Copyright
©The Author(s) 2024.
World J Gastrointest Pathophysiol. Aug 22, 2024; 15(4): 93606
Published online Aug 22, 2024. doi: 10.4291/wjgp.v15.i4.93606
Published online Aug 22, 2024. doi: 10.4291/wjgp.v15.i4.93606
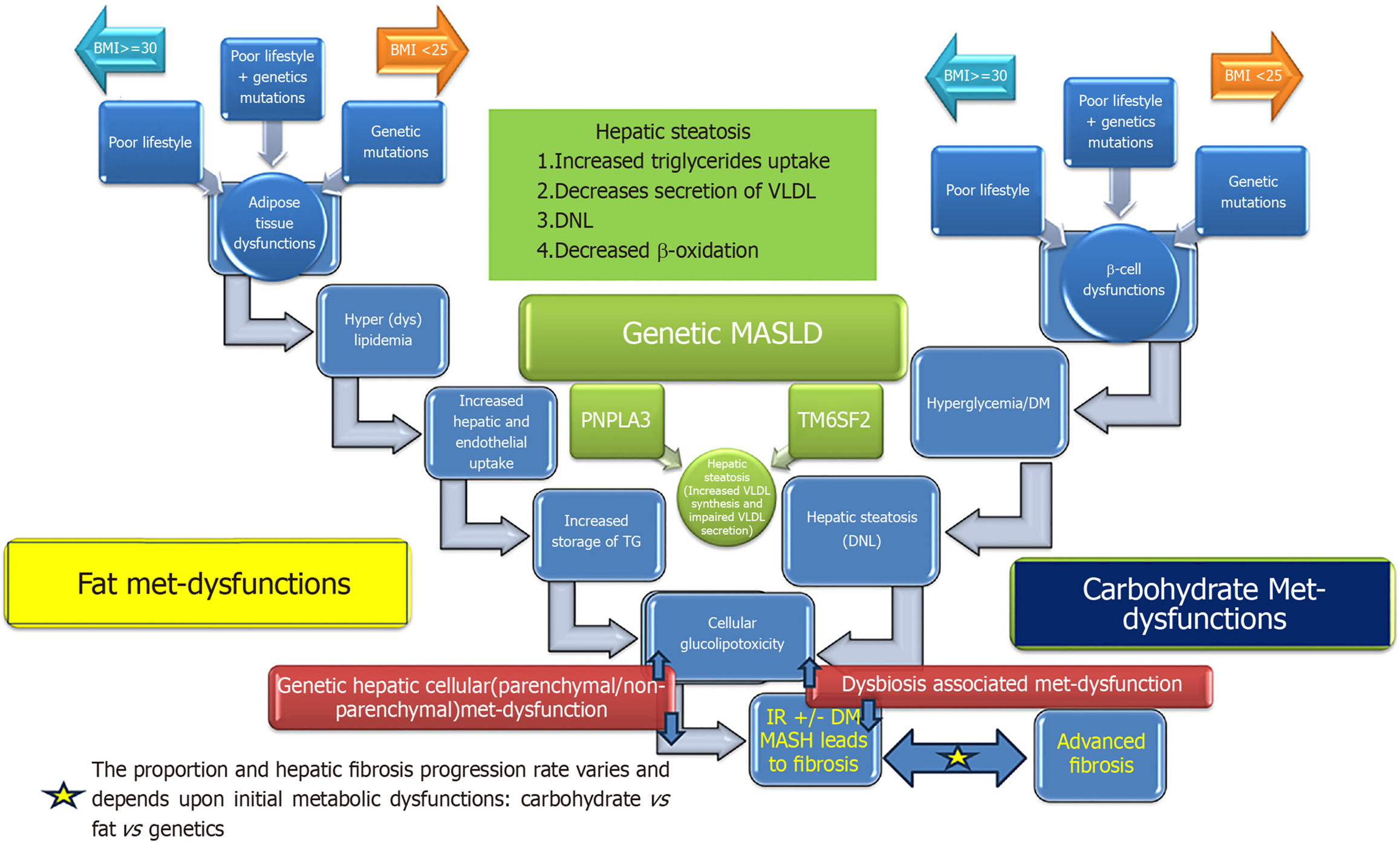
Figure 1 Pathogenetic mechanisms causing metabolic dysfunction-associated steatotic liver disease[254].
DNL: De novo lipogenesis; TG: Triglyceride. Citation: Habib S. Metabolic dysfunction-associated steatotic liver disease heterogeneity: Need of subtyping. World J Gastrointest Pathophysiol 2024; 15: 92791. Copyright © The Baishideng Publishing Group 2024. Published by Baishideng Publishing Group.
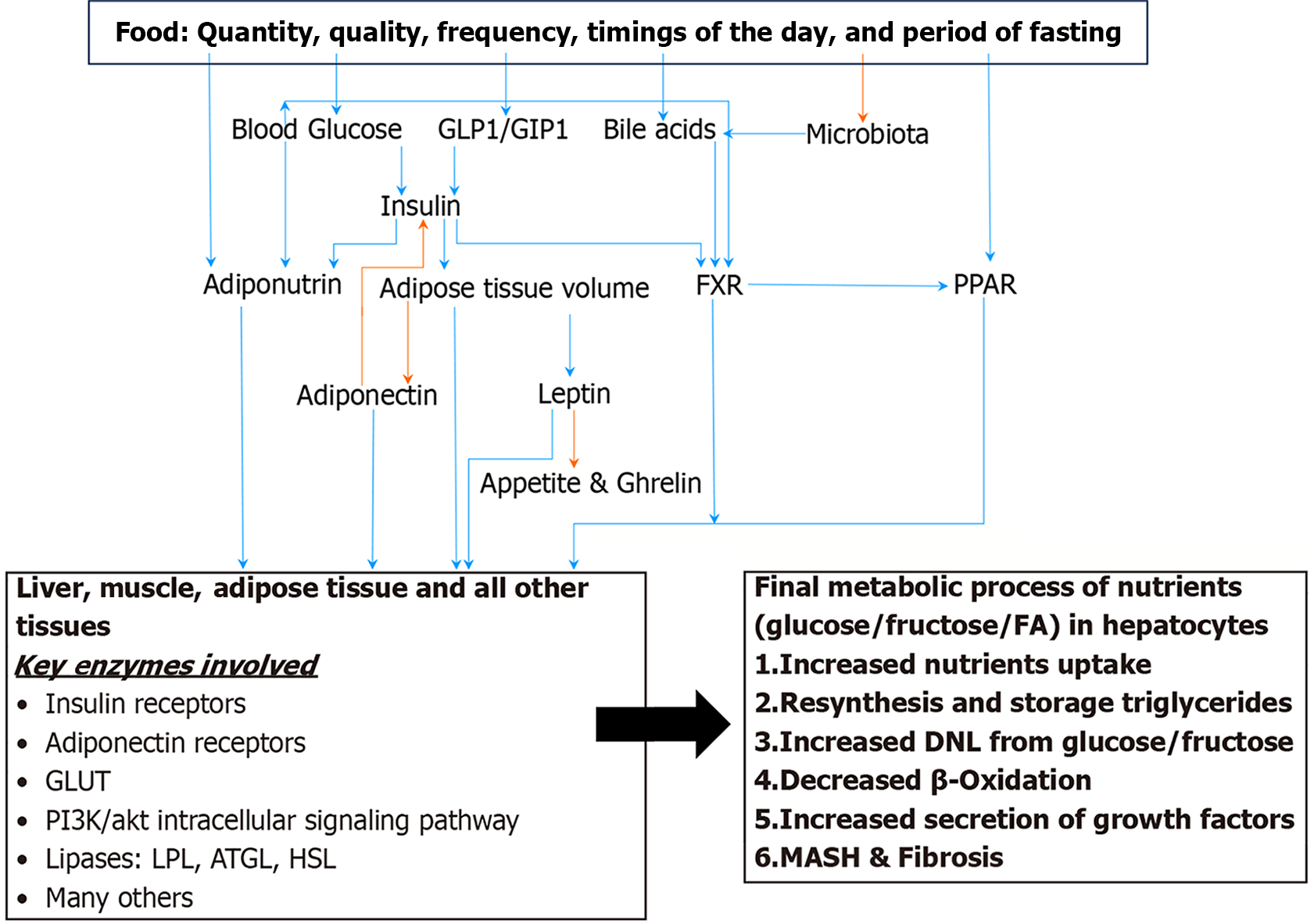
Figure 2 The key modulators of carbohydrate and fat metabolism.
Orange arrow indicates negative relationship and blue arrow indicates positive relationship[254]. FA: Fatty acid; GLP1: Glucagon like peptide 1; GIP1: Gastric inhibitory peptide 1; FXR: Farnesoid X receptor; PPAR: Peroxisome proliferator-activated receptor; GLUT: Glucose transporters; LPL: Lipoprotein lipase; ATGL: Adipose triglycerides lipase; HSL: Hormone sensitive lipase. Citation: Habib S. Metabolic dysfunction-associated steatotic liver disease heterogeneity: Need of subtyping. World J Gastrointest Pathophysiol 2024; 15: 92791. Copyright © The Baishideng Publishing Group 2024. Published by Baishideng Publishing Group.
Figure 3 Pathophysiology of gut-liver-axis[31].
The microbiome sets the stage for the gut-liver-axis, representing an excessive source of bacterial products and metabolites in terms of both quantity and diversity. In conditions of increased intestinal permeability, the epithelial barrier is crossed more than in healthy conditions by bacterial products (lipopolysaccharides, peptidoglycans, bacterial DNA, flagellin etc.), which stimulate the gut-associated lymphatic tissue to release pro-inflammatory cytokines (TNF, IL1, IL6 etc.), chemokines, as well as eicosanoids, leading to portal-venous P/MAMP- and cytokinemia. Moreover, bacterial metabolites (trimethylamine, ethanol and other volatile organoids, fatty acids, acetaldehyde etc.) increasingly permeate the epithelial barrier. Anything crossing the epithelial barrier faces the gut-vascular barrier, which determines the likely rate and size of molecules entering the portal-venous circulation. The intrahepatic effects of this portal-venous inflow of stimulants, as well as platelets on Kupffer cells and hepatic stellate cells, drives inflammation, fibrogenesis and carcinogenesis. LPS: Lipopolysaccharide; HSC: Hepatic stellate cell; MCP-1: Monocyte chemoattractant protein-1; CDCA: Chenodeoxycholic acid. Citation: Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol 2017; 67: 1084-1103. Copyright © European Association for the Study of the Liver 2017. Published by Elsevier B.V.
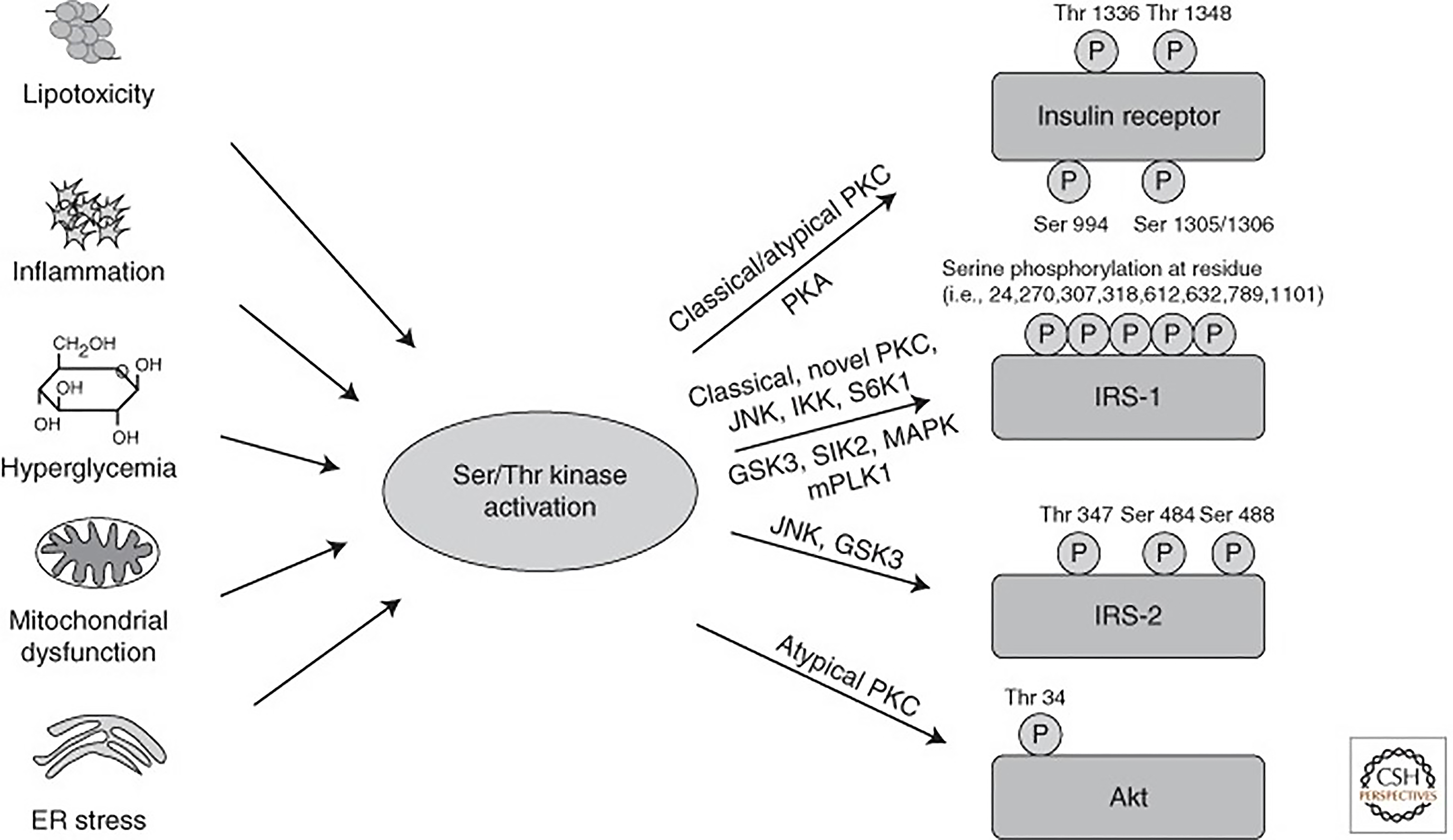
Figure 4 Activation of Ser/Thr kinases causes inhibitory phosphorylation on insulin-signaling molecules[35].
Lipotoxicity, inflammation, hyperglycemia, and subsequently oxidative stress, as well as mitochondrial dysfunction and endoplasmic reticulum stress, all converge on activation of Ser/Thr kinases, inducing inhibitory Ser/Thr phosphorylation of insulin receptor, insulin receptor substrate proteins, and Akt on multiple residues, causing insulin resistance. PKC: Protein kinase C; PKA: Protein kinase A; JNK: c-Jun N-terminal kinase; IKK: Inhibitor of nuclear factor-κB kinase; GSK: Glycogen synthase kinase 3. Citation: Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 2014; 6. Copyright © The Cold Spring Harbor Laboratory Press 2014. Published by Cold Spring Harbor Laboratory Press.
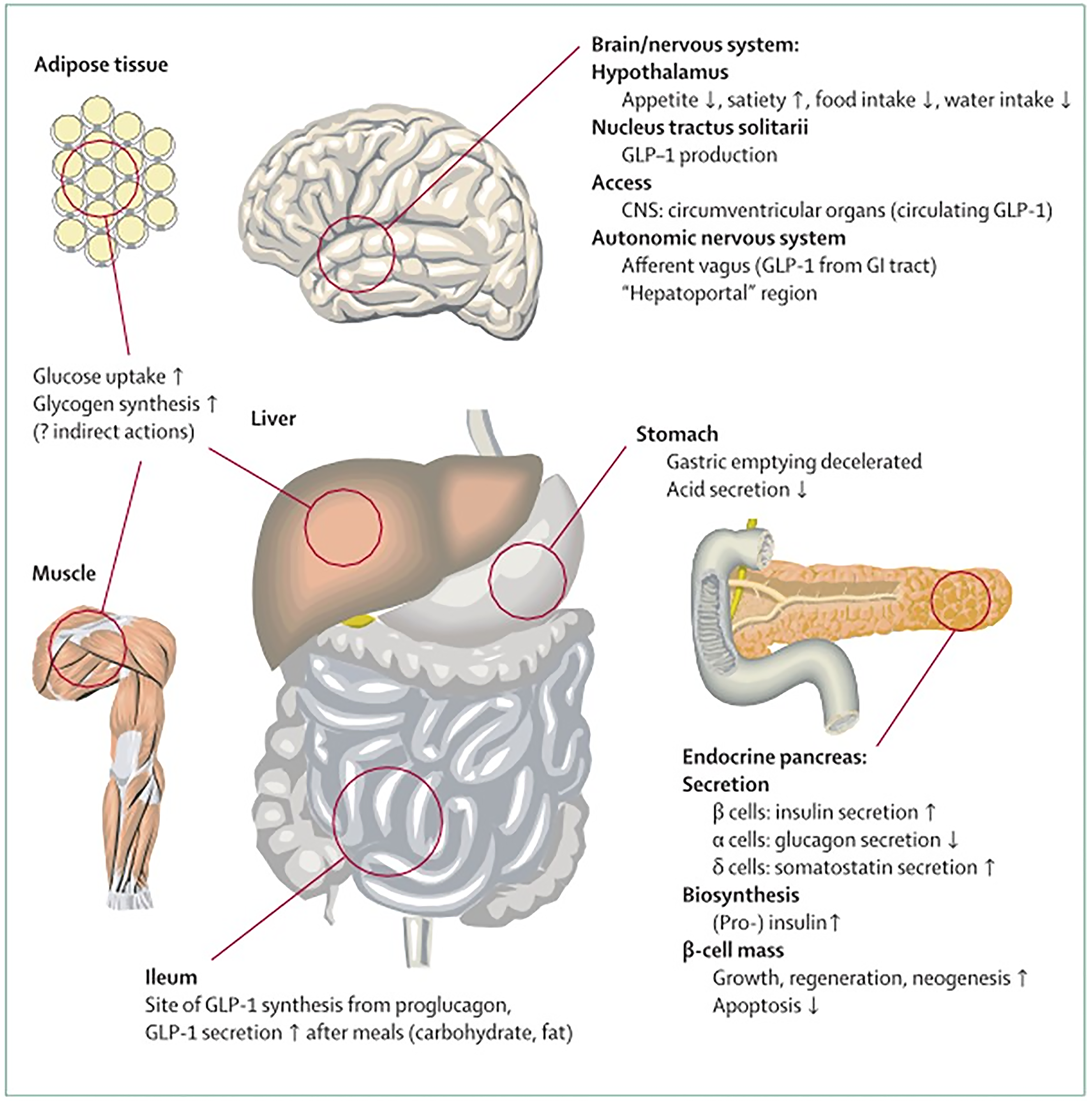
Figure 5
Physiology of GLP-1 secretion and action on GLP-1 receptors in different organs and tissues[255].
Citation: Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696-1705. Copyright © The Elsevier Ltd 2006. Published by Elsevier Ltd.
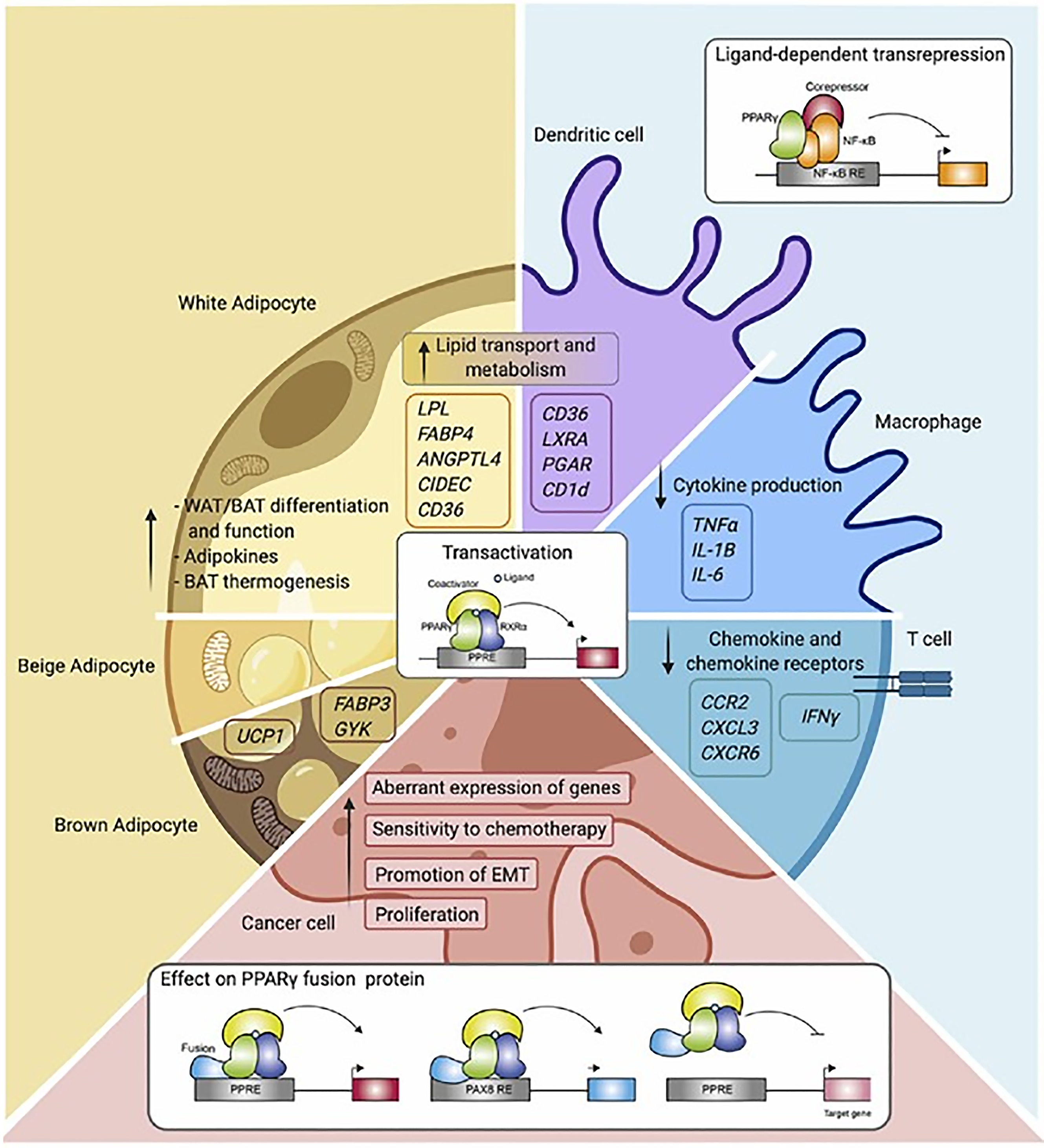
Figure 6 Overview of PPARγ function and mechanisms in the different cell types[256].
Schematic representation of PPARγ in adipocytes (white, beige, and brown adipocytes), immune cells (macrophages, dendritic cells, and T cells), and cancer cells. Indicated are different cellular processes and mechanisms of action in which PPARγ is involved. WAT: White adipose tissue; BAT: Brown adipose tissue; TNF: Tumor necrosis factor-alpha; IL: Interleukin; IFN-γ: Interferon gamma. Citation: Hernandez-Quiles M, Broekema MF, Kalkhoven E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front Endocrinol (Lausanne) 2021; 12: 624112 Copyright © Hernandez-Quiles, Broekema and Kalkhoven. Published by Hernandez-Quiles, Broekema and Kalkhoven.
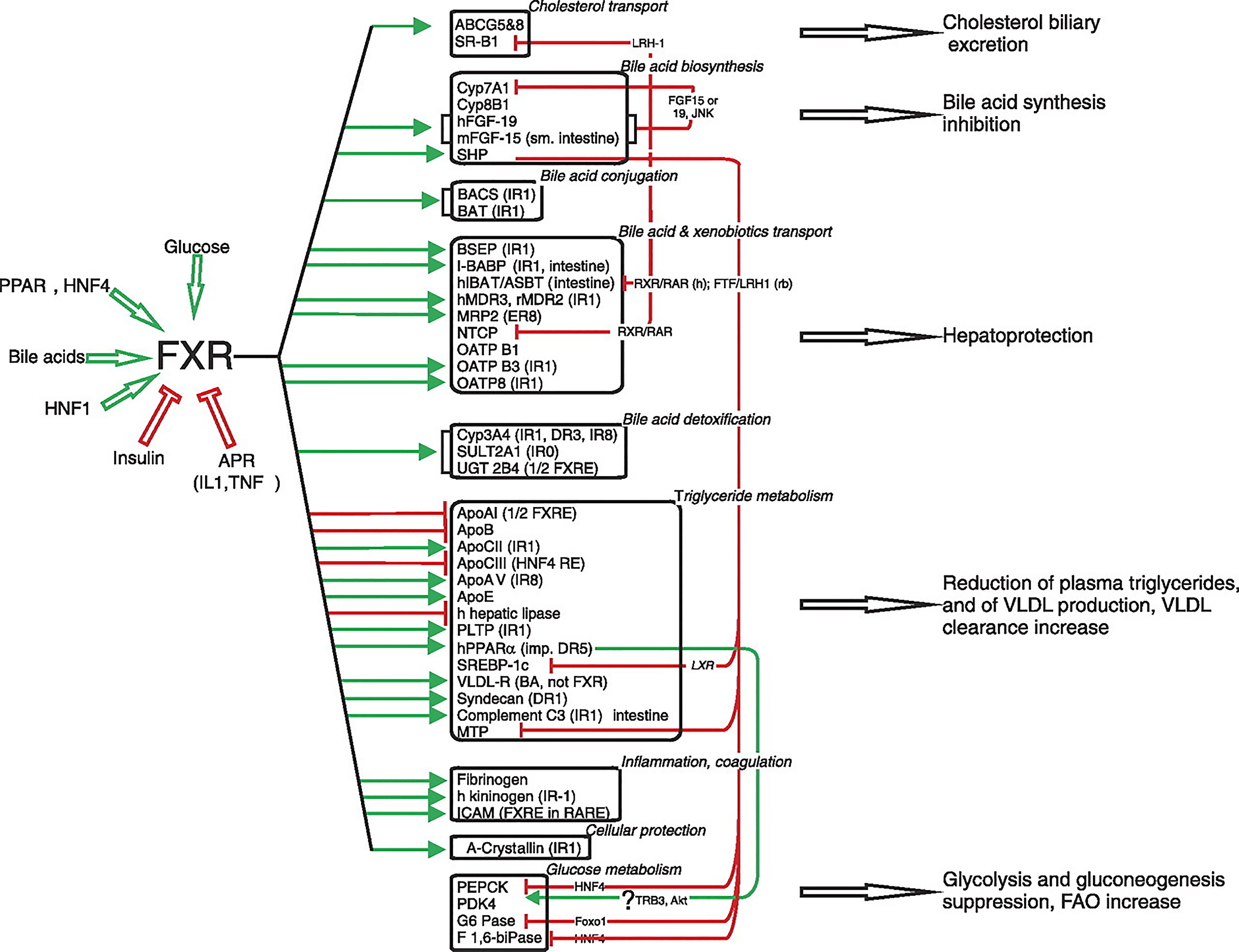
Figure 7 The FXR-controlled gene regulation network.
The main biological functions regulated by FXR are indicated on the right[117]. Genes upregulated by FXR are indicated by green arrows, and those that are downregulated by red arrows. Indirect regulation modes are indicated on the right, underlining the important role of SHP in this pathway. When known, the intermediate signaling molecule or the component(s) of the signaling cascade are indicated. Species-specific regulation is mentioned when known (h, human; Rb, rabbit). When known, the type of FXRE is indicated. IR: Inverted repeat; DR: Direct repeat; ER: Everted repeat. Citation: Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 2009; 89: 147-191. Copyright © The American Physiological Society 2009. Published by The American Physiological Society.
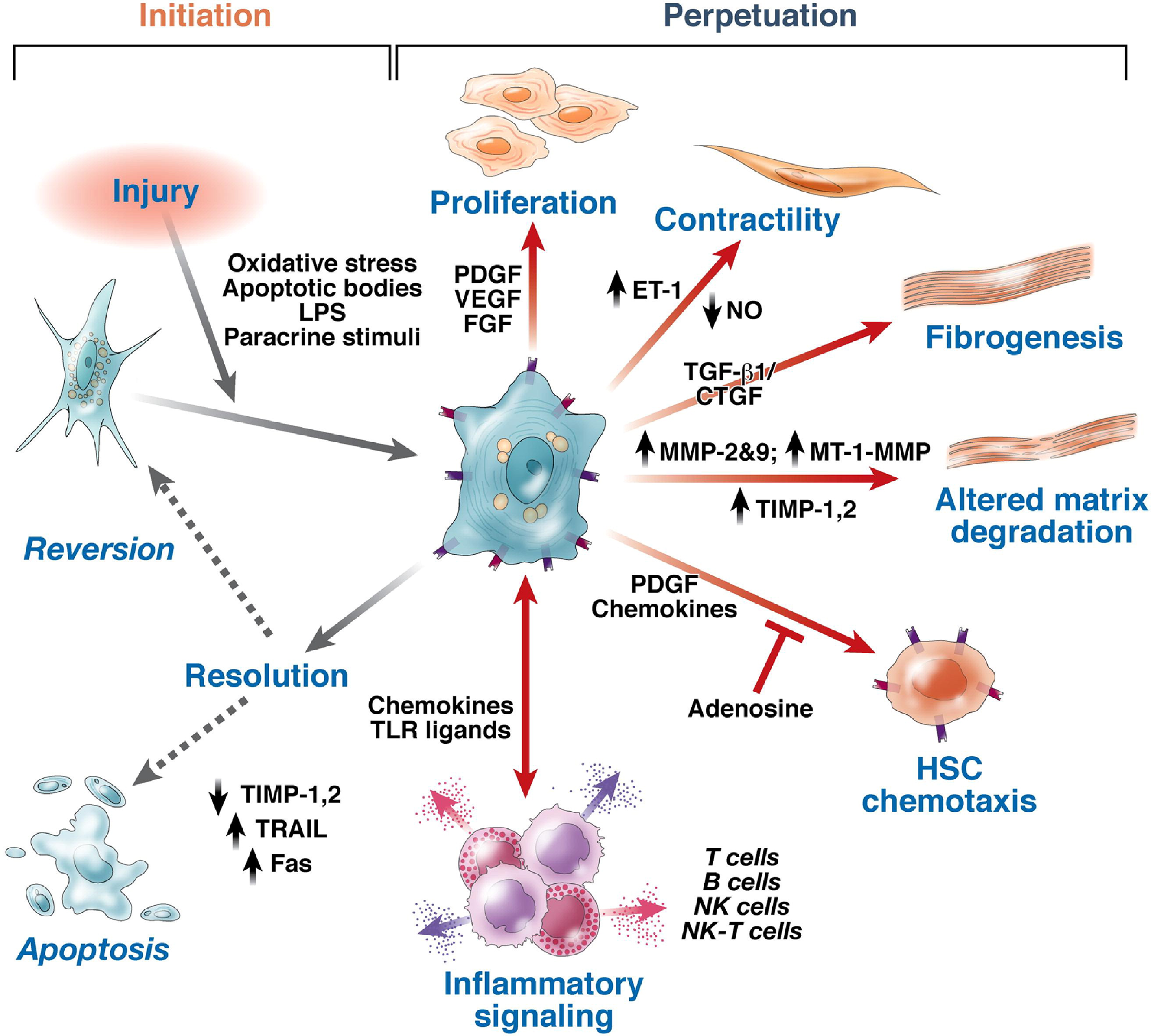
Figure 8 Pathways of hepatic stellate cell activation[139].
Features of stellate cell activation can be distinguished between those that stimulate initiation and those that contribute to perpetuation. Initiation is provoked by soluble stimuli that include oxidant stress signals (reactive oxygen intermediates), apoptotic bodies, lipopolysaccharide, and paracrine stimuli from neighboring cell types including hepatic macrophages (Kupffer cells), sinusoidal endothelium, and hepatocytes. Perpetuation follows, characterized by a number of specific phenotypic changes including proliferation, contractility, fibrogenesis, altered matrix degradation, chemotaxis, and inflammatory signaling. FGF: Fibroblast growth factor; ET-1: Endothelin-1; NK: Natural killer; NO: Nitric oxide; MT: Membrane type. Citation: Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008; 134: 1655-1669. Copyright © AGA Institute 2008. Published by Elsevier Inc.
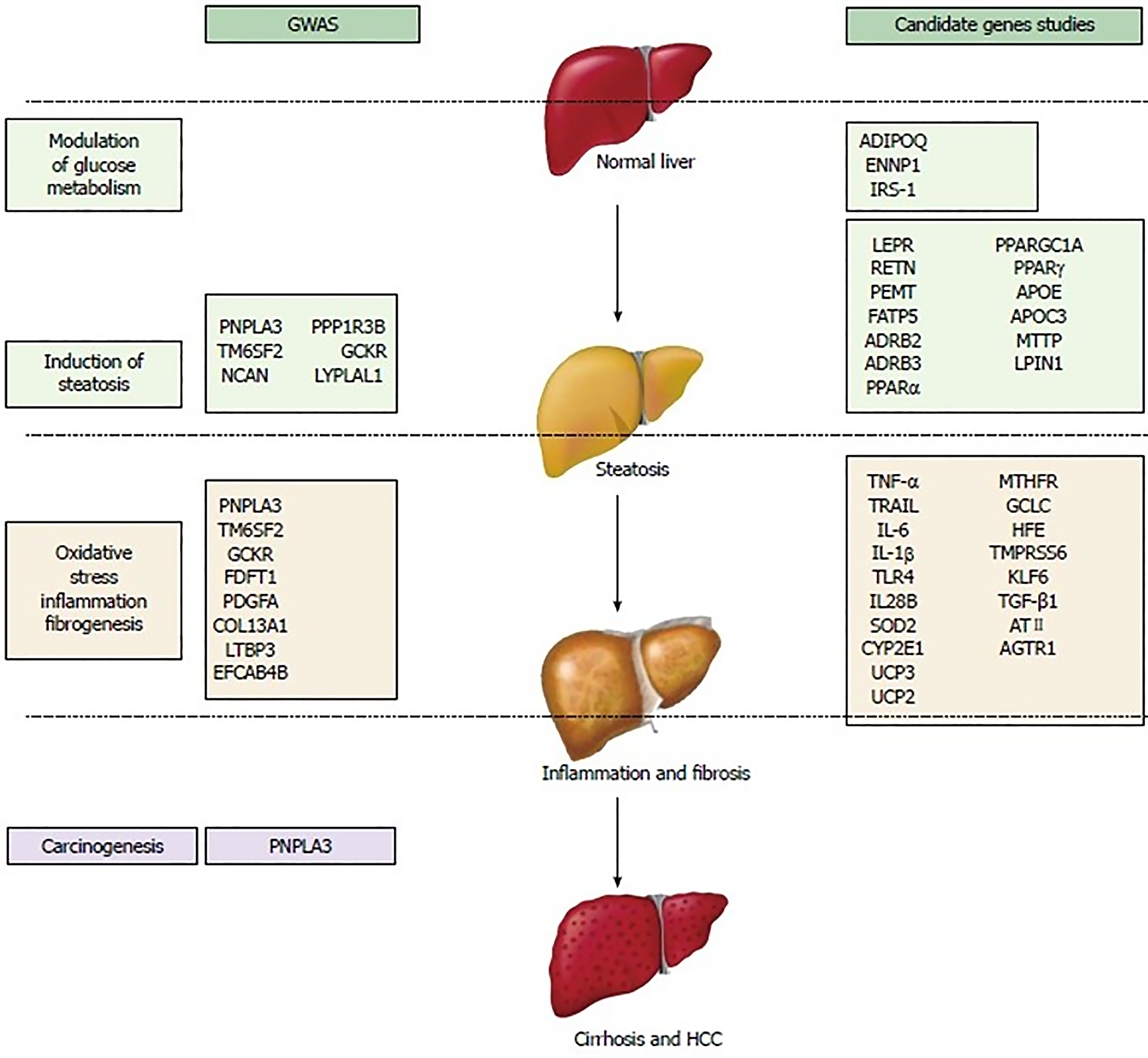
Figure 9 Schematic overview of the main genetic variants potentially involved in nonalcoholic fatty liver disease/nonalcoholic steatohepatitis susceptibility and progression[216].
GWAS: Genome-wide association studies; HCC: Hepatocellular carcinoma. Citation: Macaluso FS, Maida M, Petta S. Genetic background in nonalcoholic fatty liver disease: A comprehensive review. World J Gastroenterol 2015; 21: 11088-11111. Copyright © The Baishideng Publishing Group 2015. Published by Baishideng Publishing Group.
- Citation: Habib S. Team players in the pathogenesis of metabolic dysfunctions-associated steatotic liver disease: The basis of development of pharmacotherapy. World J Gastrointest Pathophysiol 2024; 15(4): 93606
- URL: https://www.wjgnet.com/2150-5330/full/v15/i4/93606.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v15.i4.93606