Published online Dec 15, 2010. doi: 10.4291/wjgp.v1.i5.154
Revised: November 24, 2010
Accepted: December 1, 2010
Published online: December 15, 2010
AIM: To test the hypothesis that histamine 3 receptor (H3R) activation during Helicobacter infection inhibits gastric acid secretion in vivo and in vitro.
METHODS: Helicobacter felis (H. felis) infected and uninfected C57Bl/6 mice were infused with either PBS or the H3 receptor antagonist thioperamide (THIO) for 12 wk. After treatment, mice were analyzed for morphological changes and gastric acid content. Total RNA was prepared from the stomachs of each group and analyzed for changes in somatostatin and gastrin mRNA abundance by real time-polymerase chain reaction (RT-PCR). Location of H3 receptors in the stomach was analyzed by co-localization using antibodies specific for the H3 receptor and parietal cell marker H+, K+-ATPase β subunit.
RESULTS: Inflammation and parietal cell atrophy was observed after 12 wk of H. felis infection. Interestingly, treatment with the H3R antagonist thioperamide (THIO) prior to and during infection prevented H. felis-induced inflammation and atrophy. Compared to the uninfected controls, infected mice also had significantly decreased gastric acid. After eradication of H. felis with THIO treatment, gastric acidity was restored. Compared to the control mice, somatostatin mRNA abundance was decreased while gastrin gene expression was elevated during infection. Despite elevated gastric acid levels, after eradication of H. felis with THIO, somatostatin mRNA was elevated whereas gastrin mRNA was suppressed. Immunofluorescence revealed the presence of H3 receptors on the parietal cells, somatostatin-secreting D-cells as well as the inflammatory cells.
CONCLUSION: This study shows that during H. felis infection, gastric acidity is suppressed as a consequence of an inhibitory effect on the parietal cell by H3R activation. The stimulation of gastric mucosal H3Rs increases gastrin expression and release by inhibiting release of somatostatin.
-
Citation: Zavros Y, Mesiwala N, Waghray M, Todisco A, Shulkes A, Merchant JL. Histamine 3 receptor activation mediates inhibition of acid secretion during
Helicobacter -induced gastritis. World J Gastrointest Pathophysiol 2010; 1(5): 154-165 - URL: https://www.wjgnet.com/2150-5330/full/v1/i5/154.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v1.i5.154
Histamine 3 receptor (H3R) activation has been implicated as a mediator for altered acid secretion that is observed during Helicobacter pylori (H. pylori) infection[1-4]. Courillon-Mallet et al first revealed elevated concentrations of H3R agonist Nα-methylhistamine (Nα-MH) in the gastric mucosa of H. pylori infected patients[1,5,6]. Nα-MH has an indirect inhibitory effect on acid secretion by inhibiting the acid secretagogue histamine from the ECL-like cells[3,5]. Stimulation of H3Rs on ECL-like cells eventually inhibits histidine decarboxylase (HDC) activity[3,5]. Down-regulation of HDC causes a decrease in the amount of histamine produced and decreased acid secretion[3,5]. Early in vivo experiments using H3R agonist Rα-methylhistamine (Rα-MH) also potently inhibits gastric secretion by a number of indirect stimuli[7,8]. However, experiments using in vitro techniques are quite controversial. In isolated rat fundic ECL-like cells, Rα-MH is likely to inhibit acid secretion via the suppression of histamine[2,4] whereas increased acid secretion secondary to reduced somatostatin secretion is reported in isolated mouse stomach[9]. Overall, the role of H3Rs in the regulation of hormonal and paracrine influences on acid secretion during Helicobacter infection is unclear.
The exact cellular location of the H3R in the stomach is not known since its presence is based on pharmacological studies[9,10], thus making it difficult to understand the direct role of the H3R in physiological function. Here, we show that during H. felis infection, gastric acidity is suppressed, a response that is mediated by an inhibitory effect on the parietal cell by H3R activation. The stimulation of gastric mucosal H3Rs increases gastrin expression and release by inhibiting release of somatostatin.
Helicobacter felis (H. felis) (American Type Culture Collection, Rockville, MD) was inoculated on Campylobacter selective agar supplemented with 5% sterile horse blood (BD Diagnostics, Bedford, MA), trimethoprim (5 μg/mL), vancomycin (10 μg/mL) and nystatin (10 μg/mL)[11]. Cultures were incubated for two days in a humidified microaerophilic chamber (BBL Gas System, with CampyPak Plus packs, BD Microbiology, Sparks, MD). H. felis was harvested and used to inoculate mouse stomachs by oral intubation. C57BL/6 mice were orally inoculated with a catheter once daily over 3 d with 108H. felis organisms per 200 μL of brain heart infusion. Mice were divided into four groups: PBS (n = 8), thioperamide (n = 8), H. felis (n = 8) and H. felis plus thioperamide (n = 8). For the thioperamide treatment, mice were injected with thioperamide (100 μg/kg per mouse per day, i.p.) during the 12 wk H. felis infection subsequent to a 7 d pretreatment.
A standard curve was generated by extracting total RNA, using Trizol Reagent (Life Technologies, Gaithersburg, MD), from H. felis bacterial cultures with densities ranging from 103 to 109 total bacteria. Total RNA was also isolated from stomach tissue using Trizol Reagent. Primer pairs C97 and C98 were used to amplify the 16S rRNA species that is specific for Helicobacter and generates an amplicon of approximately 400 base pairs[12]. PCR amplifications were performed in a total volume of 25 μL, containing 10 × PCR buffer with 10-9 moles/L MgCl2, dNTPs, 200 nmol/L primers, 5 μL of cDNA, 10-7 moles/L Taq polymerase GOLD and 2.5 μL of Sybr Green (Molecular Probes). Each PCR amplification was performed in duplicate wells in a Biorad I-Cycler (Biorad Laboratories, I-Cycler IQ Real-Time PCR Detection System, Hercules, CA) using the following conditions: 94°C for 10 min followed by 35 two-temperature cycles (94°C for 1 min and 55°C for 1 min).
Merino-Corriedale cross sheep were administered a local anesthetic (2 mL of 1% xylocaine) in the skin surrounding the jugular vein. The method of cannulation involved the insertion of a hypodermic needle into the jugular vein and directed toward the head. The cannula was passed through the hypodermic needle and into the vein. Following removal of the needle, adhesive plaster was used to secure the cannula to the skin with sutures. The same procedure was followed for the contralateral jugular vein except the cannula was directed toward the heart. Each cannula was flushed with 2 mL heparin, capped, wrapped with sterile gauze and tied to the sheep’s neck and secured with elastic bandage. The jugular vein cannula directed up was used for blood sampling (10 min intervals) and the cannula directed down for agonist (pentagastrin, Bachem, 5 μg/kg per hour) and antagonist (thioperamide, Sigma, 100 μg/kg per hour) infusions. A recovery period of 24 h was allowed. Sheep were housed in metabolism cages for the duration of the experiment[13].
During the sheep infusions, blood was sampled every 10 min into tubes containing 100 μmol/L Na2EDTA (10 μL/mL blood), centrifuged at 3000 rpm for 15 min at 4°C and plasma somatostatin concentration was measured in ethanol extracted plasma by radioimmunoassay (RIA) as previously described[14]. Mice were euthanized and approximately 1 mL of blood was collected by cardiac puncture, aliquoted into tubes containing 100 μmol/L Na2EDTA (10 μL/mL blood) and centrifuged at 3000 rpm for 15 min at 4°C. Plasma was collected immediately and stored at -20°C until assayed for gastrin by RIA as previously described[15].
Gastric acidity was measured as previously published[15]. Briefly, after mice were fasted for 16 h the stomachs were opened along the greater curvature and washed with 2 mL normal saline (pH 7.0). The contents were centrifuged at 3000 rpm for 5 min and supernatant was collected. The supernatant was titrated by using 0.005N NaOH and gastric acidity was expressed as μEq.
RNA extraction was performed on all antral and fundic tissue samples from the mice. Tissue was homogenized with a polytron PT-2000 (Kinematica, Cincinnati, OH) in 1 mL of TRIzol (Sigma) reagent according to the manufacturer’s protocol. Once RNA was extracted, it was resuspended in 50 μL of DEPC H2O and stored at -80°C.
RNA from all antral and fundic tissue samples underwent quantitative testing using a spectrometer and then was converted to cDNA using the reverse transcriptase kit (Promega). RNA polymerase chain reaction (PCR) amplifications were performed in a total volume of 25 mL containing 10 × PCR buffer with MgCl2, 10 nmol/L dNTPs, 200 nmol/L primers, 5 mL of cDNA, 100 nmol/L Taq polymerase GOLD and 2.5 mL of Sybr green (Molecular Probes). Each PCR amplification was performed in duplicate wells in a Bio-Rad I-Cycler (Bio-Rad, I-Cycler IQ Real-Time PCR Detection System) with the following conditions: 94°C for 10 min followed by 35 two-temperature cycles (94°C for 1 minute and 55°C for 1 min). Sequences for somatostatin, gastrin, Helicobacter and HPRT primers are given in Table 1.
| Primer | Sequence (5’ to 3’) |
| Somatostatin | FW: TGCTGTCCTGCCGTCTCC |
| RV: ATCATTCTCTGTCTGGTTGG | |
| Gastrin | FW: ACACAACAGCCAACTATTC |
| RV: CAAAGTCCATCCATCCGTAG | |
| Helicobacter | FW: GCTATGACGGGTATCC |
| RV: GATTTTACCCCTACACCA | |
| HPRT | FW: AGTCCCAGCGTCGTGATTAGC |
| RV: ATAGCCCCCCTTGAGCACACAG |
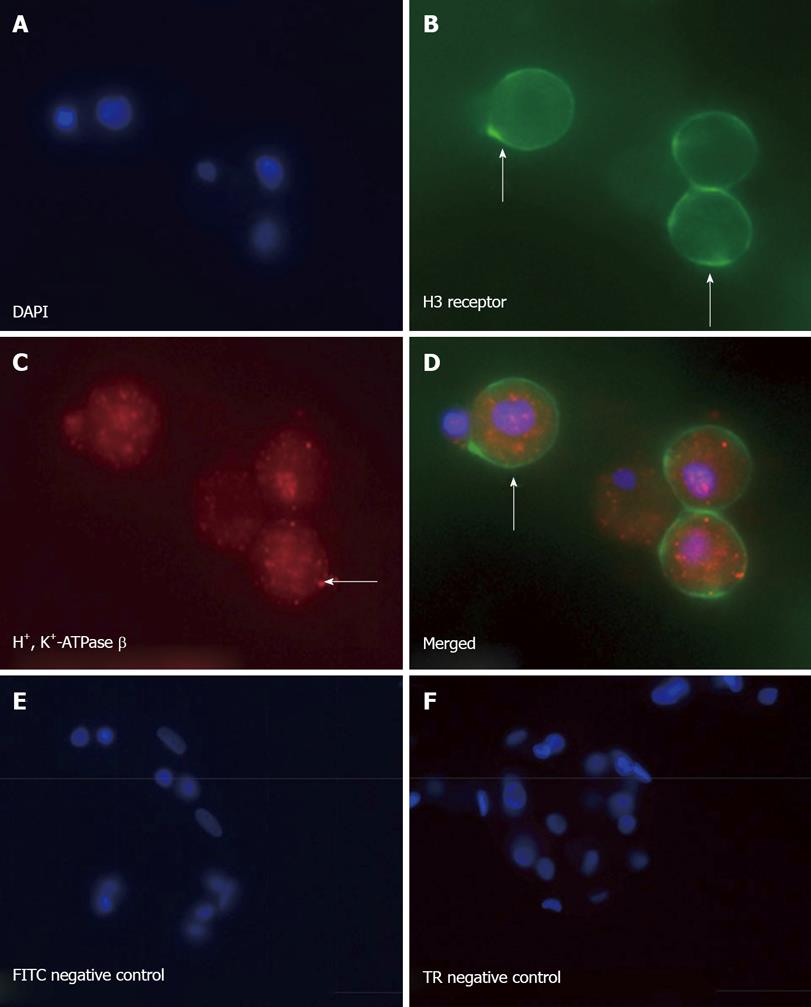
Parietal- and somatostatin-secreting D-cells were isolated from canine oxyntic mucosa based on a modified elutriation method by Soll et al[16]. The isolated parietal cells (2 × 106 cells/well) were cultured in Ham’s F-12/Dulbecco’s modified Eagle’s medium (1:1) containing 0.1 mg/mL gentamicin, 50 U/mL penicillin G, 0.01 mg/mL ciprofloxacin and 2% DMSO (Sigma) on either 35 mm culture dishes or chamber slides coated with 150 or 50 μL of growth factor reduced Matrigel (BD Biosciences) respectively[17]. The cultures were 95% to 98% homogenous for parietal cells[18]. The elutriated fraction containing D-cells was plated onto Matrigel (2.0 × 106 cells/well) in culture medium containing Dulbecco’s modified Eagle’s medium: Ham’s F-12 medium (50:50) supplemented with 10% heat-inactivated dog serum, gentamicin, penicillin, insulin and hydrocortisone[19]. D cells were enriched to 50%-60% purity by washing away non-adherent mucous cells with culture medium and analyzed for the presence of H3 receptors by flow cytometry.
Gastric acid secretion was measured according to published methods[17,20]. Briefly, accumulation of the weak base [14C]aminopyrine (Amersham Biosciences) was used to detect acid production by canine parietal cells. Cultured parietal cells were washed once with Earle’s balanced salt solution and incubated with 0.1 μCi of [14C]aminopyrine. Cells were then treated with either (Veh, DMSO/PEG), (r)-α-methylhistamine (Ρα-MH, 100 nmol/L), thioperamide (100 nmol/L) or histamine (100 μmol/L) for 30 min. Parietal cells were then lysed with 500 μL of 1% Triton X-100 and the radioactivity of the lysate was quantified in a liquid scintillation counter.
A longitudinal section of the mouse stomach (spanning both the fundic and antral regions) was fixed in 4% paraformaldehyde/PBS, paraffin-embedded and sectioned (3 mm). Parietal cells were cultured on Matrigel-coated chamber slides overnight, rinsed and fixed in methanol for 20 min. Antigen retrieval was performed on tissue after deparaffinization by heating the slides for 10 min at 100°C in 0.01 mol/L sodium citrate. Cells and tissue were then hydrated using PBS/0.01% Triton X-100 for 20 min and then blocked in 20% normal goat serum for an additional 20 min. Slides were incubated with a 1: 50 dilution of histamine 3 (H3) receptor (Santa Cruz Biotechnology) anti-rabbit antibody at 4°C overnight followed by a 2 h room temperature incubation with 1: 800 dilution of H+, K+-ATPase (MBL International, Watertown, MA) anti-mouse antibody. Stomach tissue expressing the H3 receptor were then detected by using a 1: 100 dilution of either anti-rabbit conjugated to FITC or anti-mouse conjugated to Texas red secondary antibodies for 1 h at room temperature cover-slipped and viewed under a fluorescence microscope [Olympus (Melville, NY) BX60 with Diagnostic Instruments “Spot” Camera].
In a separate experiment, the elutriated fraction containing canine D cells were then double labeled with goat polyclonal somatostatin-FITC (Santa Cruz Biotechnology) and rabbit polyclonal H3 receptor-phycoerythrin (Santa Cruz Biotechnology). FITC-conjugated goat IgG or phycoerythrin-conjugated rabbit IgG was used as the isotype controls. Labeled cells were then analyzed by cytometry using a Coulter Elite ESP Cell Sorter (Bechman-Coulter Electronics, Hialeah, FL).
The significance of the results were tested using the unpaired t-test or one-way ANOVA where appropriate, using a commercially available software (GraphPad Prism, GraphPad Software, San Diego, CA). A P value < 0.05 was considered significant.
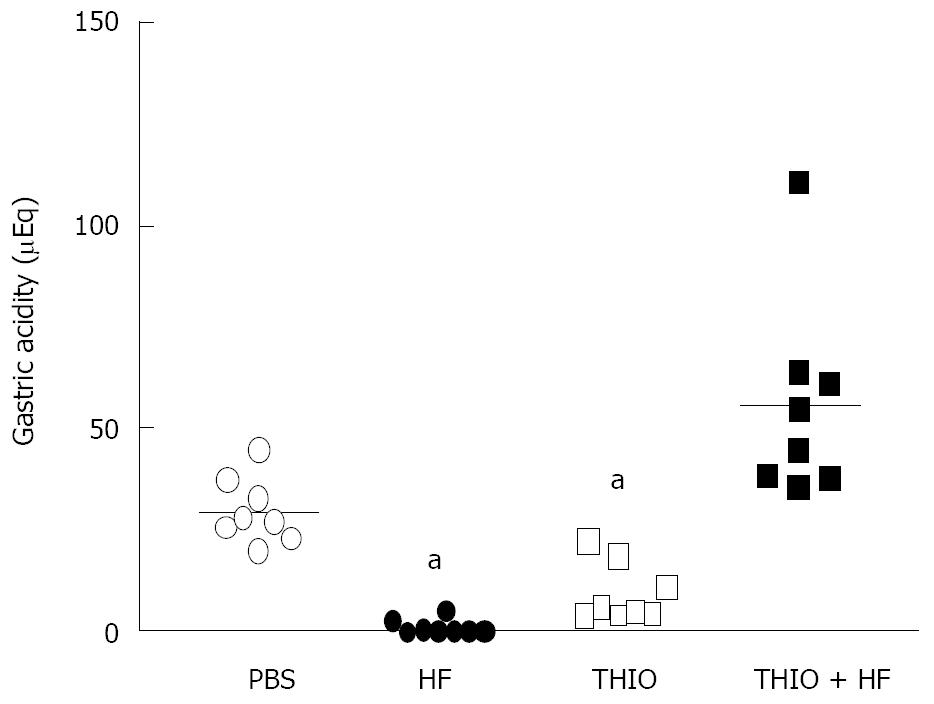
The H3R antagonist thioperamide was used to determine the role of the H3R as a mediator of the decreased gastric acidity during H. felis-induced gastritis. Gastric acidity was significantly reduced in 12 wk H. felis-infected mice compared to the PBS treated animals (Figure 1). Thioperamide alone caused a significant reduction in gastric acidity compared to PBS-treated mice (Figure 1). Thioperamide treatment during H. felis infection reversed the decrease in gastric acidity (Figure 1). These results indicate that H. felis has an inhibitory effect on gastric acid secretion in vivo and that this inhibitory pathway may be mediated by H3R activation.
H. felis-induced gastritis is reduced with thioperamide treatment
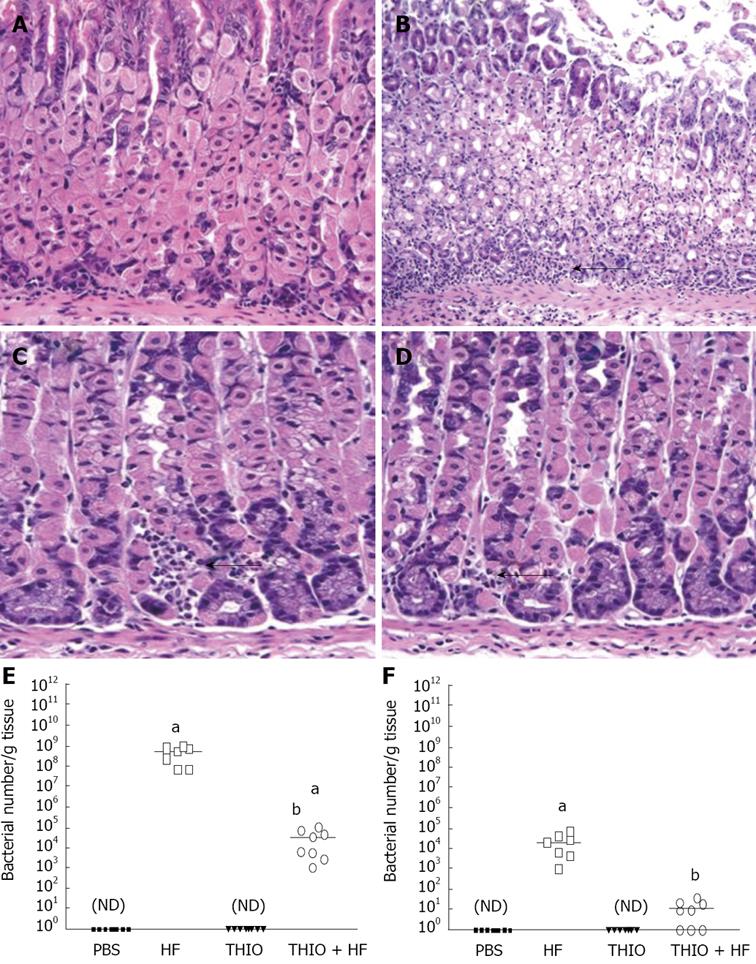
Hematoxylin/eosin (H&E)-stained secretions were examined for changes in histology in response to H. felis infection and thioperamide treatment. After 12 wk of H. felis infection, mice developed significant inflammation and atrophy compared with the PBS controls (Figure 2A, B). H. felis-infected mice treated with thioperamide showed resolution of the inflammation observed with the infection alone (Figure 2D). Minimal inflammation was observed with the thioperamide alone treatment (Figure 2C).
We considered that reduced colonization with H. felis might account for the reduced gastritis with the thioperamide treatment. Thus, bacterial numbers per gram of stomach tissue collected from each group of mice was analyzed using a H. felis specific quantitative RT-PCR assay. The data revealed that H. felis colonization was significantly reduced with thioperamide treatment in both fundic and antral stomach tissue (Figure 2E, F). The resistance to colonization with H. felis contributed to the reduced gastritis and restoration of normal gastric acidity with thioperamide treatment.
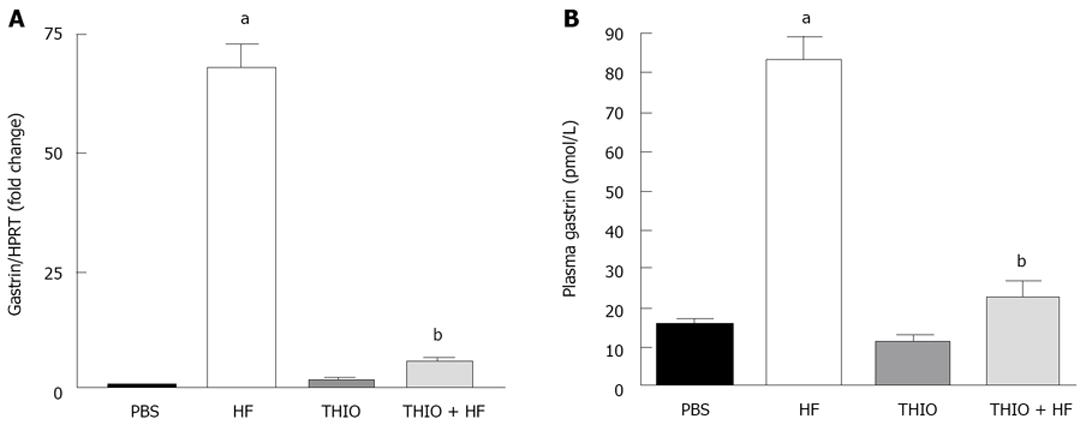
Chronic gastritis and reduced gastric acidity is accompanied by reciprocal changes in gastrin and somatostatin[21]. Analysis by qRT-PCR showed significantly higher gastrin mRNA expression in H. felis infected mice compared to untreated PBS controls (Figure 3A). Thioperamide treatment of H. felis infected mice resulted in a reversal of the increase in gastrin mRNA expression (Figure 3A), a finding consistent with the reversal of the reduced gastric acidity. Changes in circulating gastrin paralleled that of gastrin mRNA with an increase in response to H. felis infection that was resolved with thioperamide treatment (Figure 3B).
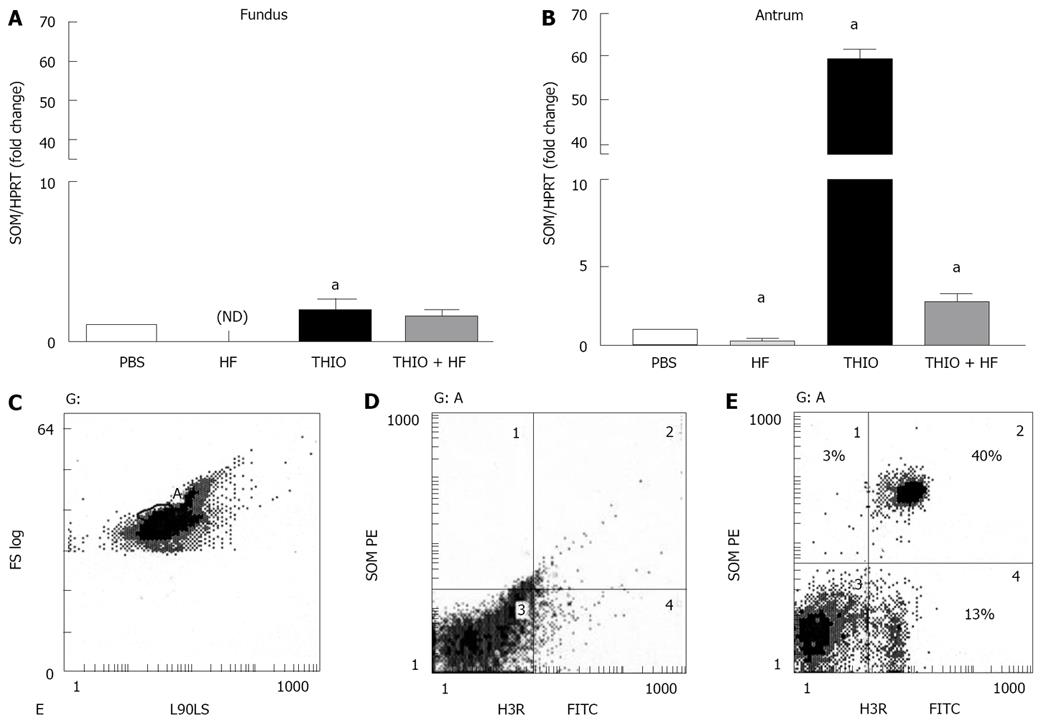
The H3 receptor antagonist thioperamide has been shown to stimulate somatostatin release in vitro[9,10,22]. In addition, prior studies have shown that somatostatin regulates the immune system, down-regulating a number of functions including lymphocyte proliferation, IFNγ production as well as a direct anti-proliferative effect on Helicobacter[21,23-25]. Given that we observed a significant reduction in H. felis-induced gastritis with thioperamide treatment, we then measured changes in somatostatin mRNA expression by qRT-PCR in both the fundic and antral mucosa of treated mice (Figure 4). Significantly decreased somatostatin mRNA expression in both the fundic and antral mucosa was observed in the stomachs of H. felis infected mice (Figure 4A, B). Although thioperamide alone had a minimal stimulatory effect on fundic somatostatin mRNA expression (Figure 4A), thioperamide treatment significantly increased antral somatostatin levels (Figure 4B). Reduced fundic and antral somatostatin mRNA expression observed with H. felis infection was restored to baseline after thioperamide treatment (Figure 4A, B). Using flow cytometry, we identified a subset of somatostatin expressing D-cells that also expressed the H3R comprising 40% of the entire cell preparation (Figure 4C-E) supporting a possible direct activation of H3R by thioperamide on D-cells. There was a 13% population of somatostatin expressing cells that were negative for the H3R (Figure 4E).
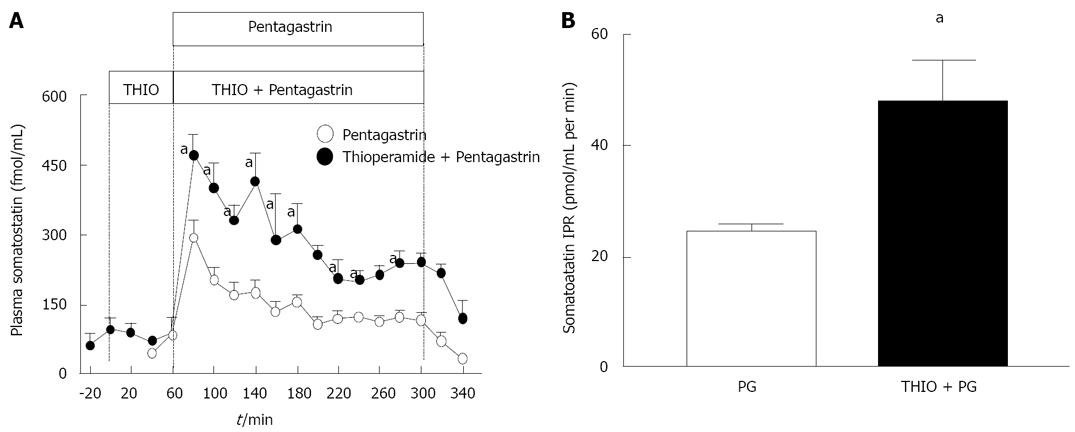
In order to determine if the thioperamide induced increase in somatostatin mRNA was associated with a functional secretory response in somatostatin, plasma somatostatin was measured in sheep treated with pentagastrin and thioperamide. Previous studies have shown that sheep have a similar reciprocal gastrin/somatostatin response to changes in gastric acidity[13,26,27]. Thioperamide augmented the secretion of somatostatin (Figure 5A, B), indicating a functional relationship between the H3R and the D cell.
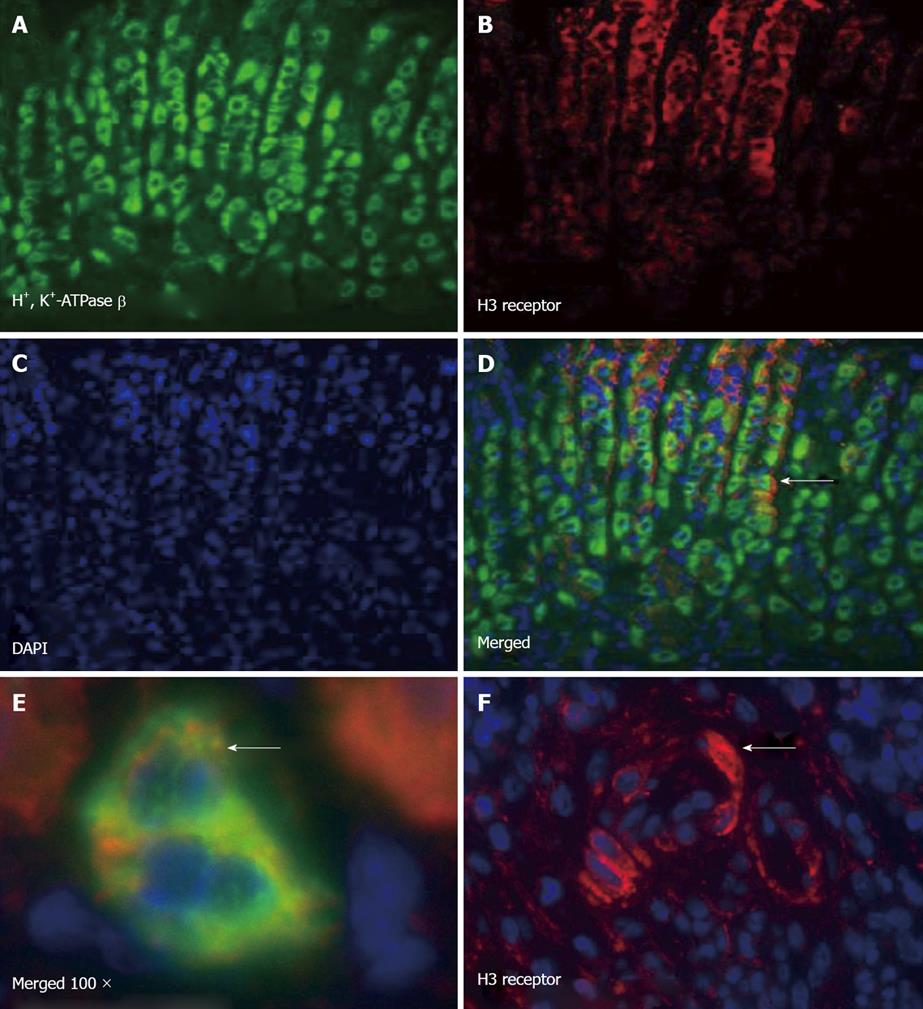
The localization of the H3R in the gastric mucosa has not been fully determined. We used immunofluorescence to determine the expression pattern of the H3R in the stomachs of H. felis-infected and uninfected animals. Immunofluorescence showed co-localization of the H3R on mouse H+, K+-ATPase positive parietal cells (Figure 6A-E). Using a primary canine parietal cell culture system, immunofluorescence data supported Figure 5 and showed H3R co-localization on H+, K+-ATPase expressing parietal cells (Figure 6A-F). Interestingly, in H. felis-infected mice, immunostaining for the H3R was also observed within the inflammatory infiltrate (Figure 6F), suggesting a potential direct interaction between thioperamide and the parietal cells and infiltrating immune cells.
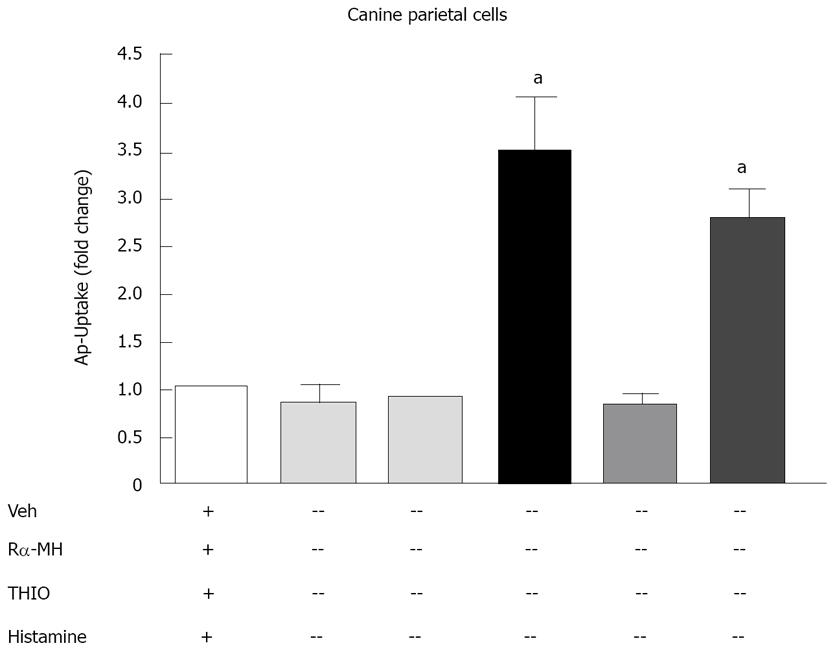
We tested the effect of H3R activation on acid secretion from the isolated canine parietal cells, the H3R agonist Rα-MH and the H3R antagonist thioperamide. The H3R agonist or antagonist alone had no effect on radiolabeled aminopyrine uptake (Figure 8). Histamine, which potently stimulates acid secretion through the activation of the H2R, significantly induced aminopyrine uptake (Figure 8). Rα-MH blocked the stimulatory effect of histamine on aminopyrine uptake agonist (Figure 8). The H3R antagonist thioperamide reversed the inhibitory effect of Rα-MH on histamine-induced aminopyrine uptake (Figures 7 and 8). Thus, activation of the H3R on parietal cells results in the inhibition of secretagogue-stimulated acid secretion.
We have used in vivo and in vitro models to identify the physiological function of H3R activation in the stomach. In particular, H3R activation contributed to decreased acid secretion observed during H. felis infection. H. felis-induced hypochlorhydria was associated with reciprocal changes in gastrin and somatostatin secretion and expression. Findings reported here demonstrate an important role for the H3R in gastric acid and somatostatin regulation during Helicobacter infection.
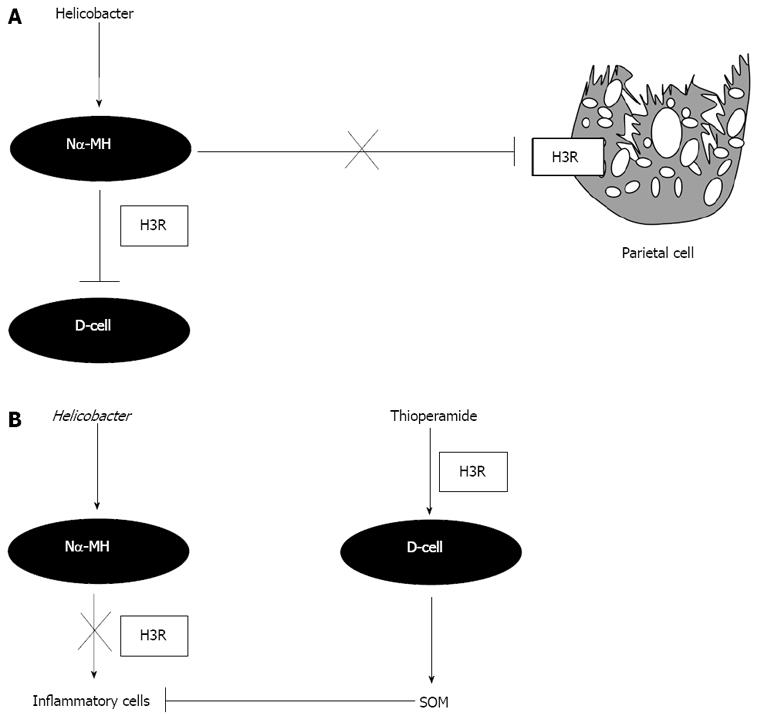
H. felis-induced gastritis was associated with significantly decreased gastric acidity 12 wk after infection, as previously reported[21]. The H3R antagonist thioperamide reversed suppressed gastric acidity observed during H. felis infection. Our data was consistent with other in vivo observations. In humans it has been reported that gastric acid secretion increases 4-8 wk after eradication therapy, suggesting that Helicobacter inhibits acid secretion[28]. In cats, acid secretion was inhibited within 3 wk of H. pylori infection[3]. The inhibition of gastric acid secretion may also be accounted for by suppression of histidine decarboxylase (HDC) activity that is important in the production of the major acid secretagogue histamine[5,29,30]. The inhibition of HDC is brought about by the production of the potent H3R agonist Nα-MH secreted from the bacterium itself[5,31]. This is supported by previous evidence showing H3R located on histamine-producing cells that reduce excess acid[1]. Our in vitro and immunofluorescence data demonstrating H3R on the parietal cells are consistent with previous observations made in the rabbit[1]. The expression of the H3R on the gastric parietal cell supports the hypothesis of a direct inhibitory effect of the Helicobacter metabolite Nα-MH on parietal cell acid secretion (Figure 9A).
The H3R antagonist thioperamide has been shown to stimulate somatostatin release in vitro[32] and this may account for the decreased gastric acidity observed. H. felis alone also caused a significant decrease in gastric acidity perhaps due to the parietal cell atrophy observed with Helicobacter infection. Thioperamide treatment during H. felis infection reversed the decrease in gastric acidity observed with the bacterial infection alone. Given that thioperamide is a H3R antagonist, these results may indicate that H. felis has an inhibitory effect on gastric acid secretion in vivo and that this inhibitory pathway may be mediated by H3R activation. Thioperamide treatment also suppressed bacterial colonization and this may contribute to the normalization of gastric acidity observed with the thioperamide and H. felis group. It may be that with continued thioperamide treatment a further decrease in gastric acidity would have been observed in the H. felis-infected group.
In the gastrointestinal tract, H3R are located in cholinergic and NANC neurons of the myenteric plexus, in endocrine and paracrine cells of the gastric mucosa and on rabbit parietal cells[1]. The cellular location of H3Rs in the stomach mucosa is uncertain. Our data suggests that in addition to parietal cells, H3Rs are located on gastric D cells. This finding confirms previous pharmacological studies where it has been assumed that the H3R exists on the D-cell[9,10]. Consistent with our findings in vivo in mouse and sheep where H3R antagonism stimulated somatostatin mRNA and somatostatin secretion, in vitro studies using isolated mouse antral mucosa demonstrated a similar increase with thioperamide[32]. In vivo, stimulation of somatostatin expression with thioperamide during H. felis infection resulted in suppression of hypergastrinemia. Collectively, our study extends these in vitro findings by demonstrating the expression of the H3R on the D-cell and suggests a direct inhibitory effect of Nα-MH on somatostatin secretion during H. pylori infection (Figure 9A).
Treatment of H. felis infected mice with thioperamide abolished the H. felis-induced gastritis consistent with a reduction in bacterial colonization. Thus, we considered that resistance to colonization with Helicobacter might account for the reduced gastritis with the thioperamide treatment. Infected mice were also hypergastrinemic and exhibited suppressed gastric somatostatin mRNA expression. Reciprocal changes in gastrin and somatostatin expression are characteristic of Helicobacter infection or bacterial overgrowth in the stomach and this has been explained by elevation of IFNγ secreted by inflammatory cells within the mucosa[15,33]. With thioperamide treatment, both gastrin and somatostatin levels returned to baseline. In particular, thioperamide alone caused a significant increase in antral somatostatin mRNA expression. Antral somatostatin mRNA expression remained significantly higher compared to the untreated mice. Interestingly in our previous study, we show that somatostatin is both necessary and sufficient to prevent colonization and subsequent development of Helicobacter-induced gastritis[21]. Somatostatin is a known modulator of the immune system that is capable of down-regulating a number of functions including lymphocyte proliferation, IFNγ, TNFα and IL-1β production[25,34]. Perhaps the stimulation of somatostatin by thioperamide reduced the colonization of H. felis in addition to directly block the gastritis (Figure 9B). It is also important to note that H3Rs are located on inflammatory cells within the gastric mucosa of H. felis infected animals. This raises the question of whether Nα-MH is stimulating proliferation of the inflammatory cells and whether this response is blocked with thioperamide.
Most patients with peptic ulcer disease are currently treated with proton pump inhibitors or H2R antagonists. The long-term use of these compounds has been associated with potential problems such as ECL-cell hyperplasia, bacterial overgrowth and persistent inflammation of the gastric mucosa[15,35,36]. Thus, there is a rationale for the development of new antisecretory agents. The H3R antagonist thioperamide decreases acid secretion by elevating somatostatin release. The elevated somatostatin concentrations may be of benefit due to the potent inhibitory effect that somatostatin has on gastritis and bacterial colonization[21]. The therapeutic implications of H3R antagonists deserve further study.
Histamine is produced by enterochromaffin-like (ECL) cells and by mast cells in the setting of gastric inflammation such as observed with Helicobacter infection. Prior studies have shown that the histamine 3 receptor (H3R) is present on endocrine cells specifically on ECL cells. It is known that inhibitors of the H3R will inhibit acid secretion but the mechanism has been poorly understood. The current article demonstrates that blocking H3Rs will increase somatostatin which in turn can then suppress gastric acid secretion through a paracrine mechanism.
Important areas in this field are to better understand how inflammation in the stomach regulates parietal cell acid production. Also to identify new targets for the development of acid suppressing therapies given the emerging side effects with current proton pump inhibitors in particular.
Prior studies have focused on the cellular location of the H3R and the development of agonists and antagonists for this receptor. Apparently, antagonists inhibit acid secretion but the mechanism has not been well-described. Most of the studies have focused on the effect of H3R activation on ECL cells which have been reported to express 90% of the H3Rs. However, the study here shows that D and parietal cells as well as immune cells also express H3Rs. This novel finding should make one rethink the mechanism by which the H3R affects acid secretion, especially during Helicobacter infection.
Most human subjects with peptic ulcer, esophageal reflux disease or bleeding from the stomach are treated with H2R antagonists or proton pump inhibitors. However, chronic use of these drugs is now found to have side effects, e.g. fundic gland hyperplasia, bacterial overgrowth and metabolic disturbances such as hip fractures related to calcium absorption. Therefore, other mechanisms should be explored to block acid secretion such as increasing tissue levels of somatostatin that in turn would inhibit gastrin-producing (G) cells. The study here demonstrates that blocking the H3R can directly increase somatostatin and provide a different mechanism to block acid.
The manuscript by Zavros et al demonstrates an inhibitory effect of H3R activation induced by Helicobacter infection on gastric secretion in vivo and in vitro. The mechanism involves decreased expression of somatostatin and increased expression of gastrin. It can be accepted after minor revision.
Supported in part by Public Health Service Grants R37-DK 45729 (JLM), Michigan Gastrointestinal Peptide Research Center Pilot Feasibility Grant P30- DK34933 (YZ) and National Health and Medical Research Council of Australia Grant 350234 (AS)
Peer reviewers: Prabhakar Reddy Veerareddy, Professor and Principal, St. Peter’s Institute of Pharmaceutical Sciences, Vidyanagar, Hanamkonda, Warangal, Andhra Pradesh 506001, India; Sharon DeMorrow, PhD, Digestive Disease Research Center, Department of Internal Medicine, Scott and White Hospital and Texas A&M Health Science Center, 702 SW H.K Dodgen Loop, Temple, TX 76504, United States
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
| 1. | Coruzzi G, Morini G, Adami M, Grandi D. Role of histamine H3 receptors in the regulation of gastric functions. J Physiol Pharmacol. 2001;52:539-553. |
| 2. | Prinz C, Kajimura M, Scott DR, Mercier F, Helander HF, Sachs G. Histamine secretion from rat enterochromaffinlike cells. Gastroenterology. 1993;105:449-461. |
| 3. | Sobhani I, Canedo S, Alchepo B, Vissuzaine C, Chevalier C, Buyse M, Moizo L, Laigneau JP, Mignon M, Lewin JM. Putative effect of Helicobacter pylori and gastritis on gastric acid secretion in cat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G727-G734. |
| 4. | Bertaccini G, Coruzzi G. An update on histamine H3 receptors and gastrointestinal functions. Dig Dis Sci. 1995;40:2052-2063. |
| 5. | Courillon-Mallet A, Launay JM, Roucayrol AM, Callebert J, Emond JP, Tabuteau F, Cattan D: Helicobacter pylori infection: Physiopathologic implication of n alpha-methyl histamine. Gastroenterology. 1995;108:959-966. |
| 6. | Murray S, Taylor GW, Karim QN, Bliss P, Calam J: N alpha-methylhistamine: Association with helicobacter pylori infection in humans and effects on gastric acid secretion. Clin Chim Acta. 2000;301:181-192. |
| 7. | Soldani G, Mengozzi G, Intorre L, De Giorgi G, Coruzzi G, Bertaccini G. Histamine H3 receptor-mediated inhibition of gastric acid secretion in conscious dogs. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:61-65. |
| 8. | Coruzzi G, Bertaccini G, Schwartz JC. Evidence that histamine H3 receptors are involved in the control of gastric acid secretion in the conscious cat. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:225-227. |
| 9. | Vuyyuru L, Schubert ML. Histamine, acting via H3 receptors, inhibits somatostatin and stimulates acid secretion in isolated mouse stomach. Gastroenterology. 1997;113:1545-1552. |
| 10. | Vuyyuru L, Harrington L, Arimura A, Schubert ML. Reciprocal inhibitory paracrine pathways link histamine and somatostatin secretion in the fundus of the stomach. Am J Physiol. 1997;273:G106-G111. |
| 11. | Lee A, O’Rourke J, Ungria MCd, Robertson B, Daskalopoulos G, Dixon MF: A standardized mouse model of helicobacter pylori infection: Introducing the sydney strain. Gastroenterology. 1997;112:1386-1397. |
| 12. | Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536-542. |
| 13. | Zavros Y, Shulkes A. Cholecystokinin (CCK) regulates somatostatin secretion through both the CCK-A and CCK-B/gastrin receptors in sheep. J Physiol. 1997;505:811-821. |
| 14. | Reasbeck PG, Burns SM, Shulkes A. Calcitonin gene-related peptide: enteric and cardiovascular effects in the dog. Gastroenterology. 1988;95:966-971. |
| 15. | Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL. Genetic or chemical hypochlorhydria is associated with inflammation that modulates parietal and G-cell populations in mice. Gastroenterology. 2002;122:119-133. |
| 16. | Soll AH. The actions of secretagogues on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978;61:370-380. |
| 17. | Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem. 2005;280:15700-15708. |
| 18. | Soll AH, Amirian DA, Park J, Elashoff JD, Yamada T. Cholecystokinin potently releases somatostatin from canine fundic mucosal cells in short-term culture. Am J Physiol. 1985;248:G569-G573. |
| 19. | Soll AH, Yamada T, Park J, Thomas LP. Release of somatostatinlike immunoreactivity from canine fundic mucosal cells in primary culture. Am J Physiol. 1984;247:G558-G566. |
| 20. | Kaise M, Muraoka A, Yamada J, Yamada T. Epidermal growth factor induces H+,K+-ATPase alpha-subunit gene expression through an element homologous to the 3’ half-site of the c-fos serum response element. J Biol Chem. 1995;270:18637-18642. |
| 21. | Zavros Y, Rathinavelu S, Kao JY, Todisco A, Del Valle J, Weinstock JV, Low MJ, Merchant JL. Treatment of Helicobacter gastritis with IL-4 requires somatostatin. Proc Natl Acad Sci USA. 2003;100:12944-12949. |
| 22. | Vuyyuru L, Schubert ML, Harrington L, Arimura A, Makhlouf GM: Dual inhibitory pathways link antral somatostatin and histamine secretion in human, dog , and rat stomach. Gastroenterology. 1995;109:1566. |
| 23. | Yamashita K, Kaneko H, Yamamoto S, Konagaya T, Kusugami K, Mitsuma T: Inhibitory effect of somatostatin on helicobacter pylori proliferation in vitro. Gastroenterology. 1998;115:1123-1130. |
| 24. | Krantic S. Peptides as regulators of the immune system: Emphasis on somatostatin. Peptides. 2000;21:1941-1964. |
| 25. | Muscettola M, Grasso G. Neuropeptide modulation of interferon gamma production. Int J Neurosci. 1990;51:189-191. |
| 26. | Zavros Y, Fleming WR, Hardy KJ, Shulkes A. Regulation of fundic and antral somatostatin secretion by CCK and gastrin. Am J Physiol. 1998;274:G742-G750. |
| 27. | Zavros Y, Fleming WR, Shulkes A. Concurrent elevation of fundic somatostatin prevents gastrin stimulation by GRP. Am J Physiol. 1999;276:G21-G27. |
| 28. | McColl KE, el-Omar E, Gillen D. Interactions between H. pylori infection, gastric acid secretion and anti-secretory therapy. Br Med Bull. 1998;54:121-138. |
| 29. | El-Omar EM, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, Williams C, Ardill JE, McColl KE: Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology. 1997;113:15-24. |
| 30. | Queiroz DMM, Moura SB, Mendes EN, Rocha GA, Barbosa AJA, Carvalho AST: Effect of helicobacter pylori eradication of g-cell and d-cell density in children. Lancet. 1994;343:1191-1193. |
| 31. | Beales IL, Calam J. Effect of N alpha-methyl-histamine on acid secretion in isolated cultured rabbit parietal cells: implications for Helicobacter pylori associated gastritis and gastric physiology. Gut. 1997;40:14-19. |
| 32. | Schubert ML, Saffouri B, Makhlouf GM: Identical patterns of somatostatin secretion from isolated antrum and fundus of rat stomach. Am J Physiol. 1988;254:G20-G24. |
| 33. | Beales I, Calam J, Post L, Srinivasan S, Yamada T, DelValle J: Effect of transforming growth factor alpha and interleukin 8 on somatostatin release from canine fundic d cells. Gastroenterology. 1997;112:136-143. |
| 34. | Chowers Y, Cahalon L, Lahav M, Schor H, Tal R, Bar-Meir S, Levite M. Somatostatin through its specific receptor inhibits spontaneous and TNF-alpha- and bacteria-induced IL-8 and IL-1 beta secretion from intestinal epithelial cells. J Immunol. 2000;165:2955-2961. |
| 35. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. |