular damage of cells and subsequently inflammation and thrombosis. Damaged acinar cells are unable to regulate trypsin activity leading to further inflammation and eventual tissue damage through excessive amounts of activated enzymes within the pancreas. Lipase in particular, induces necrosis in fat cells within the pancreas leading to local recruitment of proinflammatory markers including cytokines[7,13].
Identifying prognostic markers of AP would ensure early patient stratification. Markers such as C-reactive protein (CRP), nuclear factor kappa B (NF-κB), and IL-6 have been identified as potential prognostic markers in AP. CRP, an acute-phase reactant produced by the liver and induced by IL-6, is well described as an inflammatory marker for the disease. It has been demonstrated as an effective prognostic marker of AP severity at 48 h after admission, although other studies found that its strength as a prognostic marker is prominent only at 72 h after admission[11,14-15]. NF-κB, on the other hand, is a transcription factor involved in cell proliferation[13]. This molecule is responsible for cellular responses to free radicals such as reactive oxygen species, production of inflammatory cytokines (IL-2, IL-6, TNF-α, IL-1β, and IL-8), and excess production of calcium within acinar cells, which results in premature activation of trypsinogen[13]. NF-κB is also responsible for activating the cytokine cascade that manifests as SIRS[13,6-17].
Considering that AP is an inflammatory disease, continuous efforts to fully understand its immunopathogenesis are critical to potentially improve management. This is due to the underlying complex pathophysiology associated with the disease[18]. For autoimmune diseases, the excessive recruitment of inflammatory mediators and subsequent increase in the production of cytokines and chemokines after an insult is responsible for inflammation[19]. This condition is further aggravated by the continued recruitment and infiltration of macrophages, neutrophils, and lymphocytes to the site of injury[19–21]. The resulting inflammation from the tissue injury, as a result of damage to the pancreas due to either obstruction or passage of gallstones, in biliary AP, can be attributed to damage-associated molecular patterns, which may result in necrosis of the pancreas in more severe forms of AP[20]. These inflammatory molecules are then recognized by pattern recognition receptors of the innate immune system. This process mobilizes the recruitment of neutrophils, macrophages, dendritic cells, and mast cells in the peripheral blood and at the site of injury, which in turn produces cytokines including IL-1, IL-6, and TNF-α[20-21]. This results in inflammation at the site of injury and phagocytosis by macrophages and neutrophils[21]. Phagocytosis activates antigen-presenting cells (APCs), which include macrophages, dendritic cells, and B cells[21]. Another cell type involved in innate immunity is natural killer (NK) cells, which help activate the adaptive immune system (AIS) by increasing the production of interferon-gamma (IFN-γ), a recognized initiator of the AIS[21-22]. This presentation process of the AIS activates T cell proliferation[22]. Naïve T cells will differentiate into cytotoxic T cells (CD8+) or T helper (Th) cells (CD4+ cells). CD8+ cells eliminate the threat of infected cells and tumorous cells[21]. Once the threat is eliminated, another group of T cells, T regulatory cells, suppress the immune response to achieve homeostasis[19].
Natural Killer cells belong to a group of cells known as innate lymphoid cells (ILCs). These cells are responsible for regulating immune responses and are mainly found within the tissues[21-22]. The ILCs have been described as mirrors of the T helper cells but within the innate immunity[23]. Three groups of ILCs produce the same cytokines as T helper cells, i.e., ILC group 1 (ILC1) produces Th1 cytokines; ILC2 produces Th2 cytokines and ILC3 produces Th17 cytokines[21-22]. As detailed in Supplementary Table 1, the group 1 ILCs produce IFN-γ and require T-box transcription factor for their proliferation; group 2 ILCs require transcription factor GATA-3 and ROR-α to develop and will produce Th2 cytokines, such as IL-4, IL-5, and IL-13[24]. Group 3, ILCs depend on the transcription factor, ROR-γt, for their development and produce IL-17 and IL-22 and granulocyte-macrophage colony-stimulating factor (GM-CSF)[22]. ILCs have also been reported to act as antagonists of both innate and adaptive immune cells[25], by mimicking the activity of T regulatory cells in achieving immune homeostasis[22].
CCR8, a chemokine receptor, is highly expressed on monocytes and cells of Th2 Lineage including ILC2 and ILC3[26]. This chemokine is also expressed on peritoneal macrophages in tissue and lymphocytes of Th2 Lineage[27]. Studies demonstrate that NF-кB is suppressed in CCR8 deficient mice and that macrophage chemotaxis in the peritoneal cavity, which includes the pancreas, is Chemokine (C-C motif) ligand 1 (CCL1), which is the ligand of CCR8 is dependent[27]. CCR8 and its ligand, CCL1, are known to recruit and activate macrophages in type 1 diabetes[28-29]. This study is the first to describe CCR8 in AP and its possible linkages to lymphocyte and monocyte cell frequencies.
This study utilized patients’ samples at different severities (MAP, MSAP, and SAP) to profile inflammatory genes, and proteins (including CCR8 and IL-6) and identified those that were distinctly upregulated or downregulated. White blood cell populations were characterised and assessed and linkages to gene and protein expression are proposed as potential prognostic markers for AP. The findings also provide insights that are more recent and contribute to the scarce literature on the prevalence, demographics, and etiology of AP in an African setting.
Ethics approval for this study was obtained from the Human Research Ethics Committee Medical of the University of the Witwatersrand (Ethics No. M180133). All patients included in the study were duly informed and written consent was received before blood samples were taken. Using the Revised Atlanta Classification (RAC) for AP[1], patients were recruited from the Hepatopancreatobiliary Unit of the Chris Hani Baragwanath Academic Hospital (CHBAH) and the Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) in Johannesburg, South Africa, from August 2018 to September 2019. The total number of patients recruited was 40 (21 MAP, 14 MSAP, and 5 SAP) and 6 healthy volunteers were recruited as controls after being age and sex-matched to recruited patients. Blood samples were collected on Day 1, 3, 5, and 7 post epigastric pain using three BD vacutainer® purple blood collection tubes (BD Biosciences, New Jersey, United States) with 4 mL of blood each. Patients on average presented at the hospital approximately after 72 h of pain (day 3 of post epigastric pain). Clinicians within the Gastrointestinal Unit of the respective hospitals diagnosed patients and classified them into the three groups (MAP, MSAP, and SAP). The stratification of severity was determined using the RAC guidelines.
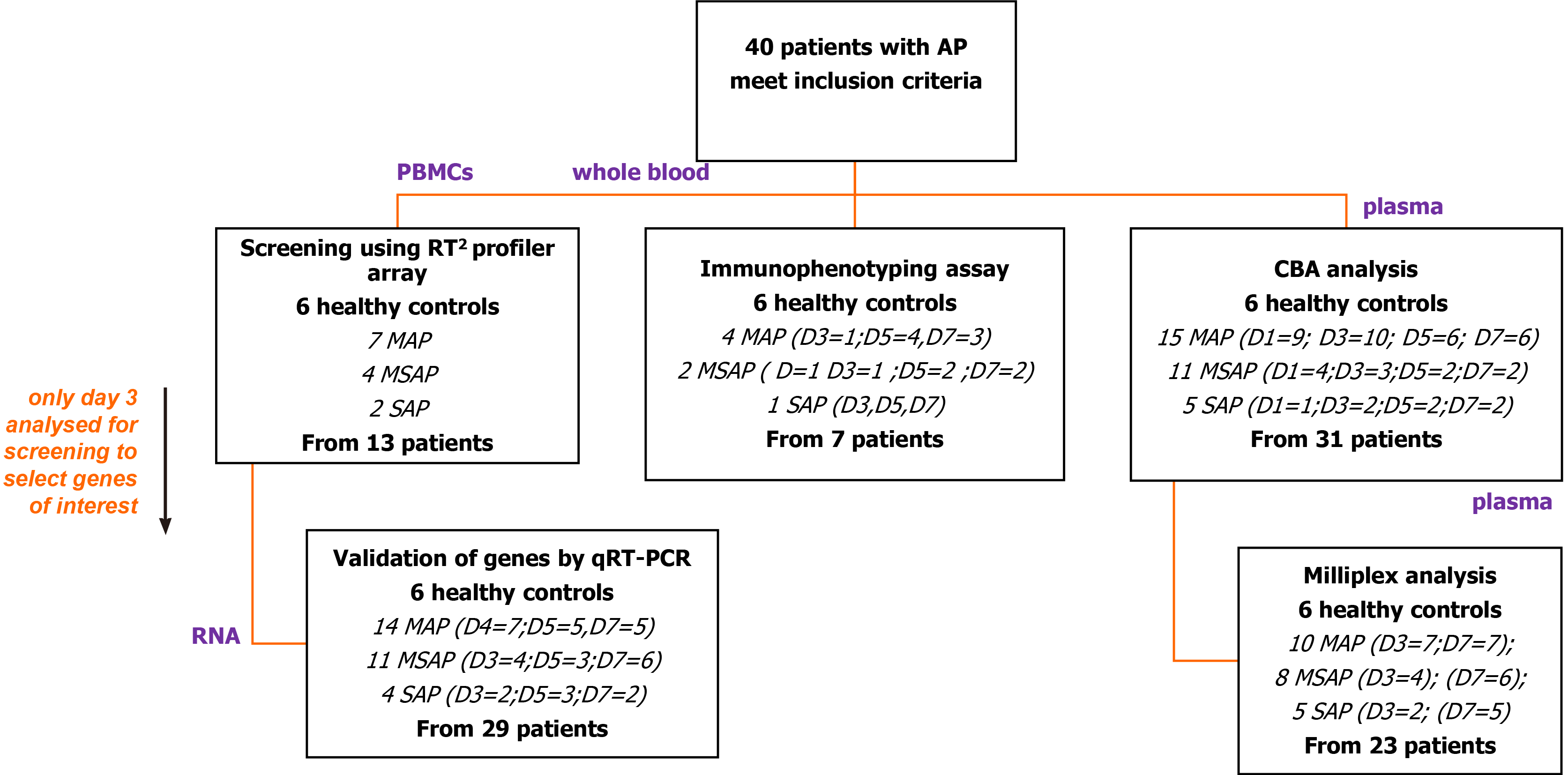
The different aspects of the study included different numbers of patients as illustrated (Figure 1). From the 40 patients, plasma and cell samples were processed in the laboratory within 4 h of phlebotomy. Plasma from a total of 31 out of 40 patients was analysed using the Th1/Th2/Th17 cytometric bead array (CBA) kit in an initial exploratory study. Based on this analysis, plasma samples from 23 patients were randomly selected for analysis of selected Th17 related cytokines including IL-6 using the MILLIPLEX® MAP Human Th17 Magnetic Bead Panel kit (Millipore™, Massachusetts, United States).
RNA was extracted from peripheral blood mononuclear cells (PBMCs) using the TriReagent® (Sigma Aldrich, Missouri, United States) method from 13 patients for screening of genes with the human innate and adaptive RT2 Profiler 96-well PCR array plates (QIAGEN, Hilden, Germany). Findings showed dose-dependent expression of the CCR8 gene with disease severity, prompting further analysis in 29 patients using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) to verify its roles. To characterize cell types into monocytes, lymphocytes, and granulocytes, seven patients were included in an antibody specific multicolour immunophenotyping flow cytometry experiment.
From the blood samples, plasma was isolated by gravity separation for 45 min at room temperature followed by centrifugation at 1500 r/min for 30 min. Plasma samples were aliquoted (200 µL) in single use vials and stored at -80 °C until needed.
Using Ficoll-Paque™ (GE Healthcare, Illinois, United States) separation method, as per the manufacturer’s instructions, PBMCs were separated and stored in single use aliquots in liquid nitrogen in a freezing medium (10% dimethyl sulphoxide in fetal bovine serum, Sigma Aldrich, Missouri, United States) until required. Samples were only thawed once to preserve integrity.
Protein expression analysis was performed using two methods as depicted in Figure 1. The first was a BD BioSciences cytometric bead array Th1/Th2/Th17 kit that served as an exploratory step to determine the concentration of interleukin (IL-2), IL-4, IL-6, IL-10, TNF, IFN-γ, and IL-17A cytokines. The assay was done on 31 AP patients (15 MAP, 11 MSAP, and 5 SAP) and 6 healthy control donor samples on days 1, 3, 5, and 7 post epigastric pain (see the supplementary section for detailed protocol). The second analysis was done using a MILLIPLEX® MAP Human Th17 Magnetic Bead Panel kit (Millipore™, Massachusetts, United States).
In the MILLIPLEX® assay, preselected cytokines, based on the performance of the CBA analysis and based on literature and previous work from the research group were used[30-32]. These cytokines were; IL-17A, IL-21, and IL-6, IFN-γ, IL-23, IL-28λ, and TNF-β measured from 23 randomly selected AP patient samples (10 MAP, 8 MSAP, and 5 SAP) from the pool of 31 patient samples tested in the CBA assay on days 3 and 7 post epigastric pain. Six healthy controls were included.
A solid 96 well plate was prepared using the manufacturer’s instructions. Plates were run on BioPlex® 2200 system (BioRAD, California, United States) and data were collected and analysed using BioPlex® Manager 5.0 software (BioRad, California, United States). All samples and controls were measured in duplicate to minimize errors. Controls included quality control (QC) 1 samples (low level) and QC2 samples (high level) as well as standards with the lowest dilution at 4:1. The observed concentration of cytokines was determined by excluding outliers and values extrapolated beyond the standard range. Values designated by an asterisk as per the BioPlex® Manager 5.0 software, were inputted as zero while values labeled as Out of Range were not considered in the analysis.
Total RNA was extracted using the TriReagent® (Sigma Aldrich, Missouri, United States) protocol, according to the manufacturer’s instructions, from the isolated PBMCs on Day 3, 5, and 7 samples. However, initial screening was performed on 13 (MAP, n = 7; MSAP, n = 4; SAP, n = 2) Day 3 samples only. The quality of RNA was measured using a NanoDrop ND-1000 Spectrophotometer (Thermo Fischer Scientific, Massachusetts, United States), and samples with an A260/280 ratio > 1.8 were observed across all samples[33-34].
Complementary DNA synthesis (cDNA) was performed from 250 ng/µL of total RNA using the RT2 First Strand Kit (QIAGEN, Hilden, Germany), according to the manufacturers’ instructions. A genomic DNA elimination mix was first prepared and incubated for 5 min at 42 °C in a SimpliAmp™ thermocycler (ThermoFischer Scientific, Massachusetts, United States), which was subsequently placed on ice for 1 min. Following this, a 20 µL cDNA synthesis reaction was prepared and run at 42°C for 15 min followed by incubation at 95 °C for 5 min. From the cDNA, 102 µL was added to the PCR mixture and loaded onto the human innate and adaptive RT2 Profiler 96-well PCR array plates (QIAGEN, Hilden, Germany). The mixture was amplified on Quant Studio 1 Real-Time System (Thermo Fischer Scientific, Massachusetts, United States) the PCR reaction was run for 40 cycles including a 10 min hot start at 95 ºC for 1 cycle; 95 ºC for 15 s and 60 ºC for 1 min. The human innate and adaptive RT2 Profiler array includes 96 genes, 5 of which are reference genes and 3 reverse-transcription controls, 3 positive PCR controls, and 1 human genomic DNA control. Using the QIAGEN GeneGlobe online tool (https://geneglobe.qiagen.com/za/analyze/), a fold-change of 2 was applied as the cut-off for differential analysis comparing the expression level of genes in the 3 severity groups to healthy control.
After screening of Day 3 samples for early immune markers with the RT2 Profiler PCR Array Human Innate and Adaptive Immune Responses (QIAGEN, Hilden, Germany) the CCR8 gene was selected for further analysis. Twenty-nine patients (MAP = 14, MSAP = 11, SAP = 4) were included in this assay as stated in Figure 1 and Table 1. The TaqMan® Fast Advanced Master Mix (Thermo Fischer, Massachusetts, United States) was used to perform duplex qRT-PCR. The PCR reaction was run for 40 cycles including a 2 min hold at 95ºC for 1 cycle; 95ºC for 1 s and 60 ºC for 20 s. Normalisation was done using RPL13A on VIC (assay ID Hs04194366_g1, Thermo Fischer Scientific, Massachusetts, United States) as the reference gene. This gene is well established in AP disease models as a reference gene[35]. The target gene was CCR8 on FAM (assay ID: Hs00174764_m1, Thermo Fischer Scientific, Massachusetts, United States). The Quant Studio™ 1 Real-Time System (Thermo Fischer Scientific, Massachusetts, United States) was used to run the RT-qPCR reactions. The 2-ΔΔCT method was used to calculate relative changes in gene expression[36].
| Parameter | Value [n, %] |
| AP patient demographics | n = 29 |
| MAP | 14 (48) |
| MSAP | 11 (38) |
| SAP | 4 (14) |
| Age (yr), [median (IQR)] | 41 (23, 76) |
| Male (n, %) | 17 (49) |
| Female (n, %) | 12 (51) |
| AP etiology/risk factor | |
| Biliary (n, %) | 13 (45) |
| Alcohol (n, %) | 13 (45) |
| ERCP (n, %) | 1 (3) |
| Antiretroviral (n, %) | 2 (7) |
| Healthy control Demographics | |
| Age (yr), [median (IQR)] | 36.5 (23, 55) |
| Male (n, %) | 3 (50) |
| Female (n, %) | 3 (50) |
Selected blood samples from days 3, 5, and 7 of onset of AP symptoms were analysed using multicolour flow cytometry to determine immune cell frequency levels to make a correlation to protein production or expression. The sampled patients included 4 in the MAP group, 2 patients from the MSAP group; 1 patient from the SAP group. Six healthy participant samples were used as controls. While the numbers here are small, given the well characterized levels of monocytes, lymphocytes, and granulocytes in AP patients from the literature[18,20,37-38], inferences from this preliminary data will be discussed with reference to the literature.
A 12-colour panel was established to characterize heterogeneous cell populations in the three risk categories of AP. Using the lyse/wash method, whole blood was used to isolate white blood cells from 100 µL of blood from an EDTA blood tube within 6 h of phlebotomy. Antibodies were optimized by titration to optimally stain lymphocytes populations and subpopulations using CD3 BD Horizon Brilliant™ Ultraviolet (BUV); CD4 Alexa flour; CD8 Brilliant Violet™ 605; CD56 PE Phycoerythrin Cyanine 7 (PECy7), CD16 PECy5) and monocyte populations using CD16PECy5 and CD14 Peridinin-Chlorophyll-protein cyanine 5.5 (PerCPCy5.5), and CD14PerCP Cy5.5 and human leukocyte D related (HLA-DR BV650). Other antibodies that were included in the 12 colour panel but not reported in the study are listed in Supplementary Table 2. All antibodies were from BD Biosciences, (New Jersey, United States).
Cells were prepared both as fully stained samples and as unstained samples. Fully stained samples were suspended in BD Horizon brilliant buffer (BD Biosciences, New Jersey, United States) and stained with selected antibodies (see Supplementary Table 2). The cells were then incubated in the dark for 20 min and thereafter fixed with 2 mL of diluted BD FACS Lyse (BD Biosciences, New Jersey, United States) and incubated for 12 min with intermittent mixing with a pipette. The cells were then washed with diluted Dulbecco's Phosphate Buffered Saline (Sigma Aldrich, Missouri, United States) at 150 x g for 5 min. Approximately 100000 cells were acquired on BD LSRFortessa™ II flow cytometer (BD Biosciences, New Jersey, United States) for each sample at a threshold of 5,000 after the necessary quality controls using FACSDiva™ software version 5 (BD, Biosciences, New Jersey, United States). The controls included voltages optimization using single stains, compensation for spillover was done using CompBeads (Anti-Mouse Ig, κ/Negative Control Compensation Particles Set; BD Biosciences, New Jersey, United States) and 8 peak beads (BD Biosciences, New Jersey, United States) were used to determine linearity in fluorescence detection channels on every sample run.
Data was further analysed using FlowJo LLC version 10 (BD, Biosciences, New Jersey, United States) with previously linked compensation controls from FACSDiva™ software. Cells were gated as singlets, then further as granulocytes, lymphocytes, and monocytes using forward scatter and side scatter properties as well as fluorescent antibody stains for specific subsets. Doublets were excluded using Forward scatter height (FSC-H) and FSC area (FSC-A), then FSC and side scatter (SSC) were used to discriminate white blood cells namely lymphocytes, granulocytes, and monocytes. All populations were represented as percentages of parent populations. Of the 12 antibodies used for cell differentiation, analysis was done for CCR8 associated cell populations. These include lymphocytes and monocytes[39]. These populations were lymphocyte subpopulations (CD3-CD16+ CD56+/-, CD3-CD16+CD57+/-) and monocyte populations and subpopulations (CD14+/- CD16+/- and CD14+HLA-DR+/-).
The cytokine data and qRT-PCR data were analysed using GraphPad Prism™ software version 8 (GraphPad Software Inc, California, United States). A Shapiro-Wilk test was used to test for normality. Once data was determined to be non-parametric, a Kruskal Wallis test was used to determine significant differences between the healthy control groups and between the MAP, MSAP, and SAP groups. The P values were considered significant at P < 0.05. A Dunn’s Multiple Comparison Test was used to perform a post hoc analysis to eliminate type 1 errors. Immunophenotyping data focused on lymphocytes and monocytes as they relate to CCR8 expression[39] and were presented as percentages and ratios. The statistical methods of this study were reviewed by Mr. Glory Chidumwa from the Division of Epidemiology and Biostatistics, School of Public Health, Faculty of Health Sciences, University of the Witwatersrand.
A total of 40 patients were included in the overall study using prescribed inclusion criteria from 1 August 2018 to 22 August 2019 from CHBAH and CMJAH in Johannesburg, South Africa. Of these 40 patients, 29 were reported in the gene expression studies (Figure 1). The gender distribution of the 29 patients was 41% females and 59% males. The most common etiologies of AP were alcohol and biliary-related with each category consisting of 45% of the recruited patients (Table 1). The median age of the patients was 36.5 years. The MSAP group age range was between 26 to 76 years and that of the SAP group was between 40 and 69 years old.
In the exploratory CBA assay, data were expressed as Mean Fluorescent Intensity (MFI) as shown in Supplementary Figure 1. In the analysis of the data, only the MFI of IL-6 revealed changes between patient plasma samples at Day 3. On Day 1, the MAP group had a high expression of IL-6 at above 5000 MFI, which was significantly different from healthy controls (P = 0.015). At Day 3 in the MAP a significant difference was reported with P = 0.004 when compared to the healthy control. In the MSAP group, there was a significant difference on Day 3 (P = 0.004) and 7 (P = 0.029). IL-6 MFI was in the region of 5000 for the SAP patient.
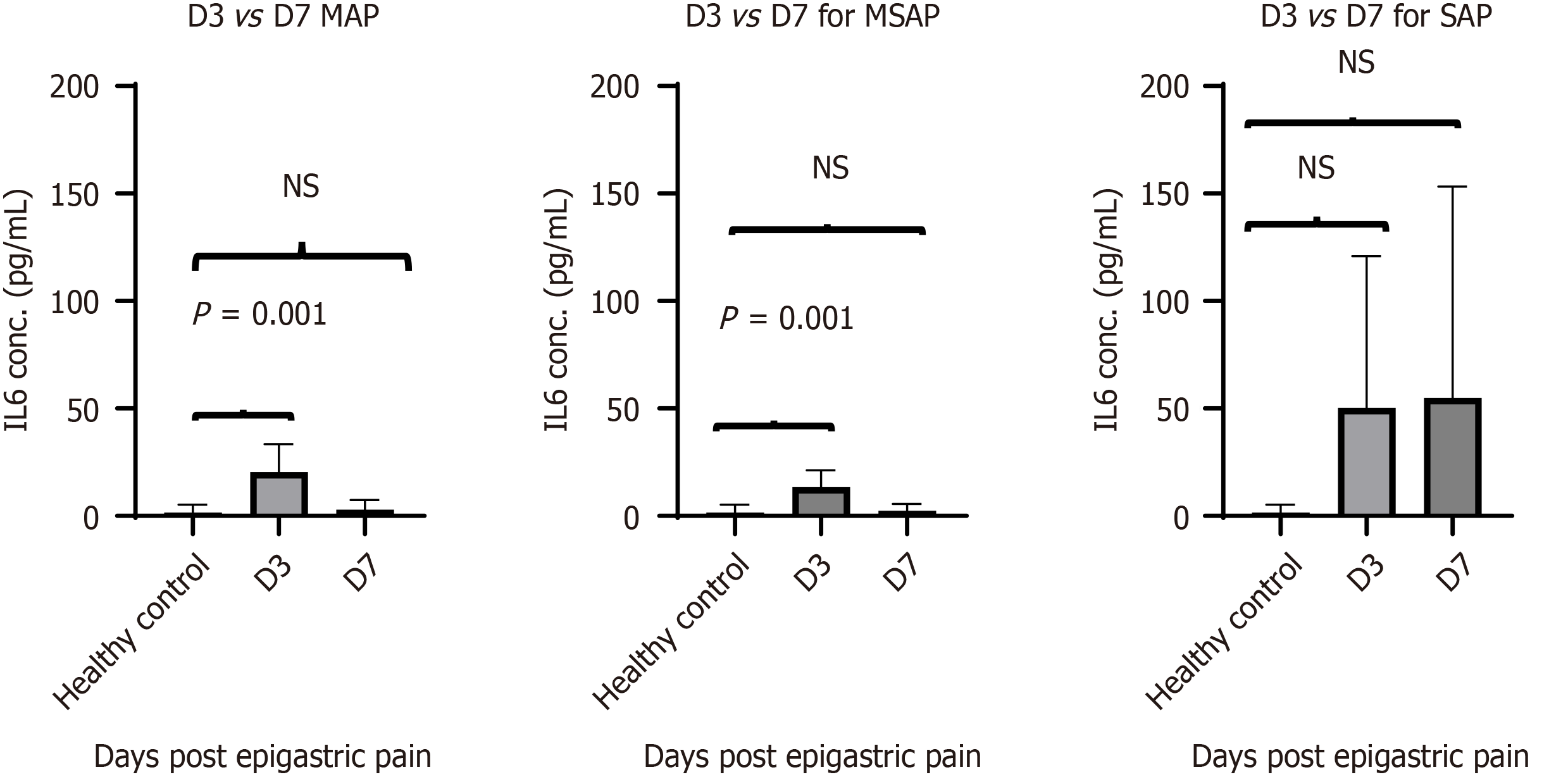
The results from the MILLIPLEX® data showed visible trends between severities over time as well as between groups. The mean concentration of IL-6 in the MAP group was 20 ± 4.9pg/mL on Day 3 and dropped to 2.9 ± 1.7pg/mL on Day 7. A similar trend was seen in the MSAP group with a drop in mean concentration from 13 ± 4 pg/mL on Day 3 to 10 ± 7.7pg/mL on Day 7. The IL-6 concentration was significantly different at D3 for MAP (n = 7) and MSAP (n = 4) compared to healthy controls with P = 0.001 and P = 0.013 respectively (Figure 2). The concentration of the SAP group was not significantly different at both Days 3 (n = 2) and 7 (n = 5) compared to healthy controls with P = 0.094 and P = 0.186 respectively. However, the mean concentration of IL-6 in the SAP group was higher compared to the MAP and MSAP groups. The concentration at Day 3 was 50 pg/mL (this included two patients with individual IL-6 concentrations of 0.13 pg/mL and 100 pg/mL). The mean concentration at Day 7 was 65 ± 62pg/mL as shown in Figure 2.
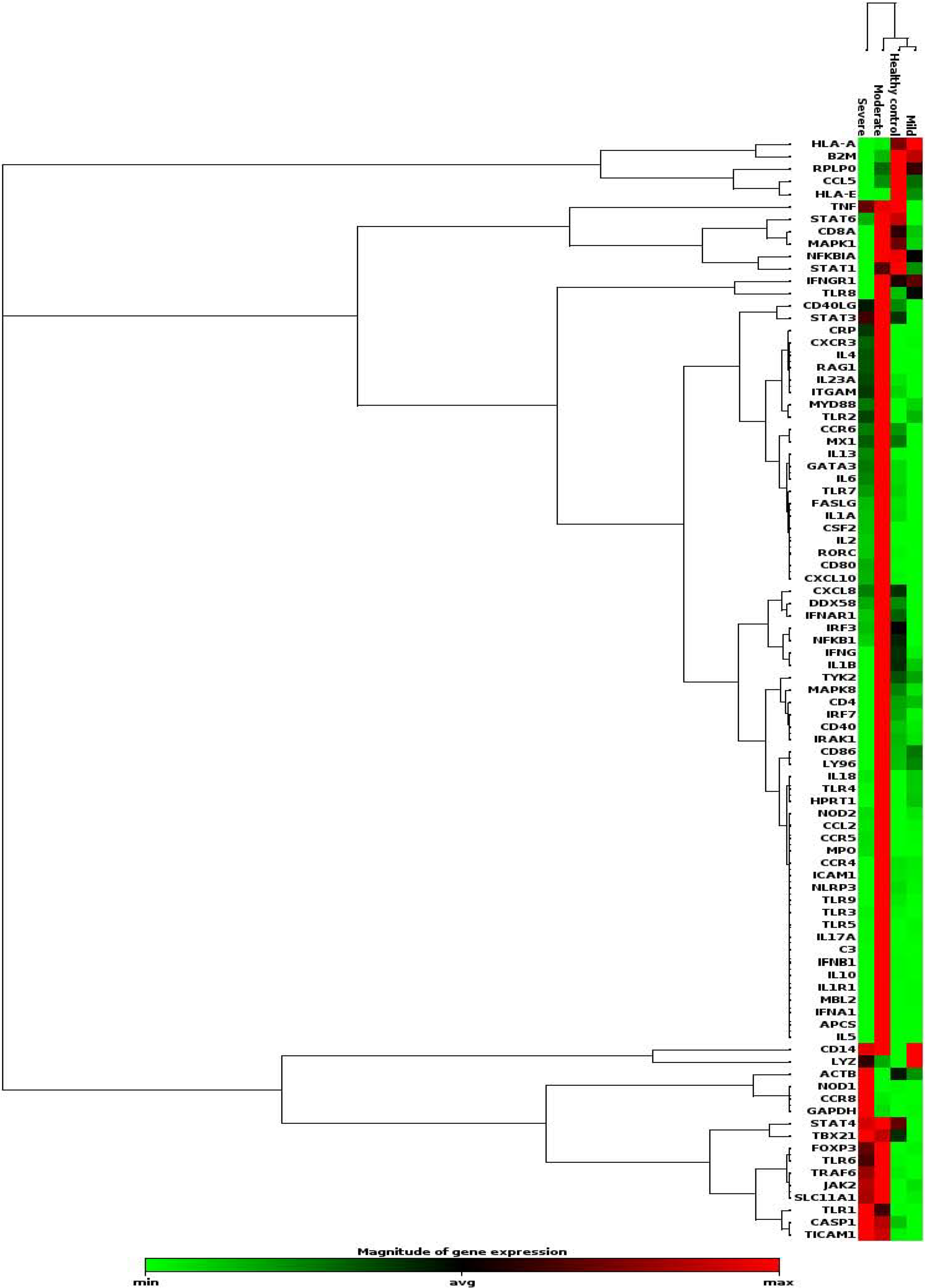
A fold change (FC) of 2 was applied to identify differentially expressed genes in the patient groups (7 MAP, 4 MSAP, and 2 SAP) at Day 3 compared to healthy controls from the RT2 First Strand Kit (QIAGEN, Hilden, Germany) assay as summarized in Table 2. Of the 96 genes analysed (represented by the heat map in Figure 3), a total of 31 genes were downregulated while 9 genes were upregulated in the MAP group with CXCL8 (fold change = -45.26) and CD14 (FC = -21.58) being the most downregulated compared to the healthy control samples. The chemokine receptor CCR6 was also downregulated in the MAP group (FC = -21.05). In the MSAP patients, 68 genes were upregulated and 4 were downregulated. The downregulated genes included CCL5 (FC = -3.76) and the upregulated genes included FOXP3 (FC = 137.02) and APCS (FC = 262.91) being the most downregulated and overexpressed, respectively. Importantly, moderately severe patients had the highest number of upregulated genes, specifically those involved in inflammation such as IL4 (FC = 108.64), IL5 (FC = 192.59), IL23A (FC = 18.07), GATA-3 (FC = 11.58), and CRP (FC = 177.42), as shown in the heat map in Figure 3. A total of 34 genes were upregulated in the SAP patients while 25 were downregulated. CCR8 (FC = 1172.45) and CD8A (FC = -74.26) were the top upregulated and downregulated genes, respectively in the SAP group. Notably, CCR8 increased steadily with disease severity producing the highest fold change across all groups. Other genes that increased with severity were GAPDH, NOD1, TRL 1 and TICAM 1, TBX21, and CASP1, which are all genes closely associated with CCR8 (Figure 3).
| Gene symbol | MAP (n = 7) | MSAP (n = 4) | SAP (n = 2) |
| CCL5 | -2.97 | -3.76 | -15.22 |
| CCR8 | 1.33 | 38.28 | 1172.45 |
| IL10 | -1.30 | 58.62 | -1.47 |
| FOXP3 | 3.90 | 137.02 | 96.27 |
| IL13 | -1.92 | 83.66 | 19.53 |
| IL17A | 1.72 | 116.93 | 2.56 |
| IL23A | -5.60 | 18.07 | 6.57 |
| IL4 | -1.13 | 108.64 | 36.83 |
| IL5 | 1.33 | 192.59 | 1.21 |
| NOD1 | -8.93 | -14.62 | 64.21 |
| MPO | 1.33 | 91.77 | 11.8 |
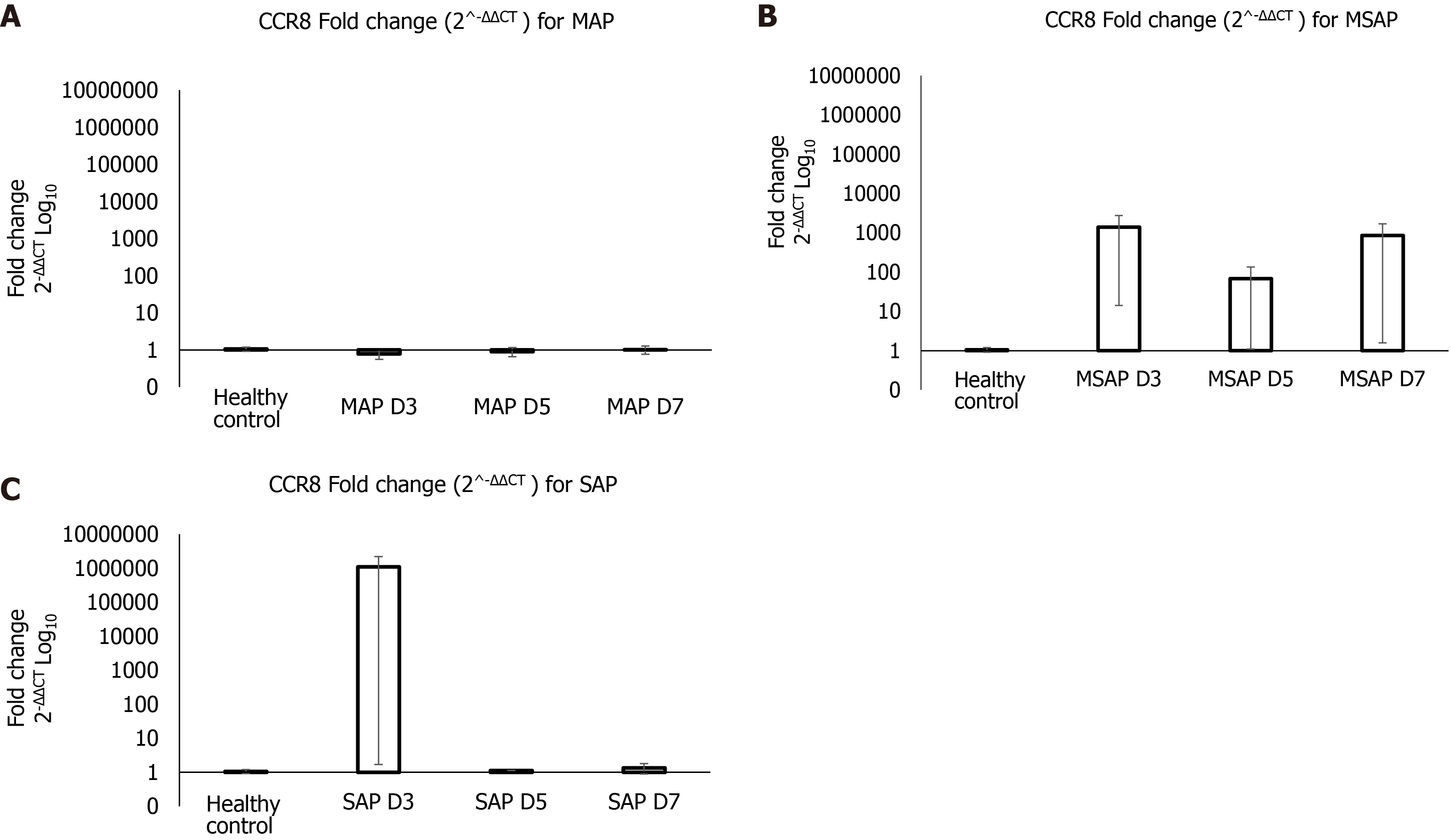
The real-time PCR verification findings were plotted as Log10 of fold change (2-ΔΔCT), shown in Figure 4. The results show that at Day 3 post epigastric pain the fold change of CCR8 for the MAP group compared with the healthy control group was almost 1 to 1 (Figure 4A). Whereas the MSAP is 1000 times more than the healthy control for the same day (Figure 4B). The SAP group was 10000000 times that of the healthy control at Day 3 (Figure 4C). This was due to an individual sample that can be considered as an outlier. This group had an FC of a 1090632 ± 1090631 (Figure 4C). On Day 5 and Day 7 the fold change dropped to almost 1:1 ratio with the healthy control in the MAP and SAP group. In the MSAP group, the FC on Day 5 was consistent with Day 3 Levels and dropped slightly to 800 ± 846 on Day 7 as observed in the comparisons of the FC of CCR8 within groups on different days (Table 3).
| Severity | Mean fold change (2^-ΔΔCT ) |
| MAP D3 | 0.8 ± 0.22 |
| MAP D5 | 0.9 ± 0.25 |
| MAP D7 | 1.0 ± 0.26 |
| MSAP D3 | 1386 ± 1372 |
| MSAP D5 | 68.0 ± 67 |
| MSAP D7 | 848 ± 846 |
| SAP D3 | 1090632 ± 1090631 |
| SAP D5 | 1.1, NA |
| SAP D7 | 1.3 ± 0.46 |
In one of the sampled patients from the MSAP group, NK cell frequencies of CD3-CD16+CD56- doubled from 12% to 27%, and those from the CD3-CD16+CD56– subsets increased in percentage from 19.8% to 49.6% from Day 3 to Day 5. NK cell subsets, which were CD57+ increased by over 30% for MSAP patients from Day 3 to Day 5 (Supplementary Figure 2).
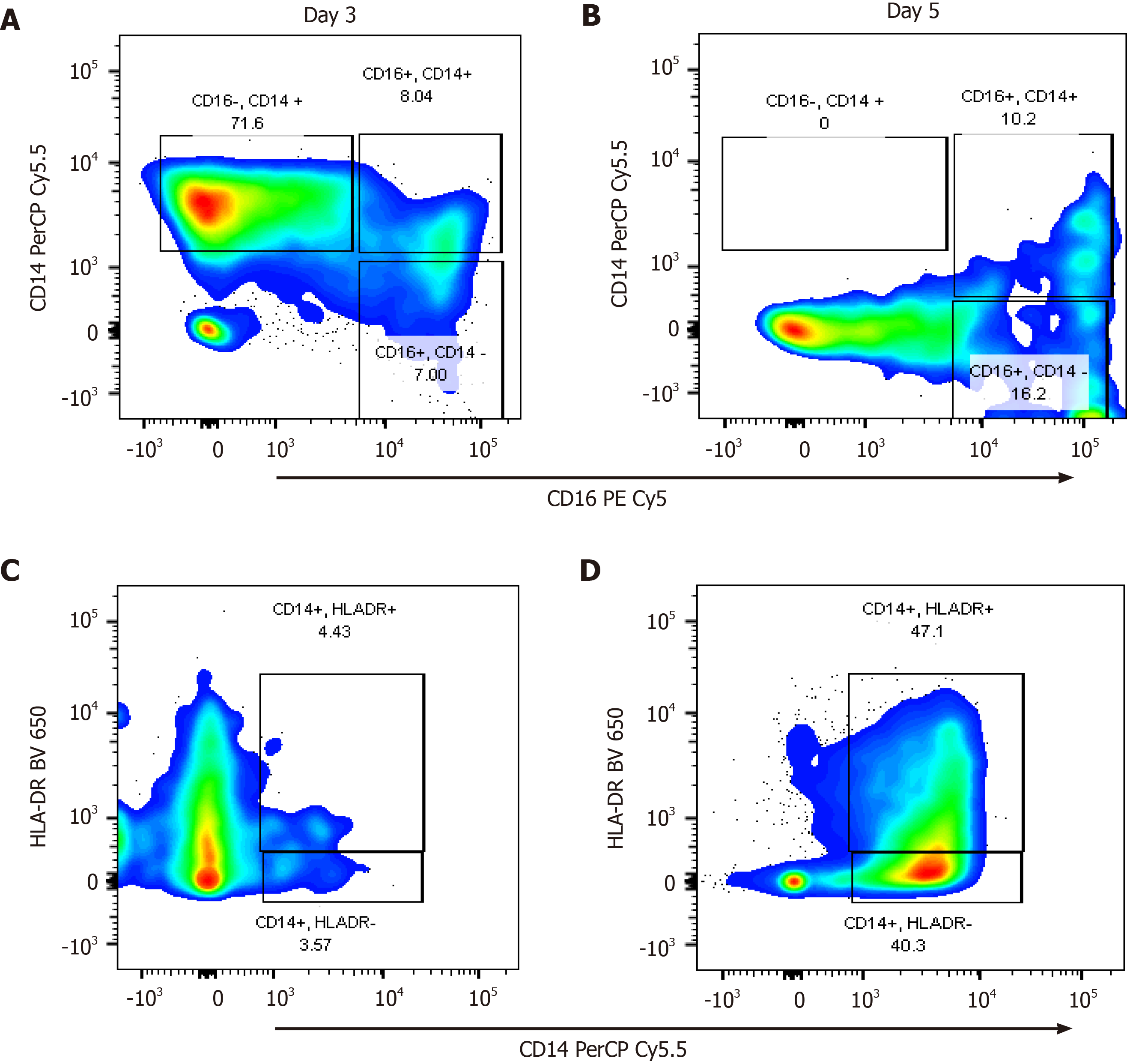
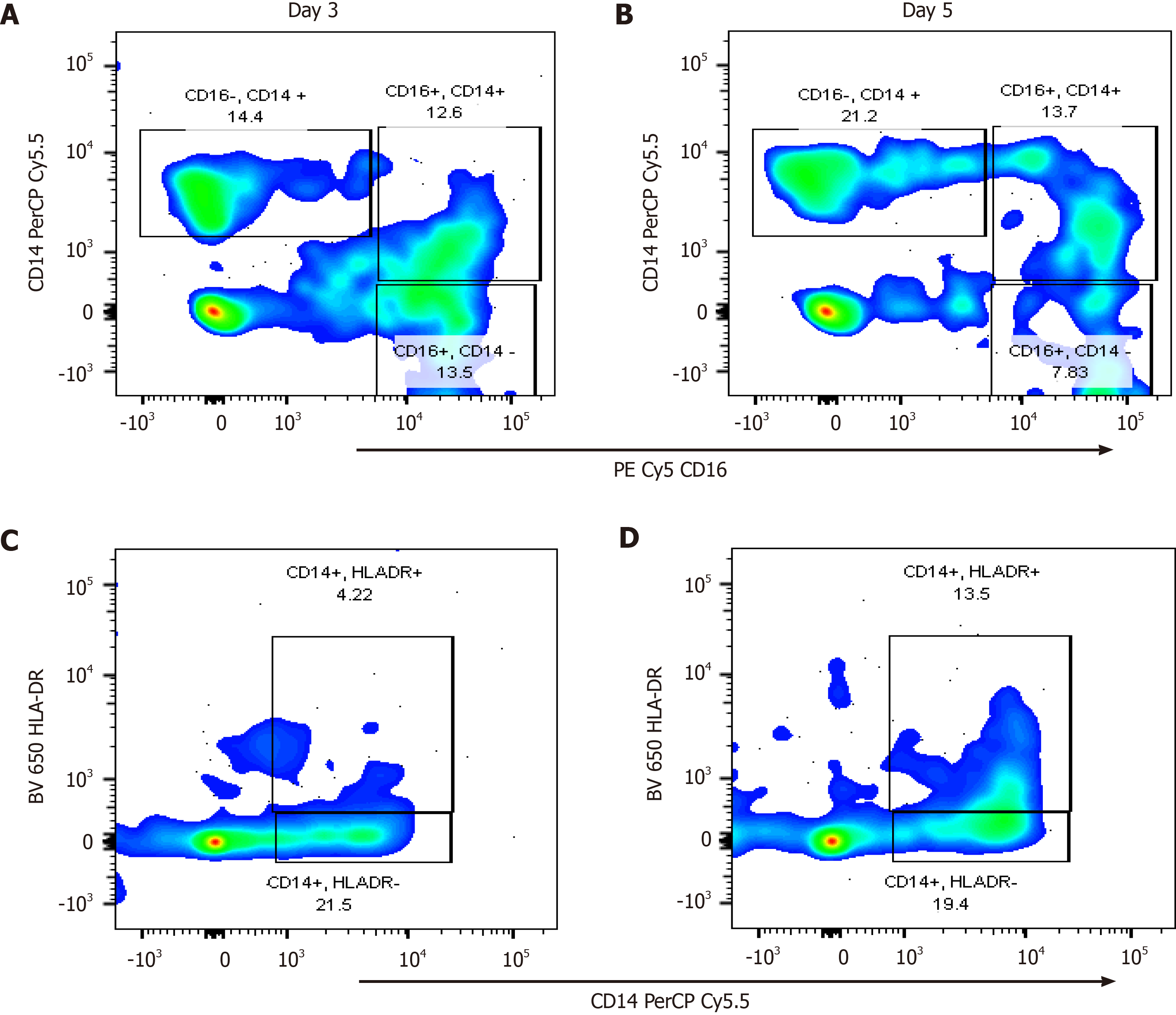
In the immunophenotyping analysis by flow cytometry, 7 patients were recruited, 4 MAP, 2 MSAP, and 1 SAP as shown in Figure 1. Cells known to express CCR8, namely lymphocytes (including NK cells belonging to ILC1) and monocytes were assessed as part of a multicolour panel using flow cytometry[39]. Classical monocyte subpopulations (CD14+CD16-) were higher in more severe patients with the MSAP patient having as much as 71.6% of the parent population on day 3, dropping to undetectable levels on day 5 (Figure 5A and B). In the SAP patients, the classical monocyte population consistently increased by more than 7% from Day 3 to 5 (Figure 6A and B). In the MSAP patient, the percentage of HLA-DR+ monocyte increased by 43% from Day 3 to Day 5 (Figure 5C and D). Whereas the percentage of HLA-DR+ monocytes increased from 4.2% on Day 3 to 13.5% on Day 5 in the SAP patient (Figure 6C and D).
In this preliminary study, the demographics of 29 AP patients, the role of CCR8, IL-6, and the frequency of cells expressing these biomolecules were explored. Patient demographics were as expected with older patients falling into more severe groups[1,40]. The study demonstrated that the increase in IL-6 Levels maintained an upward trend in the SAP group up to Day 7, compared to the healthy control group, the MAP, and MSAP group (Figure 2). The consistency in the concentration of IL-6 protein levels in the SAP group in the peripheral blood is likely as a result of the observed activated monocytes and hence CCR8 expression on these cells. CRP is a well-defined severity marker in acute pancreatitis and is initiated by elevation of IL-6[14,38]. Elevated levels of CRP, an acute phase reactant, in the pooled sample of the MSAP and SAP group (Table 2, Supplementary Table 3) may be due to increased monocyte cell populations[37]. Although, IL-6 was not shown as a useful independent marker to distinguish different risk categories of AP in this study, cells producing IL-6 such as monocytes (Figure 5A and B, Figure 6A and B) and NK cells (Supplementary Figure 2), which are part of group 1 ILCs, increased in frequency at Day 3 and 5 in the MSAP and SAP group[24,39]. A possibility exists in exploring the potential prognostic value of a lymphocyte to monocyte ratio based on the resulting difference in frequency in MAP compared to MSAP and SAP.
Our findings further show the presence of HLA-DR dim to negative monocyte subsets in an SAP patient suggesting downregulation (Figure 6C and D). This supports findings from a study that found that the presence of monocytes that do not express HLA-DR correlates with organ dysfunction in AP[37]. An important observation was that in the MSAP patient at Day 3 (Figure 5C), HLA-DR was downregulated but upregulated by Day 5 (Figure 5D) showing resolve in organ failure, supportive of the MSAP classification[1]. The presence of immunosuppressive NK cell subsets, which are CD57+ (Supplementary Figure 2) may also play an important role in this[41]. CD3-CD16-CD57+ cell subsets have a protective function in autoimmune disease[41]. This further supports the hypothesis of a possible linkage between monocyte and lymphocyte frequencies to severity based on the observed decrease of classical monocytes from Day 3 to 5 in the MSAP patient who experienced transient organ failure. These preliminary results may indicate possible links between monocytes and NK cells in the stratification of the MSAP group of patients.
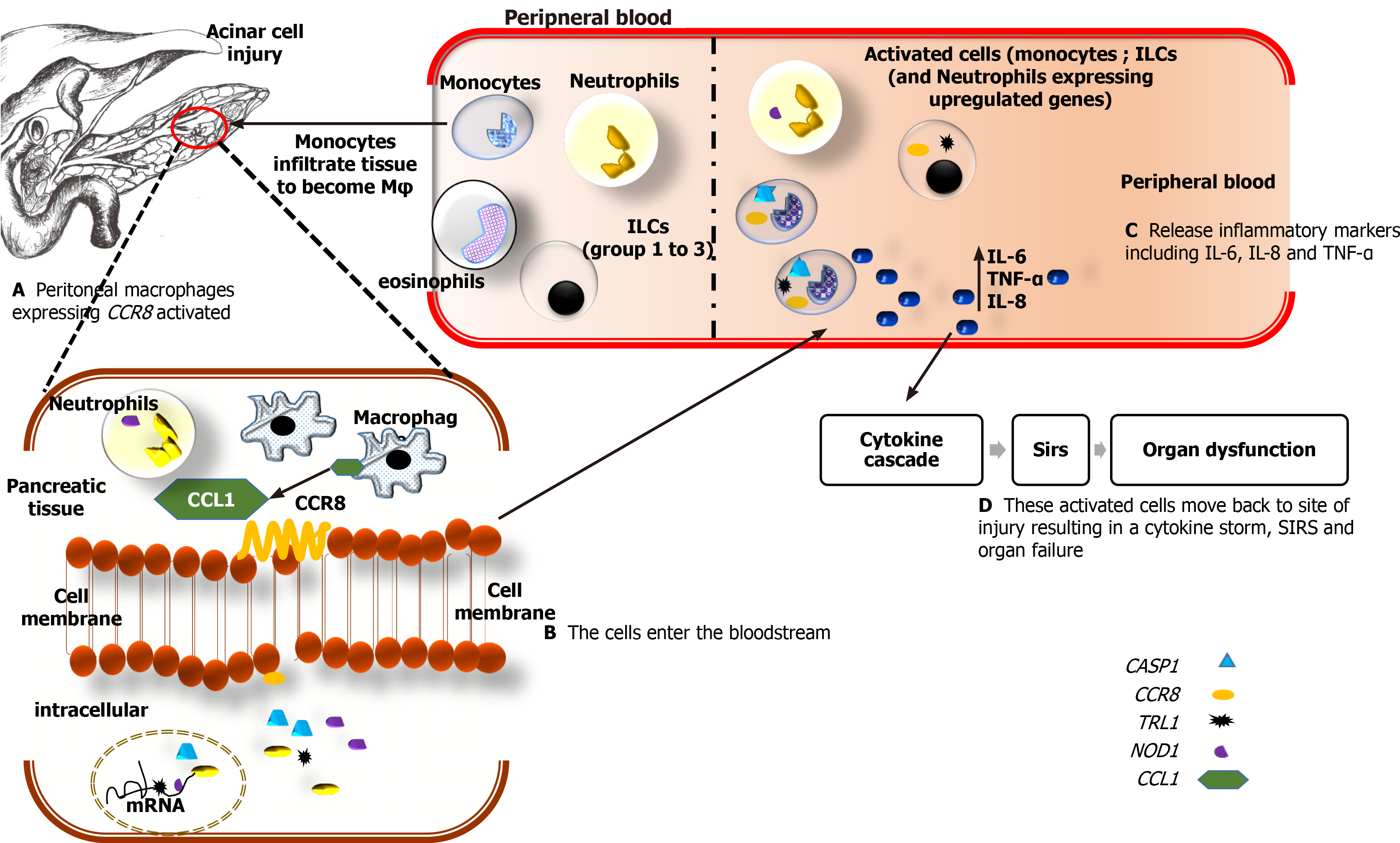
This study investigated expression patterns of several inflammatory and immune response-related molecules at the early stages of AP. We further describe a hypothetical model, which is deduced from this preliminary study and literature (Figure 7).
CCR8, a chemokine receptor, is known to be highly expressed on monocytes and cells of Th2 lineage including innate lymphoid cells group 2 (ILC2) and ILC3 cells[26,42]. Cells of the ILC1 population that are CD56- are found abundantly in peripheral blood in the disease state[17,18]. These cells are known to suppress autoimmune diseases[41]. This may explain the reason why organ failure is resolved in the MSAP patient compared to the NK cell-deficient SAP patient[1,36]. Acinar cell injury and elevation of trypsin in pancreatic tissue are followed by excessive recruitment of monocytes, neutrophils, and ILCs, to the local site of injury[42-44]. These ILCs include NK cells (ILC1), ILC2, and ILC3 cells. Once the pancreatic tissue is damaged due to AP, monocytes, and macrophages are responsible for the maintenance of inflammation[37,45]. Thus, the upregulation of CCR8 observed in this study may be due to increased levels of activated monocytes in peripheral blood. The main agonist of CCR8 is its own ligand CCL1[46]. CCL1 in the peripheral blood is highly expressed on classical, non-classical, and intermediate monocytes[39]. In other autoimmune diseases such as cancers of the renal system, CCR8 positive cells, namely monocytes and granulocytes were the most abundant in the bloodstream and contributed to prolonged inflammation within patients[47]. CCR8 is also expressed on peritoneal macrophages in tissue and lymphocytes of Th2 Lineage[27]. Oshio et al[27] demonstrated that NF-кB is suppressed in CCR8 deficient mice and that macrophage chemotaxis in the peritoneal cavity, which includes the pancreas, is CCL1 dependent. This provides a possible link between monocyte expression and upregulation of CCR8 in more severe patients observed in this study.
CCR8 gene was concomitantly upregulated with TLR1, NOD1, CASP1, and GAPDH (Table 2, Supplementary Table 3). These genes are all expressed on activated monocytes[39] and were observed in the analysis of the pooled samples (Table 2 and Supplementary Figure 3). Studies looking at inflammation in pancreatic injury have shown that continued release of proinflammatory cytokines by macrophages, increased number of neutrophils, and excess levels of nitric oxide impaired tissue regeneration and contributed to organ tissue damage[48]. This suggests that the observed increase of CCR8 levels in MSAP patients, and to an extent the SAP patients, could be associated with macrophages and monocytes levels. The 1090631 fold upregulation of CCR8 in the SAP group was due to one sample and was observed in the results of the pooled sample in the RT2 profiler analysis (Figure 4C). This means that the CCR8 expression levels in the SAP group may not necessarily be representative due to the limited number in this group suggesting the need for further research.
Several genes associated with Th2 Lymphoid cells were upregulated in the MSAP group. The upregulation of the transcription factor, GATA-3, and the IL4, IL5, and IL13 genes in Supplementary Table 3 may indicate a stronger type-2 response in MSAP patients compared to the SAP group, which is a result of excessive recruitment of macrophages and monocytes in pancreatic tissues and the bloodstream respectively[22,49]. On the other hand, upregulation of proinflammatory genes such as IL6, CRP, and FOXP3 (Supplementary Table 3) associated with CCR8/CCL1 in the MSAP group may be attributed to ILC3 and or Th17 cells. Overexpression of FOXP3 via the STAT3 pathway was directly proportional to the observed increase in fold changes for IL-17A and IL 23A genes[19]. This is expected since STAT3 is responsible for differentiation in Th17 Lineage and has been implicated in autoimmune diseases[46]. Therefore, it is likely that an elevation of these Th17 cytokines may be due to the ILC3 group[22].
This preliminary study has its limitations. Like many clinical studies, obtaining the ideal sample size, which is adequate (not too small or too big) for the interpretation of the results is important in how the results are extrapolated. Here, we sampled 40 patients overall with MAP, MSAP, and SAP at three different time points (D3, 5, and 7) and tested samples from 29 as shown in Figure 1. Due to this being a time study, we noted a trend where patients dropped out after consenting or were too weak or too sick to participate, especially from the SAP group. Presentation at the hospital was also usually delayed and this could be attributed to the socio-economic state of the patients who tend to delay seeking treatment. To circumvent this challenge, where applicable (especially for the SAP group), the results presented here have been discussed with inferences to supporting literature and further work with expanded numbers is planned.
Possible concerns about treatment affecting the expression of CCR8 and IL-6 are valid. However, the general treatment guideline for AP in the hospital unit is based on supportive care where all patients are treated according to the same protocol, none of which can influence immune responses. In mild AP, only analgesia and fluids are prescribed and nutrition is maintained with a combination of enteral and/or parenteral feeding. In the Moderate and severe group, organ support is implemented depending on the organ dysfunction. Antibiotics nor steroids are used routinely in the first phase of the inflammatory response in any of the patients and as such, we do not think that the treatment will influence the expression of IL-6 or CCR8 up to and including day 7.
This study proposes possible linkages between the upregulation of CCR8 and IL6 elevation with AP disease severity. Simultaneously, monocytes, ILCs, and Th2 Lymphocyte frequencies, found to differentiate MAP, may differentiate MSAP and SAP groups. These findings may be beneficial as prognostic parameters in early AP stratification. Despite the limitation in sample sizes, these preliminary findings are supported by the literature. The data indicate that CCR8, IL-6 Levels, and associated immune molecules and cell types may be promising parameters to improve or complement existing ones for patient risk stratification in AP. The data further contributes to the scarce literature in AP from an African setting.
Chemokine receptor 8 (CCR8) is a chemokine receptor that is highly expressed on monocytes and cells of T helper type-2 Lineage including innate lymphoid cells group 2 and 3 (ILC2 and 3). Upregulation in more severe cases of acute pancreatitis (AP) may be linked to elevated levels of interleukin (IL)-6 and upregulation of CCR8.
There is currently no known treatment for AP and no clear early immune markers to effectively distinguish between moderately severe AP and severe AP. The complex underlying pathophysiology further complicates this, necessitating studies to better understand the ensuing immune responses for improved stratification.
To identify the role of the CCR8, expressed by Th2 Lymphocytes and peritoneal macrophages, and its possible association to IL-6 as early markers to assist with AP stratification.
A total of 40 patients were recruited from the Chris Hani Baragwanath Hospital and the Charlotte Maxeke Johannesburg Academic Hospital in Johannesburg, South Africa. Bioassays were performed on 29 patients consisting of 14 mild AP (MAP), 11 moderately severe AP (MSAP), and 4 severe AP (SAP) and 6 healthy controls as part of a preliminary study. A total of 12 mL of blood samples were collected at Day (D) 1, 3, 5, and 7 post epigastric pain. Using multiplex immunoassay panels, real-time polymerase chain reaction (RT-PCR) arrays, and multicolour flow cytometry analysis, immune response-related proteins, genes, and cells were profiled respectively. The fold change (FC) analysis was used to determine differences between the groups.
This study shows possible linkages between increasing CCR8 expression and severity in mainly MSAP patients when compared to MAP. The concentration of IL-6 was significantly different at D3 post epigastric pain in both MAP group and MSAP group with P = 0.001 and P = 0.013 respectively, in a multiplex assay. CCR8 was shown to increase with severity with the following FC for MAP (1.33), MSAP (38.28) to SAP (1172.45). Further verification studies using RT-PCR showed fold change increases of CCR8 in MSAP and SAP ranging from 1000 to 1000000 times when represented as Log10, compared to healthy controls respectively at Day 3 post epigastric pain.
Notable increases in CCR8 and IL-6 in severe patients were observed. Lymphocyte and monocyte cell frequencies suggest that in MAP, IL-6 was highly expressed in lymphocytes, and the severe patients (MSAP and SAP) were highly expressed by monocytes. This provides an avenue for exploring AP stratification to improve management.
There is an opportunity to further investigate IL-6 producing cells such as T helper 2 lymphocytes, monocytes, and innate lymphoid cells group 2 and associated CCR8 increases, to determine cell-associated cytokine as a novel approach for AP risk stratification.
The support of the medical staff of Chris Hani Baragwanath Academic Hospital and Charlotte Maxeke Academic Johannesburg Hospital and the Department of Surgery of the University of the Witwatersrand are acknowledged. The Laboratory of Dr. Tanya Augustine at the School of Anatomical Sciences, for the use of the Quant Studio™ 1 Real-Time System equipment and the laboratory of Professor Raquel Duarte in the Internal Medicine Department for the use of their BioPlex® multiplex platform as well Ms. Therese Dix-Peek for the technical assistance.
Provenance and peer review: Invited article; Externally peer reviewed
Specialty type: Medicine, Research and Experimental
Country/Territory of origin: South Africa
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Zhong HJ S-Editor: Wang LL L-Editor: A P-Editor: Li JH
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4328] [Article Influence: 360.7] [Reference Citation Analysis (45)] |
| 2. | Leung PS, Ip SP. Pancreatic acinar cell: its role in acute pancreatitis. Int J Biochem Cell Biol. 2006;38:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 4. | Kylänpää L, Rakonczay Z Jr, O'Reilly DA. The clinical course of acute pancreatitis and the inflammatory mediators that drive it. Int J Inflam. 2012;2012:360685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Nesvaderani M, Eslick GD, Vagg D, Faraj S, Cox MR. Epidemiology, aetiology and outcomes of acute pancreatitis: A retrospective cohort study. Int J Surg. 2015;23:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Anderson F, Thomson SR. Pancreatitis in a high HIV prevalence environment. S Afr Med J. 2017;107:706-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Derrick D, Frandy F, Wirawan AD. Acute Pancreatitis-Etiology, Pathogenesis, Pathophysiology and the current trend in its management and prevention. Indones J Gastroenterol Hepatol Dig Endosc (Online). 2019;20:27-38. [DOI] [Full Text] |
| 8. | De Campos T, Parreira JG, Assef JC, Rizoli S, Nascimento B, Fraga GP. Classification of acute pancreatitis. Rev Col Bras Cir. 2013;40:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Dellinger EP, Forsmark CE, Layer P, Lévy P, Maraví-Poma E, Petrov MS, Shimosegawa T, Siriwardena AK, Uomo G, Whitcomb DC, Windsor JA; Pancreatitis Across Nations Clinical Research and Education Alliance (PANCREA). Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. 2012;256:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 342] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 10. | Singh P, Garg PK. Pathophysiological mechanisms in acute pancreatitis: Current understanding. Indian J Gastroenterol. 2016;35:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Leal C, Almeida N. Predicting Severity in Acute Pancreatitis: A Never-Ending Quest. GE Port J Gastroenterol. 2019;26:232-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Do JH. Mechanism of Severe Acute Pancreatitis: Focusing on Development and Progression. Korean J Pancreas Biliary Tract. 2015;20:115-123. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 14. | Stirling AD, Moran NR, Kelly ME, Ridgway PF, Conlon KC. The predictive value of C-reactive protein (CRP) in acute pancreatitis - is interval change in CRP an additional indicator of severity? HPB (Oxford). 2017;19:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Meher S, Mishra TS, Sasmal PK, Rath S, Sharma R, Rout B, Sahu MK. Role of Biomarkers in Diagnosis and Prognostic Evaluation of Acute Pancreatitis. J Biomark. 2015;2015:519534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 16. | Jakkampudi A, Jangala R, Reddy BR, Mitnala S, Nageshwar Reddy D, Talukdar R. NF-κB in acute pancreatitis: Mechanisms and therapeutic potential. Pancreatology. 2016;16:477-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 17. | Sharma D, Jakkampudi A, Reddy R, Reddy PB, Patil A, Murthy HVV, Rao GV, Reddy DN, Talukdar R. Association of Systemic Inflammatory and Anti-inflammatory Responses with Adverse Outcomes in Acute Pancreatitis: Preliminary Results of an Ongoing Study. Dig Dis Sci. 2017;62:3468-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Peng C, Li Z, Yu X. The Role of Pancreatic Infiltrating Innate Immune Cells in Acute Pancreatitis. Int J Med Sci. 2021;18:534-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Zambrano-Zaragoza JF, Romo-Martínez EJ, Durán-Avelar Mde J, García-Magallanes N, Vibanco-Pérez N. Th17 cells in autoimmune and infectious diseases. Int J Inflam. 2014;2014:651503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Fonteh P, Smith M, Brand M. Adaptive Immune Cell Dysregulation and Role in Acute Pancreatitis Disease Progression and Treatment. Arch Immunol Ther Exp (Warsz). 2018;66:199-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Marshall JS, Warrington R, Watson W, Kim HL. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 2018;14:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 531] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 22. | Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, Spits H. Innate Lymphoid Cells: 10 Years On. Cell. 2018;174:1054-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 1535] [Article Influence: 255.8] [Reference Citation Analysis (0)] |
| 23. | Panda SK, Colonna M. Innate Lymphoid Cells in Mucosal Immunity. Front Immunol. 2019;10:861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 24. | Willinger T. Metabolic Control of Innate Lymphoid Cell Migration. Front Immunol. 2019;10:2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | Crome SQ, Ohashi PS. Immunoregulatory functions of innate lymphoid cells. J Immunother Cancer. 2018;6:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Bielecki B, Mazurek A, Wolinski P, Glabinski A. Expression of chemokine receptors CCR7 and CCR8 in the CNS during ChREAE. Scand J Immunol. 2007;66:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Oshio T, Kawashima R, Kawamura YI, Hagiwara T, Mizutani N, Okada T, Otsubo T, Inagaki-Ohara K, Matsukawa A, Haga T, Kakuta S, Iwakura Y, Hosokawa S, Dohi T. Chemokine receptor CCR8 is required for lipopolysaccharide-triggered cytokine production in mouse peritoneal macrophages. PLoS One. 2014;9:e94445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Cantor J, Haskins K. Recruitment and activation of macrophages by pathogenic CD4 T cells in type 1 diabetes: evidence for involvement of CCR8 and CCL1. J Immunol. 2007;179:5760-5767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Zychowska M, Rojewska E, Piotrowska A, Kreiner G, Nalepa I, Mika J. Spinal CCL1/CCR8 signaling interplay as a potential therapeutic target - Evidence from a mouse diabetic neuropathy model. Int Immunopharmacol. 2017;52:261-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Thomson JE, Brand M, Fonteh P. The immune imbalance in the second hit of pancreatitis is independent of IL-17A. Pancreatology. 2018;18:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Kay PS, Smith M, Brand M. The Initiating Immune Response of Acute Pancreatitis May be Mediated by the T-Helper 17 Pathway. Pancreas. 2017;5. |
| 32. | Thomson JE, Nweke EE, Brand M, Nel M, Candy GP, Fonteh PN. Transient Expression of Interleukin-21 in the Second Hit of Acute Pancreatitis May Potentiate Immune Paresis in Severe Acute Pancreatitis. Pancreas. 2019;48:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Huggett JF, Foy CA, Benes V, Emslie K, Garson JA, Haynes R, Hellemans J, Kubista M, Mueller RD, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT, Bustin SA. The digital MIQE guidelines: Minimum Information for Publication of Quantitative Digital PCR Experiments. Clin Chem. 2013;59:892-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 625] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 34. | Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9915] [Cited by in RCA: 11140] [Article Influence: 696.3] [Reference Citation Analysis (0)] |
| 35. | Yan Z, Gao J, Lv X, Yang W, Wen S, Tong H, Tang C. Quantitative Evaluation and Selection of Reference Genes for Quantitative RT-PCR in Mouse Acute Pancreatitis. Biomed Res Int. 2016;2016:8367063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133837] [Article Influence: 5576.5] [Reference Citation Analysis (1)] |
| 37. | Cesar Machado MC, Mendonca Coelho AM. Role of Peritoneal Macrophages on Local and Systemic Inflammatory Response in Acute Pancreatitis. IntechOpen. 2012;. |
| 38. | Mayerle J, Dummer A, Sendler M, Malla SR, van den Brandt C, Teller S, Aghdassi A, Nitsche C, Lerch MM. Differential roles of inflammatory cells in pancreatitis. J Gastroenterol Hepatol. 2012;27 Suppl 2:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Thul PJ, Lindskog C. The human protein atlas: A spatial map of the human proteome. Protein Sci. 2018;27:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 788] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 40. | Wu BU, Banks PA. Clinical management of patients with acute pancreatitis. Gastroenterology. 2013;144:1272-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 41. | Nielsen CM, White MJ, Goodier MR, Riley EM. Functional Significance of CD57 Expression on Human NK Cells and Relevance to Disease. Front Immunol. 2013;4:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 42. | Shrivastava P, Bhatia M. Essential role of monocytes and macrophages in the progression of acute pancreatitis. World J Gastroenterol. 2010;16:3995-4002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (2)] |
| 43. | Gukovsky I, Li N, Todoric J, Gukovskaya A, Karin M. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1199-209.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 44. | Endo Y, Hirahara K, Yagi R, Tumes DJ, Nakayama T. Pathogenic memory type Th2 cells in allergic inflammation. Trends Immunol. 2014;35:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 45. | Barsheshet Y, Wildbaum G, Levy E, Vitenshtein A, Akinseye C, Griggs J, Lira SA, Karin N. CCR8+FOXp3+ Treg cells as master drivers of immune regulation. Proc Natl Acad Sci U S A. 2017;114:6086-6091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 46. | Eruslanov E, Stoffs T, Kim WJ, Daurkin I, Gilbert SM, Su LM, Vieweg J, Daaka Y, Kusmartsev S. Expansion of CCR8(+) inflammatory myeloid cells in cancer patients with urothelial and renal carcinomas. Clin Cancer Res. 2013;19:1670-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Folias AE, Penaranda C, Su AL, Bluestone JA, Hebrok M. Aberrant innate immune activation following tissue injury impairs pancreatic regeneration. PLoS One. 2014;9:e102125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Davis BP, Rothenberg ME. Inflammatory and Effector Cells/Cell Migration. Third Edition. Pediatric Allergy: Principles and Practice: Third Edition. Elsevier Inc, 2016: 41-53.e4. [DOI] [Full Text] |
| 49. | Dabrowski A, Osada J, Dabrowska MI, Wereszczynska-Siemiatkowska U. Monocyte subsets and natural killer cells in acute pancreatitis. Pancreatology. 2008;8:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |