Published online Apr 15, 2010. doi: 10.4291/wjgp.v1.i1.3
Revised: March 18, 2010
Accepted: March 25, 2010
Published online: April 15, 2010
The ability of cells to interact with extracellular matrix macromolecules is at the forefront of the regulation of cell phenotype and organization. Indeed most if not all cells bear specific cell surface receptors for these molecules, namely the integrins, which are specific for the ligation of various macromolecules such as the laminins, fibronectins and tenascins. It is now well established that integrins can regulate a variety of biological activities, most notably cell cycle and tissue-specific gene expression. In the intestine, several observations suggest functional roles for cell-matrix interactions in the regulation of epithelial cell functions. This article focuses on integrin α6β4 as a paradigm to illustrate the importance as well as the complexity of integrins in the mediation of cell-matrix interactions. Indeed, α6β4 has been well-characterized for its involvement as a link between the cytoskeleton and extracellular matrix molecules as well as in the activation of a variety of intracellular signalization processes in cooperation with growth factor receptors. Furthermore, recent studies show that distinct forms of α6 and β4 subunits are expressed in the human intestine and, more importantly, recent work provides experimental evidence that various forms of α6β4 can differentially regulate intestinal epithelial cell functions under both normal and pathological conditions. For instance, it has been discovered that colorectal cancer cells express a hybrid form of α6β4 that is never seen in normal cells. Although further work is needed, integrin α6β4 is emerging as a key regulator of intestinal functions in both intestinal health and disease.
- Citation: Beaulieu JF. Integrin α6β4 in colorectal cancer. World J Gastrointest Pathophysiol 2010; 1(1): 3-11
- URL: https://www.wjgnet.com/2150-5330/full/v1/i1/3.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v1.i1.3
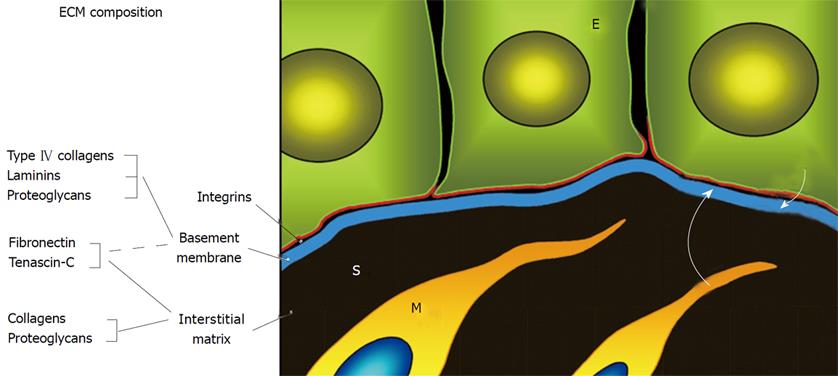
The digestive epithelium is a highly organized tissue that requires only 3 to 5 d to be completely replaced. The regulation of its renewal and expression of digestive and absorptive functions depends on a number of factors[1-3]. Among these, the extracellular matrix macromolecules such as the fibronectins, tenascins and laminins play an important role[1-5]. As for other epithelia, the intestinal epithelium lies on a specialized thin extracellular matrix, the basement membrane (BM) (Figure 1). BM composition defines the microenvironment required for the expression of cell functions such as proliferation, migration, tissue-specific gene expression and apoptosis[6]. Indeed, cell-matrix interactions are mediated by a variety of membrane receptors, many of which are members of the integrin superfamily[7-9].
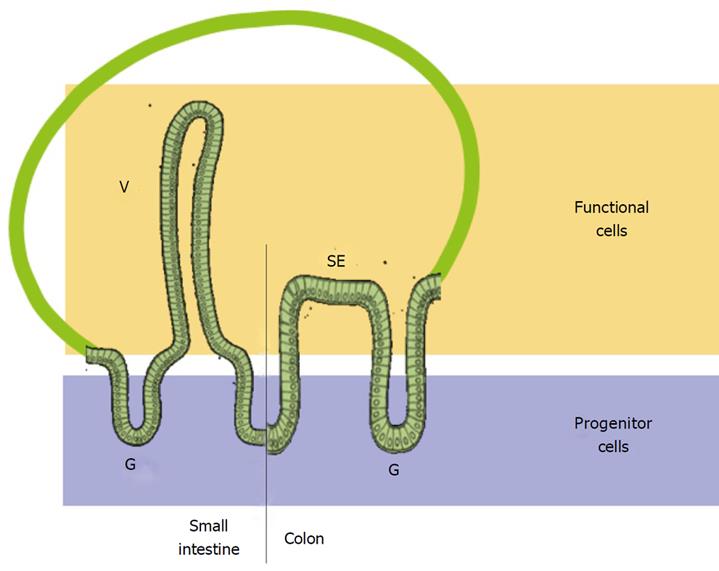
The intestinal epithelium is a particularly attractive system for deciphering the role of cell-matrix interactions in the regulation of cell functions. In both the normal small and large intestine, the epithelial renewal unit consists of spatially well-separated progenitor and functional cell populations[2,5,10,11]: the crypt-villus axis in the small intestine and the glandular-surface epithelium axis in the colon (Figure 2). Analysis of the expression patterns of integrins and their ligands along the epithelial renewal units of the healthy and pathologic human small intestine and colon has provided crucial basic information on the potential implication of each of these molecules relative to cell state and has identified fundamental differences between the human and rodents[3-5,12-15]. Used in concert with the well-characterized experimental human cell models that replicate the intestinal cell life cycle throughout the epithelial renewal unit[16], these data have led to significant progress in our understanding of the implications of cell-matrix interactions on the regulation of intestinal cell functions.
The human intestinal BM contains all major macromolecules typical of basal lamina such as the type IV collagens and laminins as well as other non-exclusive components such as the fibronectins[4,13]. An important feature of the intestinal BM is that its composition varies considerably along the renewal unit[5,17-31]. For instance, two functionally important laminins[32], LM-211 and LM-511, are subject to a reciprocal pattern of expression along the small intestinal crypt-villus axis, being restricted to the proliferative and differentiated compartments, respectively[25]. Other laminins are also subject to unique spatial and temporal patterns of expression[28,30,31]. Interestingly, patterns of laminin expression are altered in various intestinal pathologies including the chronic inflammatory bowel diseases[33] and cancer[34,35] (see[5] for a review). Taken together, these observations suggest functional roles for these interactions in the regulation of intestinal cell functions.
The biological activities of BM macromolecules depend on the repertoire of specific receptors expressed by the cells involved. Numerous receptors for extracellular matrix molecules have been identified but the integrins are considered to be the main mediators of cell-matrix interactions[8,9]. Indeed, the integrins act as fully functional membrane receptors that can trigger cytoskeletal rearrangements and a variety of intracellular signaling events leading to changes in gene expression[36-38]. Integrins are a superfamily of transmembrane αβ heterodimer glycoproteins that represent a major and ubiquitous class of receptors. There are at least a dozen of them that can interact with extracellular matrix molecules, of which many are expressed by human intestinal epithelial cells[4,5,12-14]. As for their ligands, integrin expression is highly regulated along the intestinal epithelial renewal unit. For instance, the α2β1 and α7β1 integrins are predominantly expressed in the proliferative cells of the glands[18] and newly differentiated cells[39], respectively. Further examples involve other β1 integrins such as α5β1, α8β1 and α9β1[18,40-43].
Surprisingly, α6β4, one of the best characterized integrins involved in the regulation of many cell functions such as proliferation, migration and survival in both health and disease[36,44-47], was initially found to be uniformly distributed at the base of epithelial cells from the bottom of the glands to the tip of the small intestinal villus and the surface epithelium of the colon[25,48,49]. The difficulty in interpreting the ubiquitous expression of α6β4 arose from the existence of splicing variants for both α6 (α6A-B) and β4 (β4A-E)[50] and proteolytically processed forms[51,52]. As reviewed herein, recent studies have shown that distinct forms of the α6 and β4 subunits are expressed in the intestine and, more importantly, recent work provides experimental evidence that various forms of α6β4 can differentially regulate intestinal epithelial cell functions under both normal and pathological conditions.
The α6β4 integrin is expressed at the base of most epithelial cells where it serves as a laminin receptor[53-55]. This integrin is considered to be an exception among the integrins in both structural and functional aspects. One of the most peculiar features of α6β4 is the atypically long cytoplasmic domain of its β subunit, which is involved in the formation of hemidesmosomes[56] as well as in complex signaling functions[57,58].
The cytoplasmic domain of β4 can interact with the keratin network via plectin to initiate the formation of hemidesmosomes, which are specialized structures that mediate the attachment of epithelial cells to laminins in the BM[47,59-61]. Although structurally complex, hemidesmosomes are dynamic structures that can rapidly disassemble under specific circumstances such as cell division or migration[62,63]. Cellular α6β4 redistribution[64,65] appears to be regulated by phosphorylation in response to growth factor stimulation[44,59] and involves interaction of α6β4 with the actin cytoskeletal network[45,66,67].
The signaling functions of α6β4 have received much attention. Indeed, its β subunit behaves as a binary tyrosine kinase receptor. Signal transduction is mediated by the activation of a member of the Src kinase family which combines with the juxtamembrane segment of the β4 cytoplasmic domain[64]. Then, Src kinase phosphorylates 5 major tyrosine phosphorylation sites located in the signalling domain of β4[68,69]. Phosphorylation of tyrosine 1526 mediates the recruitment of the adaptor protein Shc and activation of the Ras-MEK-Erk pathway[68] as well as the PI3-K pathway and its targets including Akt, Rac and mTOR[70-72] while phosphorylation of tyrosine 1440 induces the recruitment of Shp2 phosphatase which favours the activation of the Src kinase Shp2[73]. Furthermore, phosphorylation of serines 1356, 1360 and 1364 by PKC is involved in the disassembly of hemidesmosomes[74], the recruitment of the 14-3-3 proteins and the association with Ron[75]. Cooperation with growth factor receptors appears to play an important role in β4 signalization[37]. Indeed, α6β4 can associate with several tyrosine kinase receptors such as EGF, ErbB2, Met and Ron[64,75-77], which once activated, lead to the phosphorylation of the cytoplasmic domain of β4. Conversely, α6β4 can promote the phosphorylation of associated tyrosine kinase receptors via Src activation[73,76]. Interestingly, both series of signals appear to be necessary to generate a sustained intracellular response suggesting that in normal cells, ligation of α6β4 to laminin is required for amplifying the signal generated by the tyrosine receptor kinases. In tumor cells, receptor tyrosine kinases are often mutated or amplified and α6β4 is frequently over-expressed. Cooperation between deregulated β4 and receptor tyrosine kinases could contribute to tumoral growth and invasion[64,77-79].
Taken together, these data suggest that the α6β4 integrin plays an important role in normal cells where it is involved in the formation of hemidesmosomes as well in the regulation of a variety of intracellular signalization processes. In tumor cells, cooperation of over-expressed α6β4 with various growth factor receptors enhances signals leading to the promotion of cellular events linked to tumor progression. However, fundamental questions such as the precise mechanisms involved remain open[44,45,47].
A better understanding of how α6β4 functions under both normal and pathological conditions would have significant impact on the diagnosis and/or treatment of epithelium-related diseases. Among these, cancers in general and more particularly colorectal cancers, which is one of the major causes of death by cancer[80,81], appear the most prominent pathologies. However, until recently, α6β4 was considered to be expressed ubiquitously in the human intestinal epithelium and its up- or down-regulation in colorectal cancer was controversial[34,35,82,83]. The data obtained in our laboratory over the last few years have led to a different concept. Indeed, the discovery of distinct forms of the α6β4 integrin, which are functionally distinct and differentially expressed in relation to the cell state, suggests an additional level of complexity for this integrin.
Although important, the intrinsic signalling potential of β4 appears to be less than the complete α6β4 heterodimer, suggesting that the α6 subunit has a more important role than thought initially[84-89]. The α6 subunit is involved in signalling by different ways including via the association with proteins such as CD151[87,90], other proteins that interact with its GFFKR motif[89] or with its PDZ domain at the C-terminal end[84,88].
The α6 integrin mRNA undergoes alternative splicing to yield two distinct isoforms[91] termed α6A and α6B that exhibit distinct cytoplasmic domains and dissimilar spatial and temporal patterns of tissue expression[92-96]. For instance, α6A is found in the mammary gland and in basal keratinocytes while α6B is the predominant variant in the kidney[93]. These distinct patterns of expression for α6A and α6B have been conserved in many species[50]. Their importance is also suggested from work showing the distinct capacity of the two variants to initiate intracellular signalling events[97,98] and the ability to migrate onto laminin[99] when associated with the β1 integrin subunit; the α6Aβ1 integrin being considered to be the "active" variant relative to α6Bβ1[97-101].
Until recently, nothing was known of the importance of α6Aβ4 and α6Bβ4. Based on the fact that α6 only dimerizes with β4 in intestinal cells[102,103] and that both α6 variants are expressed in this tissue[93], the intestinal epithelium was used to characterize any functional differences between the α6Aβ4 and α6Bβ4 integrins. First, distinct patterns of expression of the α6A and α6B variants were found in the normal intestine. In both the small intestine and colon, proliferative cells of the crypt were found to predominantly express α6A while the differentiated cells of the villus, the Paneth cells, as well as the upper gland and surface epithelial cells of the colon were found to express α6B[103,104]. A similar relationship was observed in intestinal experimental cell models. Second, in addition to an upregulation of the total amounts of α6 subunit, a predominant expression of α6A in relation to α6B was found in both primary colon tumors and adenocarcinoma cell lines suggesting that the enhanced α6A/α6B ratios may be linked to the proliferative status of colon cancer cells[103]. Further studies have shown that manipulating the cellular balance of the two α6 variants can alter cell proliferation and influence transcriptional activities related to cell proliferation but not differentiation[103,104]. More specifically, the data suggest that a predominant expression of α6A could favour cancer cell proliferation by a) directly activating the TCF4/β-catenin pathway[104], a well-documented pathway for colorectal cancer progression[105-108] and b) competing with the inhibitory effect of α6B on cell proliferation and c-Myc activity[103].
The specific abilities of α6A to activate pathways linked to cell growth and of α6B to inhibit proliferation are in accordance with their predominant expression in the proliferative and quiescent compartments, respectively, of both the normal small intestine and colon. Up-regulation of α6A expression in primary colon cancers and adenocarcinoma cell lines strongly suggests that the expression and ratio of the α6A and α6B splice variants are inherent to normal intestinal homeostasis and exploited by colon cancer cells.
As mentioned above, the β4 subunit, which is ubiquitously expressed by epithelial cells[12,46,55,109], possesses an unusual β integrin cytoplasmic domain. As for its partner α6, cytoplasmic splice variants have been described but these minor forms of β4 remain poorly characterized compared to the ubiquitous β4A form[50]. On the other hand, a cytoplasmic variant of the β4A subunit that results from the proteolytic cleavage of the C-terminal domain (ctd) has been identified in the human intestinal epithelium[102]. Interestingly, this β4ctd- variant was found to be associated with the proliferative/undifferentiated cells of the crypts in both the small intestine and colon while the non-cleaved β4ctd+ form was only detected in the differentiated cells of the villus and upper gland/surface epithelium in the small intestine and colon, respectively[102,110]. Furthermore, the α6β4ctd- form was not functional for adhesion on purified laminin-332, one of the preferred ligands for α6β4[102].
In colorectal cancer, an overall up-regulation of the expression of the β4 subunit has been found in relation to c-Myc in the primary tumors but the β4ctd- form was lost in both tumors and adenocarcinoma cell lines[110]. Based on the fact that a mutation in β4 generating a deletion of this ctd domain is lethal in man[111], its function appears to be crucial. However, at present, the only potential role for the ctd domain is to self-attach to the connecting segment, a region located between the two pairs of type III fibronectin-like domains of the cytoplasmic β4 forming a loop[112,113] and/or to serve as a binding site for plectin[111,114]. The β4ctd- form, which generates an inactive α6β4 integrin for adhesion, appears to be an exclusive feature of normal proliferative intestinal cells. Its expression as a β4ctd+ form in differentiated normal intestinal cells as well as in adenocarcinoma cells thus raises fundamental questions. For instance, can the fact that the α6β4ctd- integrin is inactive for adhesion be linked to the possibility that the ctd domain is required to maintain a functional conformation of the integrin? While this hypothesis appears to be compatible with the various proposed models of α6β4[47,115-117], further work is needed to verify it. Another interesting challenge would be the identification of the precise mechanism involved in the intracellular cleavage of ctd. Indeed, the characterization of a hypothetical "β4ctd-ase" that could impair tumor growth and promotion may provide an interesting clue in the development of an anti-colorectal cancer therapy.
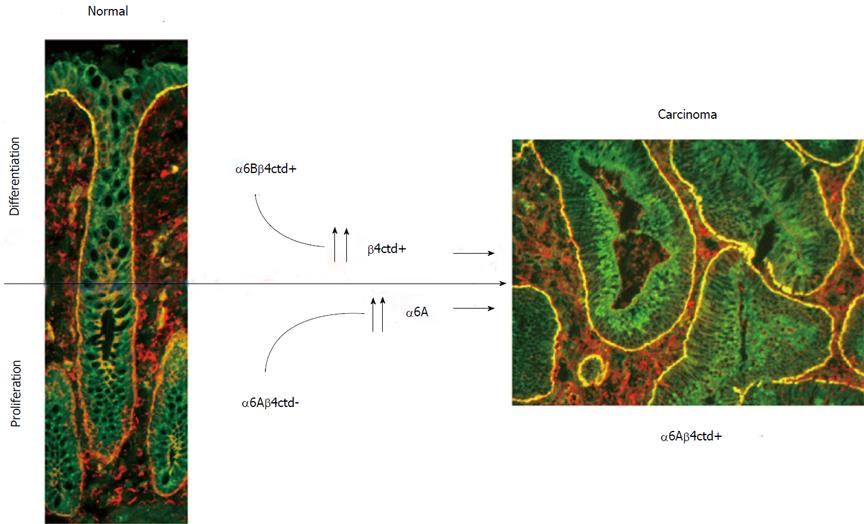
The complexity of the integrin α6β4 has only begun to be unravelled. The α6 and β4 subunits are both up-regulated in various tumor types including colorectal cancer and play key roles in the major intracellular signaling networks. Furthermore, the characterization of cytoplasmic variants for both subunits has revealed new elements to be considered in the equation. Indeed, as illustrated in Figure 3, these findings show on one hand that the α6β4 integrin is present under the α6Aβ4ctd- form (pro-proliferative but not functional for adhesion) in normal proliferative intestinal cells and under the α6Bβ4ctd+ form (anti-proliferative but functional for adhesion) in quiescent and differentiated intestinal cells. In the other hand, in human colorectal adenocarcinoma cells, the predominant form is α6Aβ4ctd+ (pro-proliferative and functional for adhesion), a hybrid form that is never seen in normal cells.
A better understanding of how α6β4 and its variants function under normal and pathological conditions, such as during the tumor progression process, would have significant impact on the diagnosis and treatment of many epithelial cancers including colorectal cancers.
Peer reviewers: Atsushi Nakajima, MD, Professor, Gastroenterology Division, Yokohama City University Hospital, 3-9 Fukuura, Kanazawaku, Yokohama, Kanagawa 236, Japan; Qing Zhu, MD, PhD, NIH/NIAID, 10/11N104, 10 Center Dr., Bethesda, MD 20814, United States
| 1. | Babyatsky MW, Podolsky DK. Growth and development of the gastrointestinal tract. Textbook of gastroenterology. 3rd ed. Philadelphia: JB Lippincott 1999; 547-584. |
| 2. | Ménard D, Beaulieu JF, Boudreau F, Perreault N, Rivard N, Vachon PH. Gastrointestinal tract. Cell Siganling and Growth Factors in Development: From molecules to Organogenesis. Weinheim: Wiley-Vch 2006; 755-790. |
| 3. | Montgomery RK, Mulberg AE, Grand RJ. Development of the human gastrointestinal tract: twenty years of progress. Gastroenterology. 1999;116:702-731. |
| 4. | Beaulieu JF. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog Histochem Cytochem. 1997;31:1-78. |
| 5. | Teller I, Beaulieu JF. Interactions between laminin and epithelial cells in intestinal health and disease. Exp Rev Mol Med. 2001;28:1-18. |
| 7. | Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816-826. |
| 8. | Tarone G, Hirsch E, Brancaccio M, De Acetis M, Barberis L, Balzac F, Retta SF, Botta C, Altruda F, Silengo L. Integrin function and regulation in development. Int J Dev Biol. 2000;44:725-731. |
| 9. | Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;201:632-641. |
| 10. | Kedinger M. Growth and development of intestinal mucosa. Small bowel enterocyte culture and transplantation. Austin TX: RG Landes Co 1994; 1-31. |
| 11. | Ménard D, Beaulieu JF. Human intestinal brush border membrane hydrolases. Membrane Physiopathology. Norwell: Kluwer Academic Publisher 1994; 319-341. |
| 12. | Beaulieu JF. Integrins and human intestinal cell functions. Front Biosci. 1999;4:D310-D321. |
| 13. | Beaulieu JF. Role of extracellular matrix proteins on human intestinal cell function: Laminin-epithelial cell interactions. Gastrointestinal Functions. Philadelphia: Vevey/Lippincott Williams & Wilkins 2001; 59-75. |
| 14. | Lussier C, Basora N, Bouatrouss Y, Beaulieu JF. Integrins as mediators of epithelial cell-matrix interactions in the human small intestinal mucosa. Microsc Res Tech. 2000;51:169-178. |
| 15. | Kedinger M, Lefebvre O, Duluc I, Freund JN, Simon-Assmann P. Cellular and molecular partners involved in gut morphogenesis and differentiation. Philos Trans R Soc Lond B Biol Sci. 1998;353:847-856. |
| 16. | Pageot LP, Perreault N, Basora N, Francoeur C, Magny P, Beaulieu JF. Human cell models to study small intestinal functions: recapitulation of the crypt-villus axis. Microsc Res Tech. 2000;49:394-406. |
| 17. | Aufderheide E, Ekblom P. Tenascin during gut development: appearance in the mesenchyme, shift in molecular forms, and dependence on epithelial-mesenchymal interactions. J Cell Biol. 1988;107:2341-2349. |
| 18. | Beaulieu JF. Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J Cell Sci. 1992;102:427-436. |
| 19. | Beaulieu JF, Jutras S, Durand J, Vachon PH, Perreault N. Relationship between tenascin and alpha-smooth muscle actin expression in the developing human small intestinal mucosa. Anat Embryol (Berl). 1993;188:149-158. |
| 20. | Beaulieu JF, Jutras S, Kusakabe M, Perreault N. Expression of tenascin in the developing human small intestine. Biochem Biophys Res Commun. 1993;192:1086-1092. |
| 21. | Beaulieu JF, Vachon PH, Chartrand S. Immunolocalization of extracellular matrix components during organogenesis in the human small intestine. Anat Embryol (Berl). 1991;183:363-369. |
| 22. | Probstmeier R, Martini R, Schachner M. Expression of J1/tenascin in the crypt-villus unit of adult mouse small intestine: implications for its role in epithelial cell shedding. Development. 1990;109:313-321. |
| 23. | Quaroni A, Isselbacher KJ, Ruoslahti E. Fibronectin synthesis by epithelial crypt cells of rat small intestine. Proc Natl Acad Sci USA. 1978;75:5548-5552. |
| 24. | Bélanger I, Beaulieu JF. Tenascin in the developing and adult human intestine. Histol Histopathol. 2000;15:577-585. |
| 25. | Beaulieu JF, Vachon PH. Reciprocal expression of laminin A-chain isoforms along the crypt-villus axis in the human small intestine. Gastroenterology. 1994;106:829-839. |
| 26. | Beaulieu JF, Vachon PH, Herring-Gillam FE, Simoneau A, Perreault N, Asselin C, Durand J. Expression of the alpha-5(IV) collagen chain in the fetal human small intestine. Gastroenterology. 1994;107:957-967. |
| 27. | Perreault N, Herring-Gillam FE, Desloges N, Bélanger I, Pageot LP, Beaulieu JF. Epithelial vs mesenchymal contribution to the extracellular matrix in the human intestine. Biochem Biophys Res Commun. 1998;248:121-126. |
| 28. | Perreault N, Vachon PH, Beaulieu JF. Appearance and distribution of laminin A chain isoforms and integrin alpha 2, alpha 3, alpha 6, beta 1, and beta 4 subunits in the developing human small intestinal mucosa. Anat Rec. 1995;242:242-250. |
| 29. | Simoneau A, Herring-Gillam FE, Vachon PH, Perreault N, Basora N, Bouatrouss Y, Pageot LP, Zhou J, Beaulieu JF. Identification, distribution, and tissular origin of the alpha5(IV) and alpha6(IV) collagen chains in the developing human intestine. Dev Dyn. 1998;212:437-447. |
| 30. | Teller IC, Auclair J, Herring E, Gauthier R, Ménard D, Beaulieu JF. Laminins in the developing and adult human small intestine: relation with the functional absorptive unit. Dev Dyn. 2007;236:1980-1990. |
| 31. | Virtanen I, Gullberg D, Rissanen J, Kivilaakso E, Kiviluoto T, Laitinen LA, Lehto VP, Ekblom P. Laminin alpha1-chain shows a restricted distribution in epithelial basement membranes of fetal and adult human tissues. Exp Cell Res. 2000;257:298-309. |
| 32. | Vachon PH, Beaulieu JF. Extracellular heterotrimeric laminin promotes differentiation in human enterocytes. Am J Physiol. 1995;268:G857-G867. |
| 33. | Bouatrouss Y, Herring-Gillam FE, Gosselin J, Poisson J, Beaulieu JF. Altered expression of laminins in Crohn’s disease small intestinal mucosa. Am J Pathol. 2000;156:45-50. |
| 34. | Lohi J, Oivula J, Kivilaakso E, Kiviluoto T, Fröjdman K, Yamada Y, Burgeson RE, Leivo I, Virtanen I. Basement membrane laminin-5 is deposited in colorectal adenomas and carcinomas and serves as a ligand for alpha3beta1 integrin. APMIS. 2000;108:161-172. |
| 35. | Sordat I, Bosman FT, Dorta G, Rousselle P, Aberdam D, Blum AL, Sordat B. Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J Pathol. 1998;185:44-52. |
| 37. | Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173-206. |
| 39. | Basora N, Vachon PH, Herring-Gillam FE, Perreault N, Beaulieu JF. Relation between integrin alpha7Bbeta1 expression in human intestinal cells and enterocytic differentiation. Gastroenterology. 1997;113:1510-1521. |
| 40. | Basora N, Desloges N, Chang Q, Bouatrouss Y, Gosselin J, Poisson J, Sheppard D, Beaulieu JF. Expression of the alpha9beta1 integrin in human colonic epithelial cells: resurgence of the fetal phenotype in a subset of colon cancers and adenocarcinoma cell lines. Int J Cancer. 1998;75:738-743. |
| 41. | Desloges N, Basora N, Perreault N, Bouatrouss Y, Sheppard D, Beaulieu JF. Regulated expression of the integrin alpha9beta1 in the epithelium of the developing human gut and in intestinal cell lines: relation with cell proliferation. J Cell Biochem. 1998;71:536-545. |
| 42. | Vachon PH, Simoneau A, Herring-Gillam FE, Beaulieu JF. Cellular fibronectin expression is down-regulated at the mRNA level in differentiating human intestinal epithelial cells. Exp Cell Res. 1995;216:30-34. |
| 43. | Benoit YD, Lussier C, Ducharme PA, Sivret S, Schnapp LM, Basora N, Beaulieu JF. Integrin alpha8beta1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK-dependent mechanism. Biol Cell. 2009;101:695-708. |
| 44. | Giancotti FG. Targeting integrin beta4 for cancer and anti-angiogenic therapy. Trends Pharmacol Sci. 2007;28:506-511. |
| 45. | Lipscomb EA, Mercurio AM. Mobilization and activation of a signaling competent alpha6beta4integrin underlies its contribution to carcinoma progression. Cancer Metast Rev. 2005;24:413-423. |
| 46. | Mercurio AM, Rabinovitz I, Shaw LM. The alpha 6 beta 4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13:541-545. |
| 47. | Wilhelmsen K, Litjens SH, Sonnenberg A. Multiple functions of the integrin alpha6beta4 in epidermal homeostasis and tumorigenesis. Mol Cell Biol. 2006;26:2877-2886. |
| 48. | Leivo I, Tani T, Laitinen L, Bruns R, Kivilaakso E, Lehto VP, Burgeson RE, Virtanen I. Anchoring complex components laminin-5 and type VII collagen in intestine: association with migrating and differentiating enterocytes. J Histochem Cytochem. 1996;44:1267-1277. |
| 49. | Simon-Assmann P, Duclos B, Orian-Rousseau V, Arnold C, Mathelin C, Engvall E, Kedinger M. Differential expression of laminin isoforms and alpha 6-beta 4 integrin subunits in the developing human and mouse intestine. Dev Dyn. 1994;201:71-85. |
| 50. | de Melker AA, Sonnenberg A. Integrins: alternative splicing as a mechanism to regulate ligand binding and integrin signaling events. Bioessays. 1999;21:499-509. |
| 51. | Giancotti FG, Stepp MA, Suzuki S, Engvall E, Ruoslahti E. Proteolytic processing of endogenous and recombinant beta 4 integrin subunit. J Cell Biol. 1992;118:951-959. |
| 52. | Potts AJ, Croall DE, Hemler ME. Proteolytic cleavage of the integrin beta 4 subunit. Exp Cell Res. 1994;212:2-9. |
| 53. | Mercurio AM. Laminin receptors: achieving specificity through cooperation. Trends Cell Biol. 1995;5:419-423. |
| 54. | Sonnenberg A. Laminin receptors in the integrin family. Pathol Biol (Paris). 1992;40:773-778. |
| 55. | Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919-3926. |
| 56. | Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res. 2007;313:2189-2203. |
| 57. | Hogervorst F, Kuikman I, dem Borne AE, Sonnenberg A. Cloning and sequence analysis of beta-4 cDNA: an integrin subunit that contains a unique 118 kd cytoplasmic domain. EMBO J. 1990;9:765-770. |
| 58. | Suzuki S, Naitoh Y. Amino acid sequence of a novel integrin beta 4 subunit and primary expression of the mRNA in epithelial cells. EMBO J. 1990;9:757-763. |
| 59. | Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16:376-383. |
| 60. | Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559-572. |
| 61. | van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet. 1996;13:366-369. |
| 62. | Geuijen CA, Sonnenberg A. Dynamics of the alpha6beta4 integrin in keratinocytes. Mol Biol Cell. 2002;13:3845-3858. |
| 63. | Tsuruta D, Hopkinson SB, Jones JC. Hemidesmosome protein dynamics in live epithelial cells. Cell Motil Cytoskeleton. 2003;54:122-134. |
| 64. | Mariotti A, Kedeshian PA, Dans M, Curatola AM, Gagnoux-Palacios L, Giancotti FG. EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol. 2001;155:447-458. |
| 65. | Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion--lessons from the alpha6beta 4 integrin. Semin Cancer Biol. 2001;11:129-141. |
| 66. | Rabinovitz I, Mercurio AM. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997;139:1873-1884. |
| 67. | Rabinovitz I, Toker A, Mercurio AM. Protein kinase C-dependent mobilization of the alpha6beta4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol. 1999;146:1147-1160. |
| 68. | Dans M, Gagnoux-Palacios L, Blaikie P, Klein S, Mariotti A, Giancotti FG. Tyrosine phosphorylation of the beta 4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J Biol Chem. 2001;276:1494-1502. |
| 69. | Shaw LM. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the alpha6beta4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol Cell Biol. 2001;21:5082-5093. |
| 70. | Chung J, Bachelder RE, Lipscomb EA, Shaw LM, Mercurio AM. Integrin (alpha 6 beta 4) regulation of eIF-4E activity and VEGF translation: a survival mechanism for carcinoma cells. J Cell Biol. 2002;158:165-174. |
| 71. | Russell AJ, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, Dey CN, Guide S, Weaver VM, Marinkovich MP. Alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. J Cell Sci. 2003;116:3543-3456. |
| 72. | Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949-960. |
| 73. | Bertotti A, Comoglio PM, Trusolino L. Beta4 integrin activates a Shp2-Src signaling pathway that sustains HGF-induced anchorage-independent growth. J Cell Biol. 2006;175:993-1003. |
| 74. | Rabinovitz I, Tsomo L, Mercurio AM. Protein kinase C-alpha phosphorylation of specific serines in the connecting segment of the beta 4 integrin regulates the dynamics of type II hemidesmosomes. Mol Cell Biol. 2004;24:4351-4360. |
| 75. | Santoro MM, Gaudino G, Marchisio PC. The MSP receptor regulates alpha6beta4 and alpha3beta1 integrins via 14-3-3 proteins in keratinocyte migration. Dev Cell. 2003;5:257-271. |
| 76. | Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489-502. |
| 77. | Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell. 2001;107:643-654. |
| 78. | Falcioni R, Antonini A, Nisticò P, Di Stefano S, Crescenzi M, Natali PG, Sacchi A. Alpha 6 beta 4 and alpha 6 beta 1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp Cell Res. 1997;236:76-85. |
| 79. | Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949-960. |
| 80. | Markowitz SD, Dawson DM, Willis J, Willson JK. Focus on colon cancer. Cancer Cell. 2002;1:233-236. |
| 81. | Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544-573. |
| 82. | Falcioni R, Turchi V, Vitullo P, Navarra G, Ficari F, Cavaliere F, Sacchi A, Mariani-Costantini R. Integrin beta4 expression in colorectal cancer. Int J Oncol. 1994;5:573-578. |
| 83. | Stallmach A, von Lampe B, Matthes H, Bornhoft G, Riecken EO. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malign tumour transformation. Gut. 1992;33:342-346. |
| 84. | El Mourabit H, Poinat P, Koster J, Sondermann H, Wixler V, Wegener E, Laplantine E, Geerts D, Georges-Labouesse E, Sonnenberg A. The PDZ domain of TIP-2/GIPC interacts with the C-terminus of the integrin alpha5 and alpha6 subunits. Matrix Biol. 2002;21:207-214. |
| 85. | Lammerding J, Kazarov AR, Huang H, Lee RT, Hemler ME. Tetraspanin CD151 regulates alpha6beta1 integrin adhesion strengthening. Proc Natl Acad Sci USA. 2003;100:7616-7621. |
| 86. | Merdek KD, Yang X, Taglienti CA, Shaw LM, Mercurio AM. Intrinsic signaling functions of the beta4 integrin intracellular domain. J Biol Chem. 2007;282:30322-30330. |
| 87. | Sincock PM, Fitter S, Parton RG, Berndt MC, Gamble JR, Ashman LK. PETA-3/CD151, a member of the transmembrane 4 superfamily, is localised to the plasma membrane and endocytic system of endothelial cells, associates with multiple integrins and modulates cell function. J Cell Sci. 1999;112:833-844. |
| 88. | Tani TT, Mercurio AM. PDZ interaction sites in integrin alpha subunits. T14853, TIP/GIPC binds to a type I recognition sequence in alpha 6A/alpha 5 and a novel sequence in alpha 6B. J Biol Chem. 2001;276:36535-36542. |
| 89. | Wixler V, Laplantine E, Geerts D, Sonnenberg A, Petersohn D, Eckes B, Paulsson M, Aumailley M. Identification of novel interaction partners for the conserved membrane proximal region of alpha-integrin cytoplasmic domains. FEBS Lett. 1999;445:351-355. |
| 90. | Lammerding J, Kazarov AR, Huang H, Lee RT, Hemler ME. Tetraspanin CD151 regulates alpha6beta1 integrin adhesion strengthening. Proc Natl Acad Sci USA. 2003;100:7616-7621. |
| 91. | Hogervorst F, Kuikman I, van Kessel AG, Sonnenberg A. Molecular cloning of the human alpha 6 integrin subunit. Alternative splicing of alpha 6 mRNA and chromosomal localization of the alpha 6 and beta 4 genes. Eur J Biochem. 1991;199:425-433. |
| 92. | de Curtis I, Reichardt LF. Function and spatial distribution in developing chick retina of the laminin receptor alpha 6 beta 1 and its isoforms. Development. 1993;118:377-388. |
| 93. | Hogervorst F, Admiraal LG, Niessen C, Kuikman I, Janssen H, Daams H, Sonnenberg A. Biochemical characterization and tissue distribution of the A and B variants of the integrin alpha 6 subunit. J Cell Biol. 1993;121:179-191. |
| 94. | Segat D, Comai R, Di Marco E, Strangio A, Cancedda R, Franzi AT, Tacchetti C. Integrins alpha(6A)beta 1 and alpha(6B)beta 1 promote different stages of chondrogenic cell differentiation. J Biol Chem. 2002;277:31612-31622. |
| 95. | Thorsteinsdóttir S, Roelen BA, Freund E, Gaspar AC, Sonnenberg A, Mummery CL. Expression patterns of laminin receptor splice variants alpha 6A beta 1 and alpha 6B beta 1 suggest different roles in mouse development. Dev Dyn. 1995;204:240-258. |
| 96. | Thorsteinsdóttir S, Roelen BA, Goumans MJ, Ward-van Oostwaard D, Gaspar AC, Mummery CL. Expression of the alpha 6A integrin splice variant in developing mouse embryonic stem cell aggregates and correlation with cardiac muscle differentiation. Differentiation. 1999;64:173-184. |
| 97. | Shaw LM, Turner CE, Mercurio AM. The alpha 6A beta 1 and alpha 6B beta 1 integrin variants signal differences in the tyrosine phosphorylation of paxillin and other proteins. J Biol Chem. 1995;270:23648-23652. |
| 98. | Wei J, Shaw LM, Mercurio AM. Regulation of mitogen-activated protein kinase activation by the cytoplasmic domain of the alpha6 integrin subunit. J Biol Chem. 1998;273:5903-5907. |
| 99. | Shaw LM, Mercurio AM. Regulation of alpha 6 beta 1 integrin-mediated migration in macrophages. Agents Actions Suppl. 1995;47:101-106. |
| 100. | Ferletta M, Kikkawa Y, Yu H, Talts JF, Durbeej M, Sonnenberg A, Timpl R, Campbell KP, Ekblom P, Genersch E. Opposing roles of integrin alpha6Abeta1 and dystroglycan in laminin-mediated extracellular signal-regulated kinase activation. Mol Biol Cell. 2003;14:2088-2103. |
| 101. | Gimond C, Baudoin C, van der Neut R, Kramer D, Calafat J, Sonnenberg A. Cre-loxP-mediated inactivation of the alpha6A integrin splice variant in vivo: evidence for a specific functional role of alpha6A in lymphocyte migration but not in heart development. J Cell Biol. 1998;143:253-266. |
| 102. | Basora N, Herring-Gillam FE, Boudreau F, Perreault N, Pageot LP, Simoneau M, Bouatrouss Y, Beaulieu JF. Expression of functionally distinct variants of the beta(4)A integrin subunit in relation to the differentiation state in human intestinal cells. J Biol Chem. 1999;274:29819-29825. |
| 103. | Dydensborg AB, Teller IC, Groulx JF, Basora N, Paré F, Herring E, Gauthier R, Jean D, Beaulieu JF. Integrin alpha6Bbeta4 inhibits colon cancer cell proliferation and c-Myc activity. BMC Cancer. 2009;9:223. |
| 104. | Dydensborg AB, Teller IC, Basora N, Groulx JF, Auclair J, Francoeur C, Escaffit F, Paré F, Herring E, Ménard D. Differential expression of the integrins alpha6Abeta4 and alpha6Bbeta4 along the crypt-villus axis in the human small intestine. Histochem Cell Biol. 2009;131:531-536. |
| 105. | Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150-158. |
| 106. | Gavert N, Ben-Ze’ev A. beta-Catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820-828. |
| 108. | Huang D, Du X. Crosstalk between tumor cells and microenvironment via Wnt pathway in colorectal cancer dissemination. World J Gastroenterol. 2008;14:1823-1827. |
| 109. | Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411-418. |
| 110. | Ni H, Dydensborg AB, Herring FE, Basora N, Gagné D, Vachon PH, Beaulieu JF. Upregulation of a functional form of the beta4 integrin subunit in colorectal cancers correlates with c-Myc expression. Oncogene. 2005;24:6820-6809. |
| 111. | Nakano A, Pulkkinen L, Murrell D, Rico J, Lucky AW, Garzon M, Stevens CA, Robertson S, Pfendner E, Uitto J. Epidermolysis bullosa with congenital pyloric atresia: novel mutations in the beta 4 integrin gene (ITGB4) and genotype/phenotype correlations. Pediatr Res. 2001;49:618-626. |
| 112. | Schaapveld RQ, Borradori L, Geerts D, van Leusden MR, Kuikman I, Nievers MG, Niessen CM, Steenbergen RD, Snijders PJ, Sonnenberg A. Hemidesmosome formation is initiated by the beta4 integrin subunit, requires complex formation of beta4 and HD1/plectin, and involves a direct interaction between beta4 and the bullous pemphigoid antigen 180. J Cell Biol. 1998;142:271-284. |
| 113. | Koster J, van Wilpe S, Kuikman I, Litjens SH, Sonnenberg A. Role of binding of plectin to the integrin beta4 subunit in the assembly of hemidesmosomes. Mol Biol Cell. 2004;15:1211-1223. |
| 114. | Rezniczek GA, de Pereda JM, Reipert S, Wiche G. Linking integrin alpha6beta4-based cell adhesion to the intermediate filament cytoskeleton: direct interaction between the beta4 subunit and plectin at multiple molecular sites. J Cell Biol. 1998;141:209-225. |
| 115. | Margadant C, Frijns E, Wilhelmsen K, Sonnenberg A. Regulation of hemidesmosome disassembly by growth factor receptors. Curr Opin Cell Biol. 2008;20:589-596. |
| 116. | Clarke AS, Lotz MM, Chao C, Mercurio AM. Activation of the p21 pathway of growth arrest and apoptosis by the beta 4 integrin cytoplasmic domain. J Biol Chem. 1995;270:22673-22676. |
| 117. | Spinardi L, Einheber S, Cullen T, Milner TA, Giancotti FG. A recombinant tail-less integrin beta 4 subunit disrupts hemidesmosomes, but does not suppress alpha 6 beta 4-mediated cell adhesion to laminins. J Cell Biol. 1995;129:473-487. |