Published online Dec 31, 2019. doi: 10.4291/wjgp.v10.i5.54
Peer-review started: October 29, 2019
First decision: November 22, 2019
Revised: December 12, 2019
Accepted: December 23, 2019
Article in press: December 23, 2019
Published online: December 31, 2019
Processing time: 61 Days and 5.3 Hours
Studies have demonstrated a potential role for fecal biomarkers such as fecal calprotectin (FC) and fecal lactoferrin (FL) in monitoring inflammatory bowel diseases (IBD) - Crohn’s disease (CD) and ulcerative colitis (UC). However, their correlation to endoscopic scores, disease severity and affected intestinal surface has not been extensively investigated.
To correlate FL, and for comparison white blood cell (WBC) and C-reactive protein (CRP), with endoscopic scores, disease extent and location in CD and UC.
Retrospective analysis in 188 patients who had FL, CRP and WBC determined within 30 d of endoscopy. Disease location, disease extent (number of intestinal segments involved), disease severity (determined by endoscopic scores), timing of FL testing in relation to colonoscopy, as well as the use of effective fast acting medications (steroids and biologics) between colonoscopy and FL measurement, were recorded.
In 131 CD and 57 UC patients, both CRP and FL - but not WBC - distinguished disease severity (inactive, mild, moderate, severe). In patients receiving fast-acting (steroids or biologics) treatment in between FL and colonoscopy, FL showed a higher correlation to endoscopic scores when tested before vs after the procedure (r = 0.596, P < 0.001, vs r = 0.285, P = 0.15 for the Simple Endoscopic Score for CD; and r = 0.402, P = 0.01 vs r = 0.054 P = 0.84 for Disease Activity Index). Finally, FL was significantly correlated with the diseased mucosal surface (colon-ileocolon > small bowel) and the number of inflamed colon segments.
FL and CRP separated disease severity categories with FL showing lower discriminating P-values. FL showed a close correlation with the involved mucosal surface and with disease extent and was more closely correlated to endoscopy when determined before the procedure – this indicating that inflammatory activity changes associated with therapy might be rapidly reflected by FL levels. FL can accurately and timely characterize intestinal inflammation in IBD.
Core tip: Studies have demonstrated a potential role for fecal biomarkers such as fecal calprotectin and fecal lactoferrin (FL) in monitoring Crohn’s disease (CD) and ulcerative colitis (UC). However, their correlation with disease burden (endoscopic scores/disease activity and disease extent) has not been extensively investigated. In our study FL separated disease severity categories based on endoscopic scores in both UC and CD patients. FL showed a close correlation with the diseased mucosal surface and with disease extent and was more closely correlated to endoscopy when determined before endoscopy. FL can accurately and timely represent intestinal inflammation in inflammatory bowel diseases.
- Citation: Rubio MG, Amo-Mensah K, Gray JM, Nguyen VQ, Nakat S, Grider D, Love K, Boone JH, Sorrentino D. Fecal lactoferrin accurately reflects mucosal inflammation in inflammatory bowel disease. World J Gastrointest Pathophysiol 2019; 10(5): 54-63
- URL: https://www.wjgnet.com/2150-5330/full/v10/i5/54.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v10.i5.54
Inflammatory bowel disease (IBD), both Crohn’s disease (CD) and ulcerative colitis (UC), are on the rise worldwide, including Asia[1-3]. In the United States, 3.1 million people are reported to be affected[4]. The annual burden of IBD is extensive, with over 2.3 million physician visits, 180000 hospital admissions, and a cost of $6.3 billion in healthcare services[5-7]. One third of the annual cost of healthcare for IBD patients is classified as outpatient services, with the major components being endoscopy and pathology[7].
IBD activity has traditionally been monitored by the severity of clinical symptoms, using clinical scoring systems such as the Crohn’s Disease Activity Index (CDAI) for CD and the clinical component of the Mayo score for UC[8]. However, these measures are subjective and correlate poorly with objective findings[9].
Endoscopy is a more objective parameter of disease activity than clinical symptoms[10,11], but it is expensive, invasive, and often unwelcomed by patients. A number of studies have shown that fecal biomarkers, specifically fecal lactoferrin (FL) and fecal calprotectin (FC), are effective indicators of mucosal inflammation and injury[8,13-16]. Fecal biomarkers have been shown to be inexpensive, noninvasive, and reproducible, and they have a strong potential for use in monitoring IBD[12]. Both FC and FL have shown similar success clinically, and levels may rise significantly before clinical relapse and may predict subsequent IBD flares[17]. FL is an iron-binding glycoprotein expressed by active neutrophils-the primary component of the active inflammatory response[18,19]. FL is stable at room temperature for weeks, resistant to proteolysis, and resilient to multiple freeze-thaw cycles[20,21].
In a recent meta-analysis evaluating the diagnostic accuracy of FL in assessing IBD activity Dai and colleagues[22] found that in ten studies comprising 773 IBD patients the pooled sensitivity and specificity values for assessing UC activity were 0.81 [95% confidence interval (CI): 0.64-0.92] and 0.82 (95%CI: 0.61-0.93), respectively. The pooled sensitivity and specificity values for assessing CD activity were 0.82 (95%CI: 0.73-0.88) and 0.71 (95%CI: 0.63-0.78), respectively.
FL appears to be equally useful in CD and UC, when active or inactive disease is present[23]. The current knowledge gap lies in an incomplete understanding of correlation between FL levels and mucosal inflammation, disease location and extent.
In this retrospective study we investigated the correlation between these parameters and FL levels and compared them to C-reactive protein (CRP) and white blood cell count (WBC), two widely used indicators of inflammation.
This retrospective study enrolled patients seen in our Center from 2008 to 2018, diagnosed with UC or CD according to widely established criteria including histology[24] and were monitored as standard of care through the measurement of FL levels. To be enrolled in the study patients had to have a colonoscopy done within 30 d of the FL test.
All clinical and laboratory data were collected from the patients’ EMR (EPIC) and endoscopy data from images and reports stored in the Olympus Endoscopy Suite Program (Endoworks 7.4). There was no direct patient involvement. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by Carilion Clinic Ethical Committee. No patient consent was deemed necessary by the Ethical Committee.
FL stool test (normal range 0-7.24 μg/mL) was measured quantitatively by the LACTOFERRIN SCAN (TECHLAB, Blacksburg, VA, United States), an enzyme-linked immunosorbent assay (ELISA). Serum measurements of CRP and WBC used established clinical laboratory methods.
For each IBD patient in this study, we reviewed endoscopic pictures and procedure reports to determine the severity of inflammation, mucosal injury, and disease location (see “Endoscopic scoring and disease extent” below). In CD patients, disease location was also based on magnetic resonance imaging (MRI) or computed tomography (CT) scan image results. The imaging reviewer was blinded to these patients FL levels at the time of the evaluation.
The Simple Endoscopic Score for CD (SES-CD) (Supplementary Table 1) and the endoscopic component of the Mayo Clinical score [Disease Activity Index (DAI)] (Supplementary Table 2) for UC were utilized to measure endoscopic disease activity[9]. The SES-CD defines: Remission (score 0-2), mild inflammation (score 3-6), moderate inflammation (score 7-15), and severe inflammation (score ≥ 16). The endoscopic component of the DAI defines: Normal or inactive disease (0), mild disease (1), moderate disease (2), and severe disease (3). Since the DAI does not include a measure of disease extent, we estimated this parameter using a simple scoring system providing one point for each colonic segment (rectum, sigmoid, descending, transverse, ascending, cecum, ileo-cecal valve) demonstrating signs of disease. A score of 0 points indicates no disease and a score of 7 points indicates pan-colitis.
Descriptive statistics were used for patients’ characteristics. Analysis of variance (ANOVA) was used on natural log-transformed values of FL, CRP and WBC to determine whether median values significantly varied according to SES-CD and DAI scores. The values were natural log transformed for use in the analysis to address the non-normality of the distributions of the biomarkers, resulting in a comparison of median values on the original scale. Post-hoc pairwise comparisons using the method of least significant differences were used to determine which levels of SES-CD and DAI significantly differed in median biomarker values from other levels. Non-parametric Spearman correlations (due to non-normality of variables) were used to quantify and test the correlation of numeric SES-CD and DAI with FL levels, separately for patients whose FL levels were determined pre- and post-colonoscopy. To contrast FL levels with the number of colonic segments involved the Kruskal Wallis test followed by pairwise Mann-Whitney comparisons was used. The 0.05 level of significance was used for all statistical tests. The statistical review of the study was performed by a biostatistician (Love K).
Using the inclusion criteria outlined in methods and data search spanning from 2008 to 2018 we identified a total of 188 IBD patients followed at Carilion Clinic IBD Center. The colonoscopy procedures were performed for standard of care indications (surveillance, monitoring or disease staging). Patient characteristics and clinical data are shown in Table 1. Overall, 59% of patients were female and 70% had CD. DAI scoring in UC patients showed mild disease in 19%, moderate disease in 48% and severe disease in 33%. Of the CD patients scored by SES-CD using the endoscopy results 10% had inactive disease, 22% had mild disease, 28% had moderate disease and 40% had severe CD. A total of 63% of UC patients had disease extending proximal to the splenic flexure (with 2/3 of patients having pancolitis), 30% had disease distal to the splenic flexure and 7% had proctitis. Of the CD patients 34% had ileal disease, 22% had ileocolonic disease and 44% had colonic involvement. In the CD group, 18% of CD patients had stricturing disease with 73% bearing non-stricturing, non-penetrating disease.
| Characteristics | UC (n = 57) | CD (n = 131) | |
| Gender | Female | 27 (47) | 83 (63) |
| Male | 30 (53) | 48 (37) | |
| Disease duration (yr) | 0-5 | 37 (65) | 72 (55) |
| 6 to 10 | 9 (16) | 16 (12) | |
| > 10 | 11 (19) | 43 (33) | |
| Smoking | Current | 1 (2) | 32 (25) |
| Former | 29 (51) | 37 (28) | |
| Never | 27 (47) | 62 (47) | |
| Biomarkers | Lactoferrin μg/g | 448 (0; 8467) | 95 (0; 9351) |
| Median (min; max) | CRP mg/dL | 0.54 (0.4; 17.3) | 1.15 (0.4; 21.1) |
| WBC (K/μL) | 8.2 (4.0; 29.9) | 7.5 (3.6; 26.4) | |
| Montreal age (yr) | A1 < 16 | 0 (0) | 4 (3) |
| A2 17-40 | 29 (51) | 47 (36) | |
| A3 > 40 | 28 (49) | 80 (61) | |
| Montreal class UC | E1 proctitis | 4 (7) | |
| E2 distal | 17 (30) | ||
| E3 extensive | 36 (63) | ||
| Disease activity by DAI | Mild (1) | 11 (19) | |
| Moderate (2) | 27 (48) | ||
| Severe (3) | 19 (33) | ||
| Montreal class CD | L1 ileal | 45 (34) | |
| L2 colonic | 57 (44) | ||
| L3 ileocolonic | 29 (22) | ||
| L4 isolated upper | 0 (0) | ||
| B1 nonstricturing | 95 (73) | ||
| B2 stricturing | 24 (18) | ||
| B3 penetrating | 12 (9) | ||
| P perianal | 4 (3) | ||
| Disease activity by SES-CD | Inactive (0-2) | 9 (10) | |
| Mild (3-6) | 20 (22) | ||
| Moderate (7-15) | 26 (28) | ||
| Severe (≥ 16) | 37 (40) |
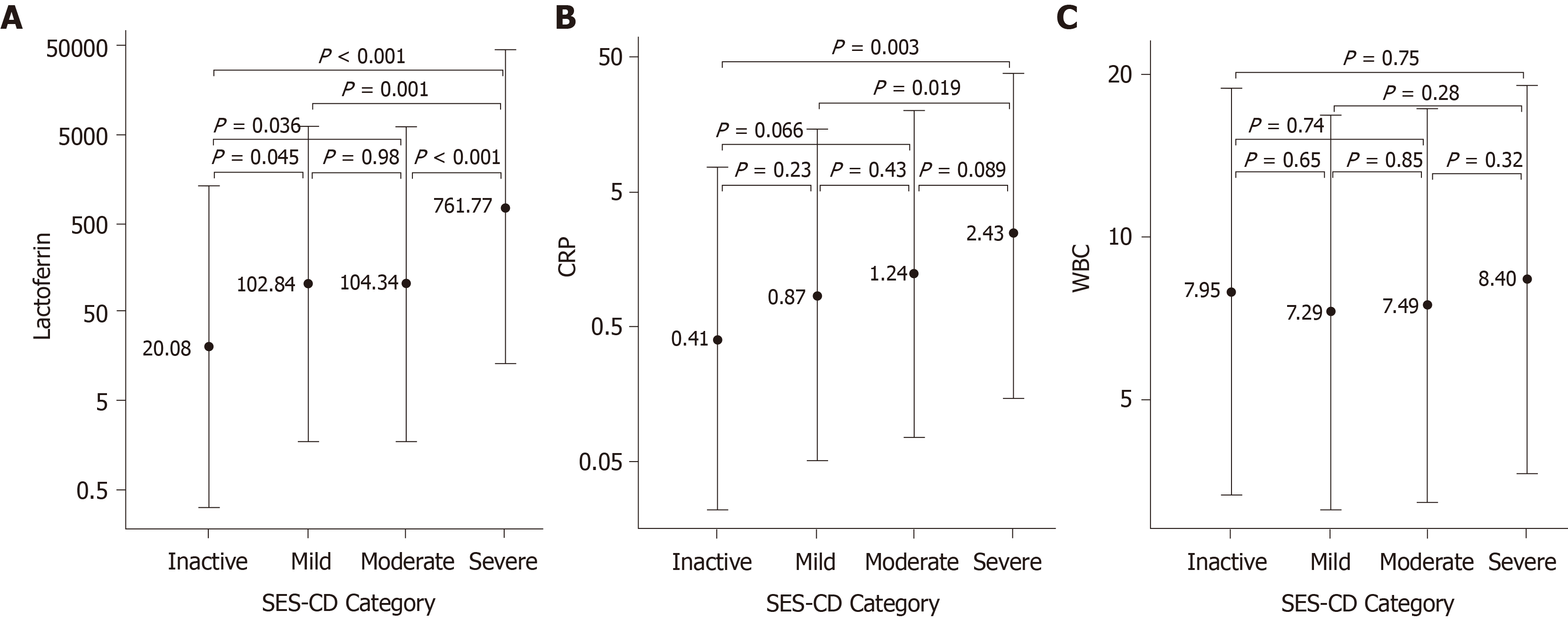
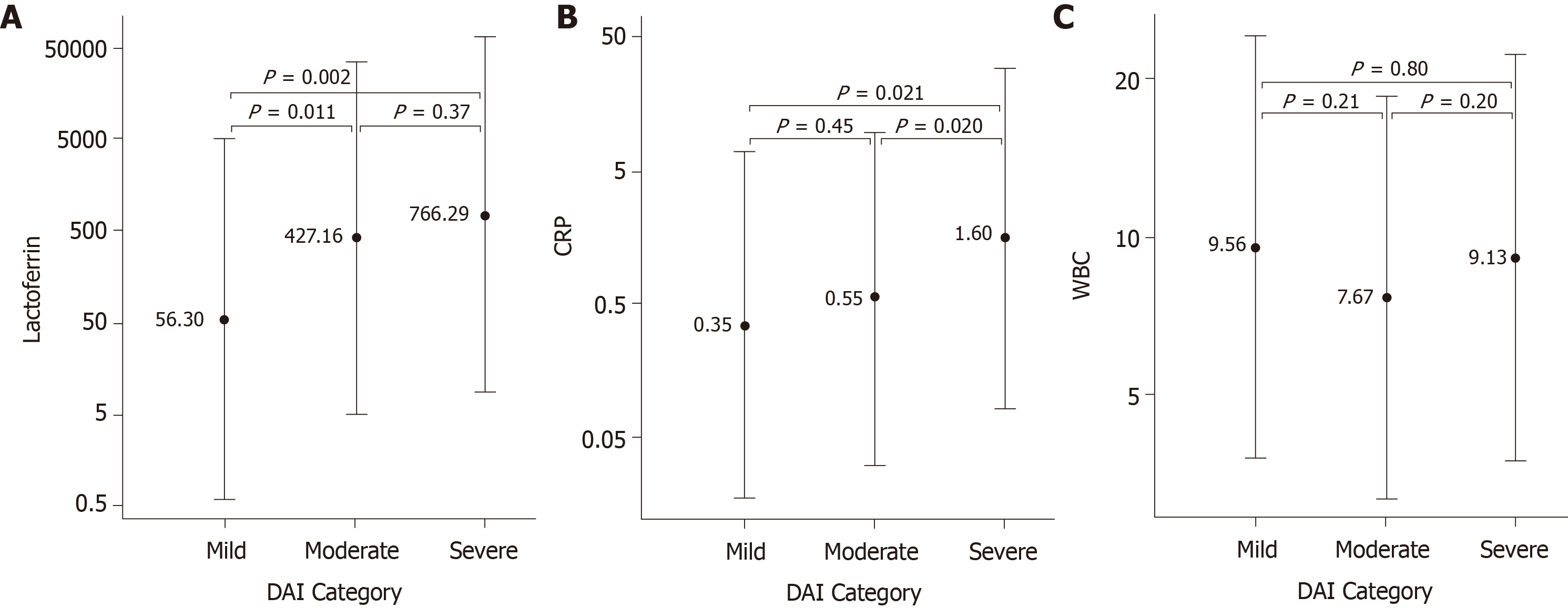
The biomarkers FL, CRP and WBC were evaluated as potential indicators of IBD disease severity as determined by endoscopic scores. In CD patients, FL median levels showed a significant difference for inactive (20 µg/g) vs mild (102 µg/g), inactive vs moderate (104 µg/g), mild vs severe (762 µg/g) and moderate vs severe activity. CRP median levels were significantly different for inactive (0.41 mg/dL) vs severe (2.43 mg/dL), and mild (0.87 mg/dL) vs severe activity (Figure 1). In UC patients, FL separated mild (56 µg/g) vs moderate (427 µg/g), and mild vs severe (766 µg/g) cases. CRP was significantly different between mild (0.35 mg/dL) vs severe (1.60 mg/dL), and moderate (0.55 mg/dL) vs severe cases (Figure 2). WBC median levels were similar across all categories for both CD and UC. Comparisons contrasting the accuracy in discriminating different disease activities showed less significant P values for CRP than for FL.
Biomarker concentrations were compared to individual endoscopic scores for CD and UC patients. Overall, both FL and CRP showed the highest Spearman correlations to SES-CD and DAI scores for all assessed patients (Table 2). WBC had a very weak correlation to both the SES-CD and DAI scores. Next, we selected patients (n = 139) in whom fast acting therapy (steroids and biologics) was successfully initiated in between FL and colonoscopy. When patients were stratified in two groups according to the timing of FL testing (Table 3) FL showed a higher correlation to SES-CD and DAI when it had been tested before the procedure compared to when it had been tested after the procedure: for SES-CD r = 0.596, P < 0.001, vs r = 0.285, P = 0.149 (n = 58 and 27, respectively); for DAI r = 0.402, P = 0.012, vs r = 0.054, P = 0.842 (n = 38 and 16, respectively).
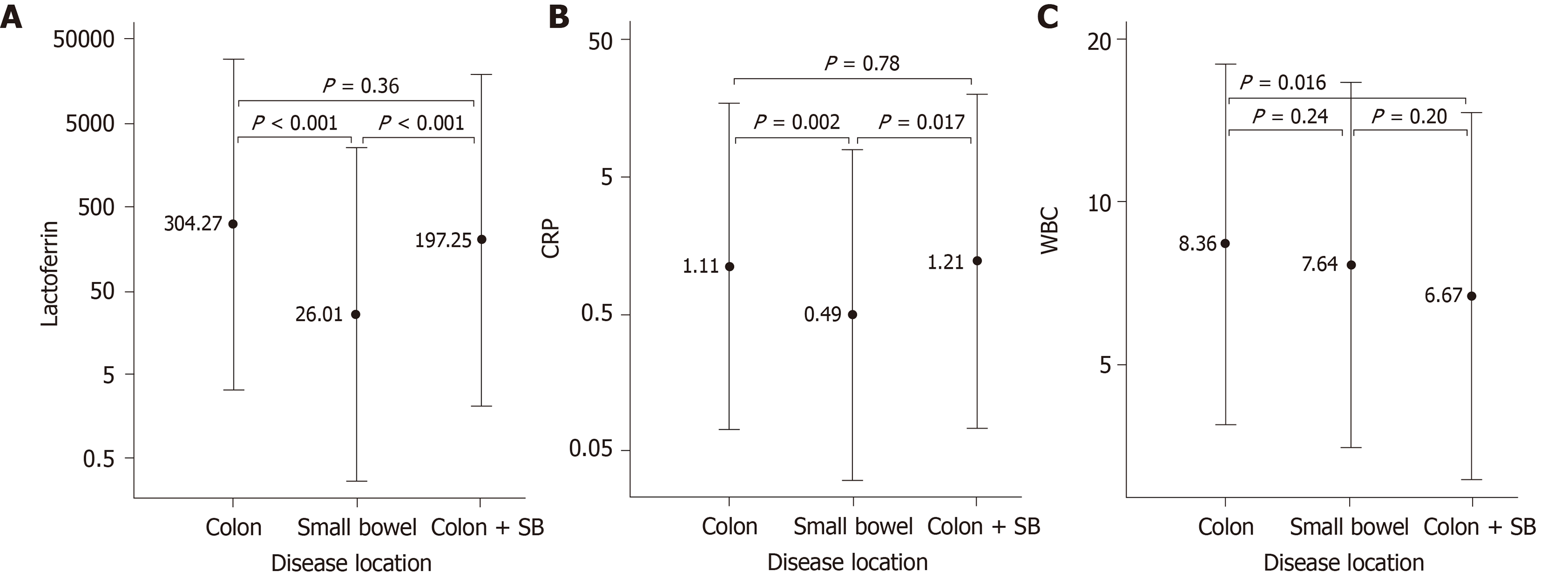
Of the 188 IBD patients included in the study, a total of 114 patients had colonic disease, 45 had isolated small bowel disease and 29 patients had both colonic and small bowel disease. FL, CRP and WBC levels relative to disease location were determined in 188, 152 and 153 patients, respectively. FL levels were significantly higher in patients with colonic and combined colonic and small bowel disease compared to patients with small bowel disease only (Figure 3A) with 69% of the latter patients having elevated (> 7.25 µg/g) levels of FL. CRP showed a similar trend with elevated levels being present in 44% of patients with small bowel disease (Figure 3B). WBC was significantly different only between patients with colonic and those with combined colonic and small bowel disease (Figure 3C). In patients with colonic disease, FL levels were associated with the number of inflamed colon segments (Table 4). Patients with 0-1 inflamed segments had a median FL level of 8 µg/g. Patients with 6 to 7 inflamed segments had a median level of 789 µg/g. Overall the association was highly significant (Kruskal Wallis test: P < 0.001).
| Number of inflamed colon segments | Number of patients | Median FL (µg/g) |
| 0-1 | 37 | 8 |
| 2-3 | 42 | 91.5 |
| 4-5 | 21 | 302 |
| 6-7 | 43 | 789 |
Colonoscopy is currently considered the standard test to diagnose and monitor IBD. However, this procedure is invasive, costly and requires sedation and an inconvenient preparation. Over the last two decades, fecal biomarkers such as FL and FC have emerged as potential substitutes of endoscopy. The advantages of fecal biomarkers are that samples (feces) are easy to obtain, can be collected at home, can be serially obtained, and are relatively easy to analyze. This allows patients to regularly monitor their disease without the need to see the clinician, by simply taking the stool sample to the laboratory. Hence, fecal biomarkers in principle offer a convenient, non-invasive and low-cost option for disease monitoring. How to best use these indicators to manage the disease is currently a subject of study and the initial results for FC are encouraging[25]. Less studies are available for FL. Gisbert et al[17] measured FL and FC in their study cohort. For FL they found that elevated values correlated with an increased risk of relapse (25% risk with a positive result vs 10% with a negative result, P < 0.05), and predicted relapse with a sensitivity of 62% and a specificity of 65%. Siponnen and colleagues reported a correlation of FL with SES-CD and with colonic histology in CD colitis, but not in small bowel disease[26]. In a recent review and metanalysis Dai et al[22] reported the results of 10 studies which used a prospective design and enrolled patients with diagnosed IBD. Overall the analysis revealed high discrimination for assessing UC and CD activity with FL concentration, with the diagnostic performance of the FL assay being apparently superior in UC patients. The authors concluded that FL is an inexpensive and useful screening marker with high sensitivity and modest specificity for assessing IBD activity[22].
In this retrospective study we investigated the correlation between FL levels and mucosal inflammation as well as disease location and extent, as assessed by endoscopy and histology in both UC and CD patients. For comparison we also tested the potential correlation of disease activity and location/extent with CRP and WBC – two blood markers also known to be elevated in inflammatory states. We only included patients who underwent endoscopy within 30 d of markers measurement. The patient population was representative of the entire spectrum of disease severity.
The results show a positive, significant correlation of FL and CRP with SES-CD and DAI. Such correlation was not seen for WBC. When stratifying patients for levels of disease activity FL was able to separate inactive vs mild, inactive vs moderate, mild vs severe and moderate vs severe activity in CD. In UC patients, FL separated mild vs moderate, and mild vs severe disease activity. Importantly, in patients with exclusive colonic involvement FL levels increased with the number of colonic segments involved. FL levels were also elevated in 69% of active small bowel-only CD patients with median FL levels significantly lower compared to patients with colonic-only disease and combined colonic and small bowel disease (26 µg/g vs 304 µg/g vs 197 µg/g respectively). This finding suggests that low FL levels might be associated with small bowel disease activity but with minimal, if any, colonic disease activity. This could be related to the different surface area of the two intestinal tracts - whereby a small extent of disease activity is relatively more significant in the small bowel than in the colon. However, the precise explanation for this finding (as well as the establishment of a FL cut-off level for inflammation in the colon vs small bowel) must await a dedicated prospective study. Nevertheless, we show here that FL is a mostly reliable indicator of small bowel disease. This finding disagrees with that of Sipponen et al[26], who did not find correlation of fecal markers with small bowel disease. However, those authors only used colonoscopy to estimate the disease presence/extent in the small bowel whereas we also relied on imaging. Another potential confounding factor in determining the accuracy of fecal markers in small bowel disease is the unclear contribution of inflammation in the deeper layers of the gut wall[27]. Regardless, the finding that FL levels are correlated with the disease extent is an important one because it does indicate that this marker might be an accurate indicator of the total disease burden - the product of severity times extent[28] - and makes it a potential candidate as an ideal therapeutic target, as already indirectly shown by others for FC[25].
Another interesting finding of our study is that FL levels obtained before colonoscopy had a better correlation with SES-CD and DAI than levels measured after the colonoscopy in patients given effective, fast acting medications (steroids and biologics) in between marker determination and the procedure. The most likely explanation of this observation is that FL is a timely indicator of changes in disease activity after therapy. FL concentration in feces is proportional to neutrophil translocation to the mucosa of the GI tract – a process that is quickly modulated by the activity of the inflammatory process[19]. Replacement of the dead epithelial cells on the other hand might be more lengthy[29]. In principle, such timeliness of FL reaction to changes in the mucosa inflammatory activity in IBD could be exploited to monitor treatment response in a number of clinical scenarios.
Our study has some obvious limitations. Firstly, it is a retrospective study. However, the data were retrieved from a single EMR and included detailed and uniform information on patients’ clinical status, disease features and treatment. Although FL was not tested for research purposes the timing and accuracy of data acquisition would not have been different in a prospective study. Secondly, our study is a single center study. As such it is possible that it might reflect the investigators’ and the institution standard of practice as they relate to several aspects of this study. However, we applied the strictest and most objective criteria to select our patients’ population – according to widely used standards. Furthermore, the size of our patient population and the representation of the spectrum of disease activity and extent are well above the average of studies focused on FL[22].
Further validation of our findings in larger scale and prospective studies might confirm that fecal markers of inflammation are accurate and inexpensive indicators of disease activity in IBD, can be used in a number of clinical scenarios and should become part of the standard armamentarium of the practicing gastroenterologist, especially in the United States where their use still lags behind most other Western countries[30].
Studies have demonstrated a potential role for fecal biomarkers such as fecal calprotectin (FC) and fecal lactoferrin (FL) in monitoring inflammatory bowel diseases (IBD) – both Crohn’s disease (CD) and ulcerative colitis (UC). However, their correlation to endoscopic scores, disease severity and affected intestinal surface has not been extensively investigated.
Achieving a better understanding of the role of fecal markers for the evaluation and mana-gement of IBD patients.
To correlate FL, and for comparison white blood cell (WBC) and C-reactive protein (CRP), with endoscopic scores, disease extent and location in CD and UC.
Retrospective analysis in 188 patients who had FL, WBC and CRP determined within 30 d of endoscopy. Disease location, disease extent (number of intestinal segments involved), disease severity [determined by endoscopic scores: Simple Endoscopic Score for CD (SES-CD) and the endoscopic component of the Mayo Clinical score/Disease Activity Index (DAI)], timing of FL testing in relation to colonoscopy, as well as the use of effective fast acting medications (steroids and biologics) between colonoscopy and FL measurement, were recorded.
In 131 CD and 57 UC patients, both CRP and FL - but not WBC - distinguished disease severity (inactive, mild, moderate, severe). In patients receiving fast-acting treatment (steroids or biologics) in between FL measurement and colonoscopy, FL showed a higher correlation to endoscopic scores when tested before vs after the procedure (r = 0.596, P < 0.001 vs r = 0.285, P = 0.15, for SES-CD; and r = 0.402, P = 0.01, vs r = 0.054, P = 0.84 for DAI). Finally, FL was significantly correlated with the diseased mucosal surface (colon-ileocolon > small bowel) and the number of inflamed colon segments. FL and CRP separated disease severity categories. FL showed a close correlation with the involved mucosal surface and with disease extent and was more closely correlated to endoscopy when determined before the procedure – this indicating that inflammatory activity changes associated with therapy might be rapidly reflected by FL levels.
The results show a positive, significant correlation of FL and CRP with SES-CD and DAI. FL showed a close correlation with the diseased mucosal surface and with disease extent and was more closely correlated to endoscopy when determined before endoscopy. FL can accurately and timely represent intestinal inflammation in IBD.
Further validation of our findings in large scale and prospective studies might confirm that fecal markers of inflammation are accurate and inexpensive indicators of disease activity in IBD, can be used in a number of clinical scenarios and should become part of the standard armamentarium of the practicing gastroenterologist.
We thank Dr. Joshua Gazo for his help with data collection.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Souza JLS, Tommasini A, Yang BL, Zhang L, Zouiten-Mekki L S-Editor: Ma RY L-Editor: A E-Editor: Wu YXJ
| 1. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 2. | Sorrentino D. The Coming of Age of Inflammatory Bowel Diseases in Asia. Inflamm Intest Dis. 2017;2:93-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Yang SK. How Does the Epidemiology of Inflammatory Bowel Disease Differ between East and West? A Korean Perspective. Inflamm Intest Dis. 2017;2:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Xu F, Dahlhamer JM, Zammitti EP, Wheaton AG, Croft JB. Health-Risk Behaviors and Chronic Conditions Among Adults with Inflammatory Bowel Disease - United States, 2015 and 2016. MMWR Morb Mortal Wkly Rep. 2018;67:190-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Sonnenberg A. Hospitalization for inflammatory bowel disease in the United States between 1970 and 2004. J Clin Gastroenterol. 2009;43:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Sonnenberg A, Chang J. Time trends of physician visits for Crohn's disease and ulcerative colitis in the United States, 1960-2006. Inflamm Bowel Dis. 2008;14:249-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kappelman MD, Rifas-Shiman SL, Porter CQ, Ollendorf DA, Sandler RS, Galanko JA, Finkelstein JA. Direct health care costs of Crohn's disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135:1907-1913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 524] [Cited by in RCA: 532] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 8. | D'Incà R, Caccaro R. Measuring disease activity in Crohn's disease: what is currently available to the clinician. Clin Exp Gastroenterol. 2014;7:151-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | D'Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, Lémann M, Marteau P, Rutgeerts P, Schölmerich J, Sutherland LR. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 795] [Article Influence: 44.2] [Reference Citation Analysis (1)] |
| 10. | Vavricka SR, Spigaglia SM, Rogler G, Pittet V, Michetti P, Felley C, Mottet C, Braegger CP, Rogler D, Straumann A, Bauerfeind P, Fried M, Schoepfer AM; Swiss IBD Cohort Study Group. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 11. | Carter D, Eliakim R. Current role of endoscopy in inflammatory bowel disease diagnosis and management. Curr Opin Gastroenterol. 2014;30:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (37)] |
| 12. | Vieira A, Fang CB, Rolim EG, Klug WA, Steinwurz F, Rossini LG, Candelária PA. Inflammatory bowel disease activity assessed by fecal calprotectin and lactoferrin: correlation with laboratory parameters, clinical, endoscopic and histological indexes. BMC Res Notes. 2009;2:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Wright EK, De Cruz P, Gearry R, Day AS, Kamm MA. Fecal biomarkers in the diagnosis and monitoring of Crohn's disease. Inflamm Bowel Dis. 2014;20:1668-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Dave M, Loftus EV. Mucosal healing in inflammatory bowel disease-a true paradigm of success? Gastroenterol Hepatol (N Y). 2012;8:29-38. [PubMed] |
| 15. | Rameshshanker R, Arebi N. Endoscopy in inflammatory bowel disease when and why. World J Gastrointest Endosc. 2012;4:201-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Koulaouzidis A, Douglas S, Rogers MA, Arnott ID, Plevris JN. Fecal calprotectin: a selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scand J Gastroenterol. 2011;46:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Gisbert JP, Bermejo F, Pérez-Calle JL, Taxonera C, Vera I, McNicholl AG, Algaba A, López P, López-Palacios N, Calvo M, González-Lama Y, Carneros JA, Velasco M, Maté J. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (3)] |
| 18. | Levay PF, Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80:252-267. [PubMed] |
| 19. | Guerrant RL, Araujo V, Soares E, Kotloff K, Lima AA, Cooper WH, Lee AG. Measurement of fecal lactoferrin as a marker of fecal leukocytes. J Clin Microbiol. 1992;30:1238-1242. [PubMed] |
| 20. | Kane SV, Sandborn WJ, Rufo PA, Zholudev A, Boone J, Lyerly D, Camilleri M, Hanauer SB. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol. 2003;98:1309-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 259] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Rogan MP, Geraghty P, Greene CM, O'Neill SJ, Taggart CC, McElvaney NG. Antimicrobial proteins and polypeptides in pulmonary innate defence. Respir Res. 2006;7:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Dai C, Jiang M, Sun MJ, Cao Q. Fecal Lactoferrin for Assessment of Inflammatory Bowel Disease Activity: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Dai J, Liu WZ, Zhao YP, Hu YB, Ge ZZ. Relationship between fecal lactoferrin and inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1440-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Benevento G, Avellini C, Terrosu G, Geraci M, Lodolo I, Sorrentino D. Diagnosis and assessment of Crohn's disease: the present and the future. Expert Rev Gastroenterol Hepatol. 2010;4:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Colombel JF, Panaccione R, Bossuyt P, Lukas M, Baert F, Vaňásek T, Danalioglu A, Novacek G, Armuzzi A, Hébuterne X, Travis S, Danese S, Reinisch W, Sandborn WJ, Rutgeerts P, Hommes D, Schreiber S, Neimark E, Huang B, Zhou Q, Mendez P, Petersson J, Wallace K, Robinson AM, Thakkar RB, D'Haens G. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 671] [Article Influence: 83.9] [Reference Citation Analysis (1)] |
| 26. | Sipponen T, Kärkkäinen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn's disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 247] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 27. | Weinstein-Nakar I, Focht G, Church P, Walters TD, Abitbol G, Anupindi S, Berteloot L, Hulst JM, Ruemmele F, Lemberg DA, Leach ST, Cytter R, Greer ML, Griffiths AM, Turner D; ImageKids study group. Associations Among Mucosal and Transmural Healing and Fecal Level of Calprotectin in Children With Crohn's Disease. Clin Gastroenterol Hepatol. 2018;16:1089-1097.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 28. | Sorrentino D, Nguyen V, Henderson C, Bankole A. Therapeutic Drug Monitoring and Clinical Outcomes in Immune Mediated Diseases: The Missing Link. Inflamm Bowel Dis. 2016;22:2527-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Blander JM. Death in the intestinal epithelium-basic biology and implications for inflammatory bowel disease. FEBS J. 2016;283:2720-2730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 30. | van Deen WK, van Oijen MG, Myers KD, Centeno A, Howard W, Choi JM, Roth BE, McLaughlin EM, Hollander D, Wong-Swanson B, Sack J, Ong MK, Ha CY, Esrailian E, Hommes DW. A nationwide 2010-2012 analysis of U.S. health care utilization in inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:1747-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |