Published online Aug 28, 2017. doi: 10.4329/wjr.v9.i8.321
Peer-review started: January 20, 2017
First decision: March 27, 2017
Revised: May 1, 2017
Accepted: May 12, 2017
Article in press: May 15, 2017
Published online: August 28, 2017
Processing time: 217 Days and 1.9 Hours
Mammographic appearance of the normal breast is altered in the post-operative setting. It is essential to be aware of the normal findings as well as to identify features of recurrent disease with particular emphasis on radiological-pathological concordance. Digital breast tomosynthesis and volumetric breast density add incremental value in this clinical setting. We present a pictorial review of various cases to illustrate normal post-operative findings as well as mammographic features suspicious for recurrent disease.
Core tip: Mammographic imaging in patients after breast conservation surgery is challenging because surgery alters the normal breast architecture. The distinction of normal post-operative changes from true findings of recurrence becomes demanding even for a breast imager making it essential to update our knowledge in the subject. In the recent times digital breast tomosynthesis and volumetric breast density are adding an incremental value in this clinical setting.
- Citation: Ramani SK, Rastogi A, Mahajan A, Nair N, Shet T, Thakur MH. Imaging of the treated breast post breast conservation surgery/oncoplasty: Pictorial review. World J Radiol 2017; 9(8): 321-329
- URL: https://www.wjgnet.com/1949-8470/full/v9/i8/321.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i8.321
Breast conservation surgery (BCS) is the most commonly employed management of breast cancer in current practice and aims at surgical excision of the tumor while conserving the patient’s breast appearance and form. Breast radiologists need to update their knowledge of typical and atypical appearances of the treated breast in order to detect abnormalities signifying recurrence as well as to not raise unnecessary concern over benign course of post-operative change.
The expected changes on mammography after breast conservative surgery include skin thickening or edema, parenchymal edema, post-operative fluid collection, scar, fat necrosis and dystrophic calcifications which are more marked up to six months after therapy. Recurrence on mammographic imaging may be observed as a mass or microcalcifications, increase in skin thickening, increase in breast density, scar enlargement, axillary nodal recurrence or Paget’s disease. We present various mammography images to illustrate findings which may be left alone and those which require further intervention.
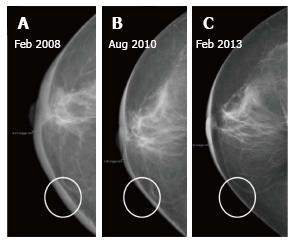
Normal skin thickness of the breast as seen on mammogram is 2 mm[1,2]. Skin thickening (more than 2 mm) is the most common finding after breast-conserving therapy (BCT), reported in up to 90% of patients[3]. On imaging it manifests as skin and trabecular thickening or overall increased breast density due to parenchymal edema which decrease on follow up studies and return to normal by 2 to 3 years (Figure 1)[2]. Post radiation edema occurs more commonly after external beam radiotherapy (EBRT) than intraoperative radiotherapy[4,5]. Less commonly the skin thickening and parenchymal edema may be a consequence of lymphedema (secondary to axillary node dissection) or mastitis[3].
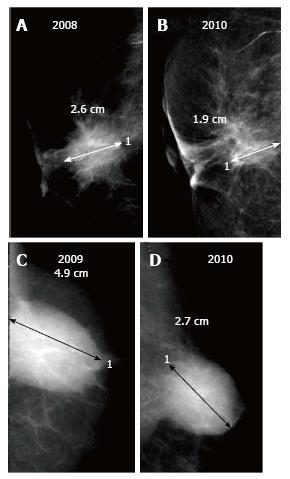
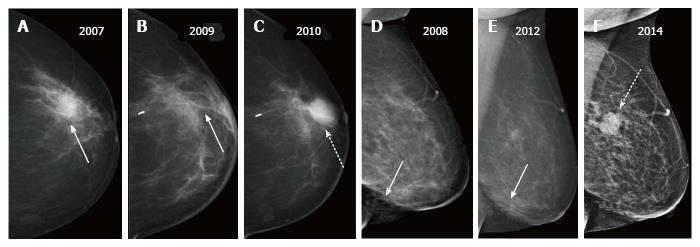
Fluid with or without blood which collects in the post-operative cavity appears as an oval or round circumscribed mass on mammography. When viewed on ultrasound, a mixed echogenic collection with variable fluid (anechoic) and haemorrhagic (echogenic) contents is observed. Post-operative fluid collections are seen in about half the patients at 1 mo after surgery and may remain in up to a fourth of cases till 6 mo[2] though in a few patients these may persist for years[6]. On sequential mammograms, the lesion becomes smaller, irregular and denser as the seroma retracts and is replaced by fibrous tissue (Figure 2). However an increase in size on follow-up merits further evaluation to exclude a recurrent mass.
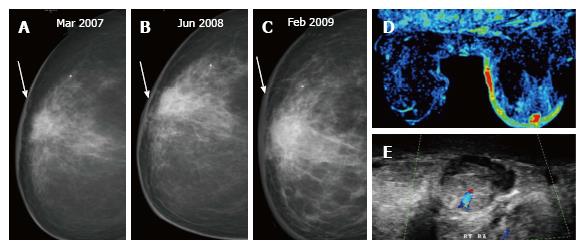
A post-surgical scar appears as an area of architectural distortion contiguous with contour deformity of surgery. In comparison to a true recurrence which appears same on all mammographic views and has a dense centre; on different projections a scar has varied appearances (appearing less distorted on one) and demonstrates fat lucencies within[3]. These features may be better imaged on spot compression or magnification views. As with a seroma, the scar usually decreases in density and/or size on serial imaging (Figure 3) or remains stable while an increase in size or density is suspicious for recurrence (Figure 4).
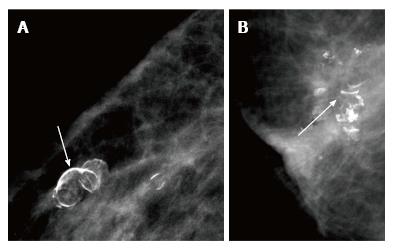
Benign calcifications are seen on mammography in about a third of treated breasts beginning 2 to 3 years after completion of therapy due to a combination of surgical trauma and radiation. Morphologically these calcifications are large (> 5 mm) and irregular in outline with central lucencies, with no associated mass/density and always occur at the site of surgery[7].
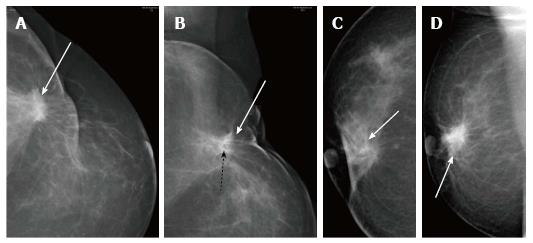
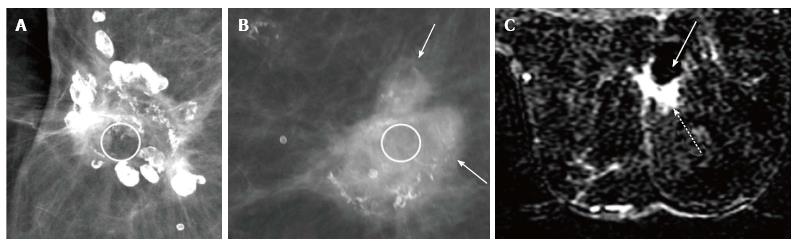
Fat necrosis is tissue necrosis resulting from damage to the intima of arteries from surgery and radiation. It more commonly manifests as an oval or round lucency with curvilinear or arc-like peripheral calcifications which are characteristic for the same (Figure 5). It is a common complication of myocutaneous flaps usually seen after 6-12 mo of treatment[3] and may clinically present as a palpable mass that is firm or hard[8]. When it presents as a palpable lesion with atypical appearance on mammography, sonography followed by biopsy may become requisite to confirm the diagnosis (Figure 6)[9].
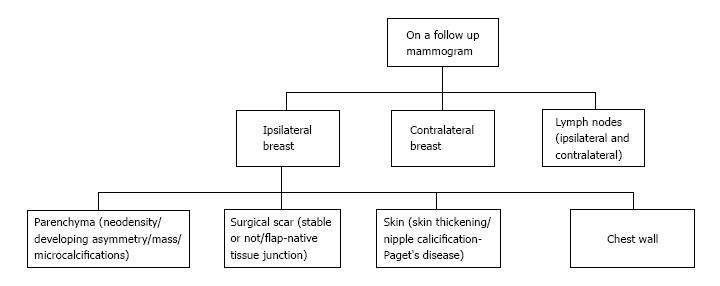
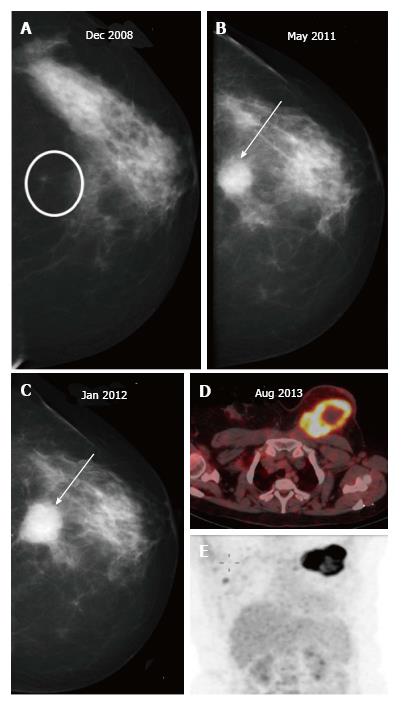
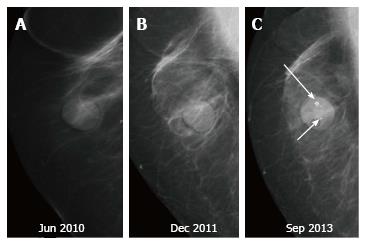
Recurrences may present at clinical examination or, may be detected only on mammography (Figure 7) as suspicious microcalcifications or masses. The rate of local recurrence after breast cancer surgery is 1%-2% per year[10]. Stability is defined as no interval change on two successive mammographic studies[7] and is generally observed at around 2-3 years after the completion of radiation therapy. Any retrograde change in imaging findings such as a new mass, microcalcifications, architectural distortion or an area of increased density at the scar site post stability should raise suspicion for tumor recurrence. Figure 8 lists the sites of recurrences to be looked for in conservatively treated breast on follow up.
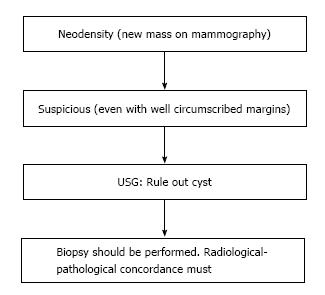
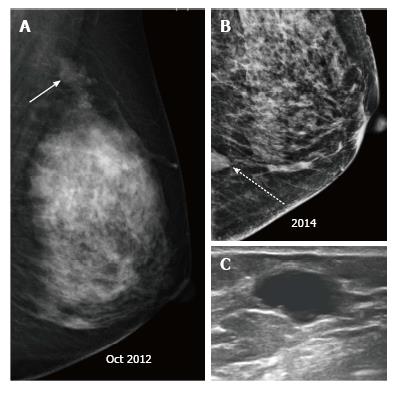
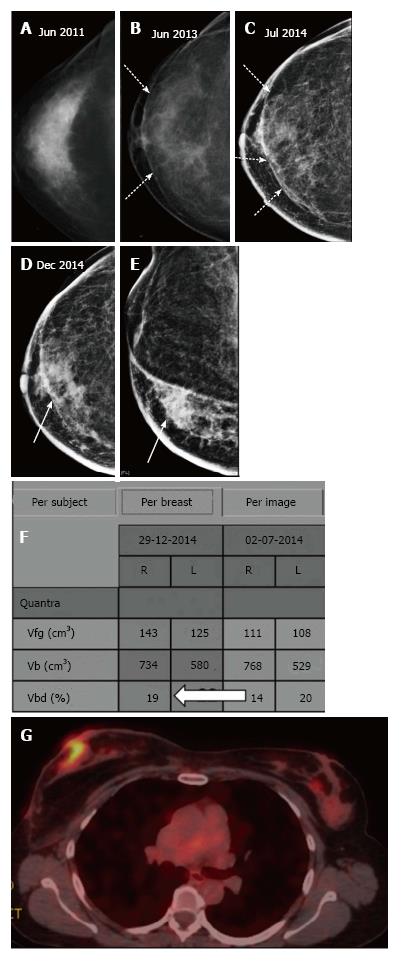
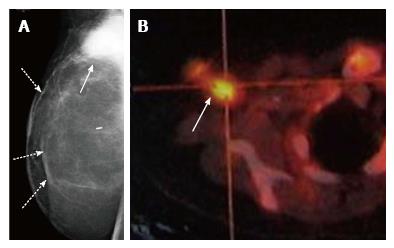
Palpable recurrences usually manifest as masses and even when seen as microcalcifications on a mammogram, they have associated densities. The temporal changes from prior mammogram determine the approach to patients[11]. Recurrences may be perceived as an increasing asymmetry or an enlarging mass within the operative bed or a new mass (neodensity) away from operative site[10] (Figure 9). Any neodensity at mammography should be evaluated on ultrasound to determine whether it is solid or cystic (Figure 10), and solid lesions should be biopsied (Figure 11).
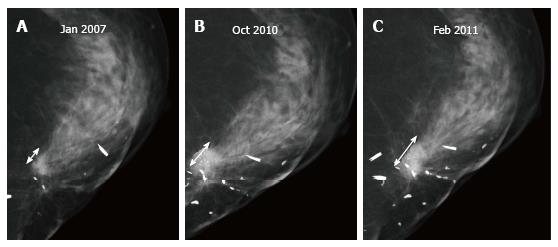
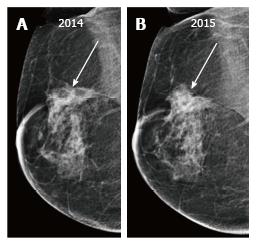
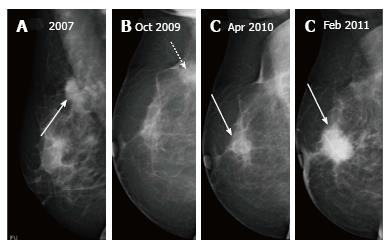
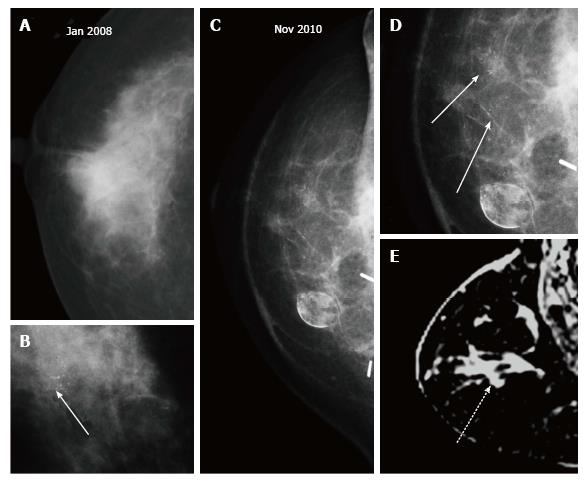
Up to 65% of early recurrences occur at or within a few centimetres of the site of original tumor, usually within 6-7 years of treatment[1]. At follow up imaging, it is essential to ensure that scar site is visible in two views (CC and MLO or additional views), at least in the first decade after surgery[6]. A new lesion or a neodensity which is suspicious may remain stable due to ongoing hormonal treatment, and stability does not indicate benign finding. Morphology is the most important criteria, and it is necessary to achieve a radiological-pathological concordance (Figure 12). Recurrence in the form of a developing asymmetry (Figure 13) has a 27% likelihood of cancer post BCT[12]. Post-oncoplastic or breast reconstruction, locoregional tumor recurrence is seen in 2.3%. The most common site of tumor recurrence is the contact line, at the junction of the flap with the native tissue[13] (Figure 14).
Microcalcifications that are casting, fine linear or linear branching and not typical of fat necrosis are suspicious. They are frequently similar in morphology to the primary cancer[7] (Figure 15). In an area of fat necrosis, fat like lucency is noted around or within the calcific densities, while in calcifications associated with recurrence, an associated mass density is seen in the region (Figure 16).
Progression of breast edema or skin thickening after the first post-surgical, post radiation therapy mammogram is abnormal and should be investigated[14]. Increasing skin thickening is better appreciated on digital mammography as compared to screen film mammography.
Radiation induced sarcoma is a rare complication which manifests as thickening of the skin or prominent trabecular pattern[15]. Angiosarcoma presents as a painless mass that may be associated with overlying blue or purplish discoloration of the skin (Figure 17).
Paget’s disease: Incidence of Paget’s disease in ipsilateral breast tumor recurrences ranges from 3.1% to 10.6%. Paget’s disease following BCT or subcutaneous mastectomy and reconstruction in patients presenting with nipple changes is not uncommon and should prompt early biopsy of what could be considered post-RT nipple changes[16]. Microcalcifications in the nipple-areola region may be seen on the mammogram (Figure 18).
Axillary nodal recurrence is relatively rare after adequate nodal dissection (of level I and II) has been done, occurring in 1%-3% of women[17]. Patients who present with axillary recurrence have metastatic disease at other sites in about fifty percent. On mammogram enlarged nodes may be seen (Figure 19).
Digital breast tomosynthesis (DBT) as a technique entails imaging of the breast tissue in multiple sections (at varied angles) instead of a two-dimensional image as with conventional mammography. The overlap of parenchymal tissues is resolved thus reducing the false positives as well as adequately identifying true lesions thus increasing the sensitivity of a mammogram. DBT not only helps in triangulation of a lesion but also reduces the requirement for additional views and lowers the patient call-back rate. Studies related to digital breast tomosynthesis to date have primarily focussed on screening with fewer reports on its utility in diagnostic mammography. Most studies have concluded a definite advantage of DBT in dense breasts (i.e., patients who have types 3 and 4 breast parenchymal density) in terms of lesion characterisation[18].
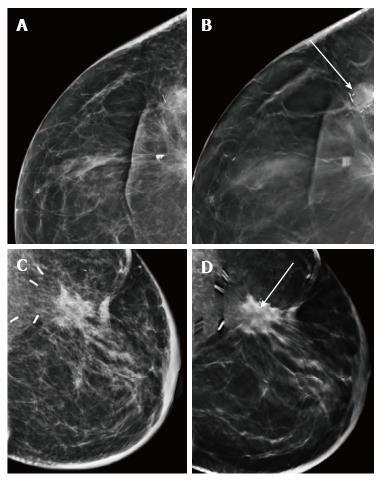
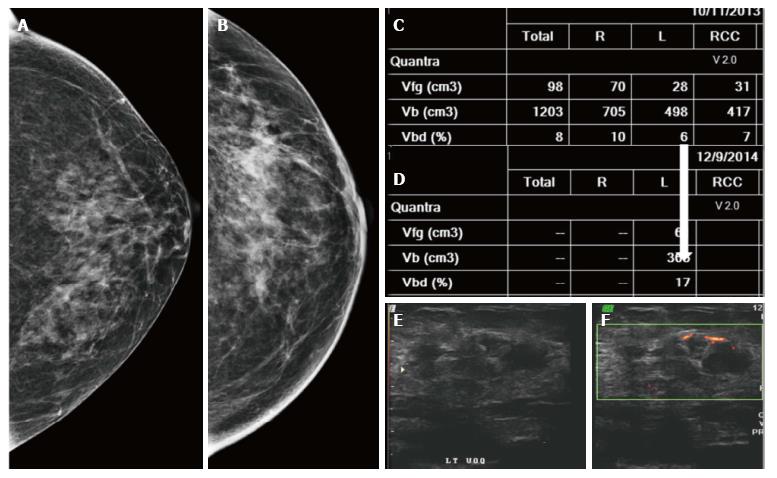
Similar to screening mammography, DBT also helps resolve post conservation changes such as a scar or other asymmetric densities due to parenchymal edema from a true recurrence[19]. A recent study by Sia et al[20] also reported that DBT decreases the rate of indeterminate findings in surveillance imaging of conservatively treated breasts. The fat density within the scar and that associated with benign calcification is better appreciated on DBT (Figure 20) whereas a true recurrence would demonstrate a mass. Increase in the volumetric breast density (Vbd) allows for a quantitative assessment for recurrences particularly where the presentation is without a definite mass (Figure 21).
To conclude, mammographic appearance of the breast is altered in the post-operative setting and breast radiologists should be cognisant of features suggestive of recurrent disease to identify it early. It is just as essential to be aware of the normal evolution of post therapy changes to avoid unnecessary further workup and stress to the patient. DBT and Vbd add incremental value in characterising normal and abnormal findings in this clinical setting. Not many studies have assessed the value of DBT over digital mammography with scope for research in this area in future.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Langdon S, Medina-Franco H, Olsha O S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Mendelson EB. Evaluation of the postoperative breast. Radiol Clin North Am. 1992;30:107-138. [PubMed] |
| 2. | Krishnamurthy R, Whitman GJ, Stelling CB, Kushwaha AC. Mammographic findings after breast conservation therapy. Radiographics. 1999;19 Spec No:S53-S62; quiz S262-S263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Chansakul T, Lai KC, Slanetz PJ. The postconservation breast: part 1, Expected imaging findings. AJR Am J Roentgenol. 2012;198:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Rivera R, Smith-Bronstein V, Villegas-Mendez S, Rayhanabad J, Sheth P, Rashtian A, Holmes DR. Mammographic findings after intraoperative radiotherapy of the breast. Radiol Res Pract. 2012;2012:758371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Elsberger B, Romsauerova A, Vinnicombe S, Whelehan P, Brown DC, Dewar JA, Thompson AM, Evans A. Comparison of mammographic findings after intraoperative radiotherapy or external beam whole breast radiotherapy. Eur J Surg Oncol. 2014;40:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Dershaw DD. Breast imaging and the conservative treatment of breast cancer. Radiol Clin North Am. 2002;40:501-516. [PubMed] |
| 7. | Gutierrez R, Horst KC, Dirbas FM, Ikeda DM. Breast imaging following breast conservation therapy. Breast surgical techniques and interdisciplinary management. New York: Springer Science Business Media 2011; 975-995. |
| 8. | Hogge JP, Zuurbier RA, de Paredes ES. Mammography of autologous myocutaneous flaps. Radiographics. 1999;19 Spec No:S63-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Hedegard W, Niell B, Specht M, Winograd J, Rafferty E. Breast reconstruction with a deep inferior epigastric perforator flap: imaging appearances of the normal flap and common complications. AJR Am J Roentgenol. 2013;200:W75-W84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Chansakul T, Lai KC, Slanetz PJ. The postconservation breast: part 2, Imaging findings of tumor recurrence and other long-term sequelae. AJR Am J Roentgenol. 2012;198:331-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Hadjiiski L, Sahiner B, Chan HP, Petrick N, Helvie MA, Gurcan M. Analysis of temporal changes of mammographic features: computer-aided classification of malignant and benign breast masses. Med Phys. 2001;28:2309-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Leung JW, Sickles EA. Developing asymmetry identified on mammography: correlation with imaging outcome and pathologic findings. AJR Am J Roentgenol. 2007;188:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Farras Roca JA, Dao TH, Lantieri L, Lepage C, Bosc R, Meyblum E, Pigneur F, Beaussart P, Assaf E, Totobenazara JL. Ipsilateral breast cancer recurrence after Deep Inferior Epigastric Perforator (DIEP) flap reconstruction: Incidence and radiological presentation. Diagn Interv Imaging. 2016;97:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Gage I, Schnitt SJ, Recht A, Abner A, Come S, Shulman LN, Monson JM, Silver B, Harris JR, Connolly JL. Skin recurrences after breast-conserving therapy for early-stage breast cancer. J Clin Oncol. 1998;16:480-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Yi A, Kim HH, Shin HJ, Huh MO, Ahn SD, Seo BK. Radiation-induced complications after breast cancer radiation therapy: a pictorial review of multimodality imaging findings. Korean J Radiol. 2009;10:496-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Plastaras JP, Harris EE, Solin LJ. Paget’s disease of the nipple as local recurrence after breast-conservation treatment for early-stage breast cancer. Clin Breast Cancer. 2005;6:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Clemons M, Danson S, Hamilton T, Goss P. Locoregionally recurrent breast cancer: incidence, risk factors and survival. Cancer Treat Rev. 2001;27:67-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Margolies L, Cohen A, Sonnenblick E, Mandeli J, Schmidt PH, Szabo J, Patel N, Hermann G, Weltz C, Port E. Digital breast tomosynthesis changes management in patients seen at a tertiary care breast center. ISRN Radiol. 2014;2014:658929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. |
Curran J.
How effective is mammography in detecting breast cancer recurrence in women after Breast Conservation Therapy (BCT) - A systematic literature review. |
| 20. | Sia J, Moodie K, Bressel M, Lau E, Gyorki D, Skandarajah A, Chua B. A prospective study comparing digital breast tomosynthesis with digital mammography in surveillance after breast cancer treatment. Eur J Cancer. 2016;61:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |