Published online Jun 28, 2017. doi: 10.4329/wjr.v9.i6.287
Peer-review started: October 19, 2016
First decision: January 16, 2017
Revised: February 24, 2017
Accepted: March 23, 2017
Article in press: March 24, 2017
Published online: June 28, 2017
Processing time: 247 Days and 17.9 Hours
To increase our insight in the neuronal mechanisms underlying cognitive side-effects of antiepileptic drug (AED) treatment.
The relation between functional magnetic resonance-acquired brain network measures, AED use, and cognitive function was investigated. Three groups of patients with epilepsy with a different risk profile for developing cognitive side effects were included: A “low risk” category (lamotrigine or levetiracetam, n = 16), an “intermediate risk” category (carbamazepine, oxcarbazepine, phenytoin, or valproate, n = 34) and a “high risk” category (topiramate, n = 5). Brain connectivity was assessed using resting state functional magnetic resonance imaging and graph theoretical network analysis. The Computerized Visual Searching Task was used to measure central information processing speed, a common cognitive side effect of AED treatment.
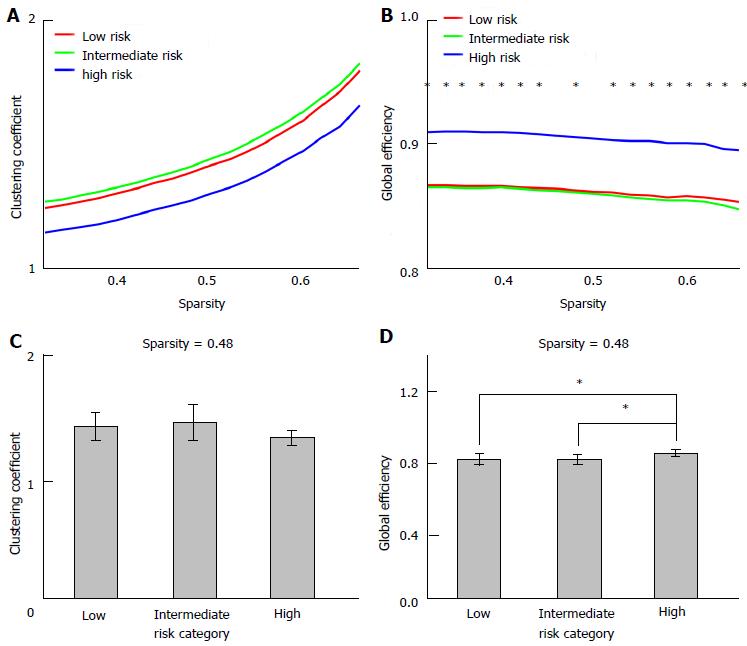
Central information processing speed was lower in patients taking AEDs from the intermediate and high risk categories, compared with patients from the low risk category. The effect of risk category on global efficiency was significant (P < 0.05, ANCOVA), with a significantly higher global efficiency for patient from the low category compared with the high risk category (P < 0.05, post-hoc test). Risk category had no significant effect on the clustering coefficient (ANCOVA, P > 0.2). Also no significant associations between information processing speed and global efficiency or the clustering coefficient (linear regression analysis, P > 0.15) were observed.
Only the four patients taking topiramate show aberrant network measures, suggesting that alterations in functional brain network organization may be only subtle and measureable in patients with more severe cognitive side effects.
Core tip: Slowed information processing is a commonly observed cognitive side-effect of antiepileptic drug (AED) treatment. We aimed to increase our insight in the neuronal mechanisms underlying this side-effect. Therefore, the relation between functional MR-acquired brain network measures, AED use, and cognitive function was investigated. No associations were found between information processing speed and graph measures, and only the four patients taking topiramate (with a high risk on cognitive side effects) showed aberrant network measures. The results suggest that alterations in functional brain network organization may be only subtle and measureable in patients with more severe cognitive side effects.
- Citation: van Veenendaal TM, IJff DM, Aldenkamp AP, Lazeron RHC, Hofman PAM, de Louw AJA, Backes WH, Jansen JFA. Chronic antiepileptic drug use and functional network efficiency: A functional magnetic resonance imaging study. World J Radiol 2017; 9(6): 287-294
- URL: https://www.wjgnet.com/1949-8470/full/v9/i6/287.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i6.287
Epilepsy is generally treated with antiepileptic drugs (AEDs). A persistent problem in AED treatment is the occurrence of adverse events among which cognitive side effects are commonly seen[1,2]. The cognitive side effects account for a high percentage of the disease burden[3] and lead to early drug discontinuation[4]. The prevalence and severity of the cognitive side effects varies among different AEDs. Several AEDs, such as topiramate, are associated with cognitive problems such as language deficit (anomia), while other AEDs such as lamotrigine seem to induce less cognitive side effects or even have activating effects[5]. Despite specific differences, a decreased central information processing speed is commonly observed among the different AEDs to some extent[2].
AEDs control epileptic seizures via several distinct mechanisms, such as enhancement of GABAergic inhibition, reduction of glutamatergic neurotransmission, or modulation of the voltage-gated ion channels[6]. Changes in brain metabolic processes also affect healthy brain activity, and are likely to be responsible for cognitive side effects[1]. Functional magnetic resonance imaging (fMRI) enables assessment of this brain activity, and can be employed to measure combined effects of different mechanism of action of AEDs[7]. Several fMRI studies have shown altered brain activity patterns in healthy participants[8] or patients with epilepsy[9,10] treated with AEDs. For instance, altered brain activity patterns appeared to be associated with language impairments when taking topiramate[11-13].
Cognitive functions are mediated by the concerted action of multiple and distributed brain regions. These brain regions show correlations of their fMRI time signals, which is commonly interpreted as functional connectivity. Collectively, these functional connections form a brain network, which can be analyzed and characterized using graph theoretical analysis. Brain networks appear to be efficient networks, characterized by a high functional segregation and integration, i.e., different brain regions form densely interconnected groups, enabling specialized information processing, and also rapid communication between distributed brain regions. Several graph measures are available to quantify these characteristics[14].
Cognitive performance has been associated with the efficiency of functional brain networks[15,16], while impaired functional brain networks have been associated with cognitive decline in epilepsy[17,18]. Furthermore, associations between drug load, cognition and graph measures were shown in one of these studies, although this was not the main focus of the current study[17]. Another study associated the use of carbamazepine with altered graph measures when compared with other AEDs, but did not investigate the relation with cognitive effects[19]. In the current study, we aim to test whether chronic use of AEDs, associated with a high risk for cognitive side-effects, affects functional resting-state network measures differently than long-term use of AEDs associated with milder cognitive side-effects. Furthermore, we will test whether functional resting-state network measures are associated with impaired cognitive functioning.
Three groups of patients with epilepsy were compared in this observational, cross-sectional study[20]. These groups were subdivided based on the AEDs that were being used, in accordance with Samarasekera et al[21]. The first group, the low risk category, consisted of patients using lamotrigine or levetiracetam. Patients taking carbamazepine, oxcarbazepine, phenytoin, or valproate were included in the intermediate risk category, while the high risk category comprised patients taking topiramate. Patients on polytherapy took at most two different AEDs and were categorized according to their AED associated with the greatest cognitive risk. By including patients with AEDs from the three risk groups, a range in slowing of information processing speed is realized.
All patients were clinically diagnosed with localization-related epilepsy and aged between 18 and 70 years. The patients were recruited from our tertiary epilepsy referral center. Participants not eligible for MRI, because of metal implants, claustrophobia, or pregnancy, were excluded from this study. Furthermore, patients did not experience seizures at least 12 h prior to MRI. This study was approved by the local Medical Ethical Committee and all participants provided written informed consent.
Cognitive functioning was assessed by two neuropsychological tasks. The Computerized Visual Searching Task (CVST) was used to measure visual (complex) information processing speed[22]. Slowing of this central information processing speed is a common side effect of AEDs[2], and therefore the CVST is considered to be sensitive for treatment effects[23]. With the CVST, a centered grid is shown surrounded by 24 other grid patterns. Participants have to find the (only) grid identical to the centered one as fast as possible.
The Raven Standard Progressive Matrices was administered to assess global cognitive performance. This is a non-verbal reasoning test which gives an indication of fluid intelligence[24]. Previous studies suggested that intelligence stays relatively unaffected by AEDs[23].
As several epilepsy related characteristics might affect functional brain networks[25], a score was composed to account for these effects. This epilepsy severity score was assessed in all patients and compared between the different risk categories. Epilepsy severity was characterized using a summarized score between zero and seven, composed by the sum of subscores for seizure type (tonic-clonic: 1, other: 0), previous occurrence of status epilepticus (yes: 1, no: 0), seizure-related injury (yes: 1, no: 0) and seizure frequency (seizure free: 0, yearly: 1, monthly: 2, weekly: 3, daily: 4).
MRI data were acquired on a 3.0T MRI scanner equipped with an 8-channel head coil (Philips Achieva, Philips Medical Systems, Best, The Netherlands). The scanning protocol included resting-state functional MRI and a T1-weighted scan. Functional MRI data were acquired using whole-brain single-shot multi-slice echo planar imaging sequence sensitive to the blood-oxygen-level-dependent (BOLD) effect (195 volumes, 32 slices, in-plane resolution 2 mm × 2 mm, 4 mm thick slices, repetition time 2000 ms, echo time 35 ms, flip angle: 90°, acquisition time: 7 min). A 3D T1-weighted scan was acquired for anatomic reference (voxel size 1 mm × 1 mm × 1 mm, repetition time 8.3 ms, echo time 4.8 ms, inversion time 1022 ms, 180 slices, flip angle 8°, acquisition time 6 min).
Preprocessing of the functional images was performed using SPM8 (Wellcome Department of Cognitive Neurology, London, United Kingdom). The functional images were corrected for differences in slice timing and head movement, coregistered to the T1 image and spatially (FWHM 6 mm) and temporally filtered (band pass 0.01-0.1 Hz). The BOLD signal originating from the white matter and ventricles, which is assumed to reflect physiological noise[26], and the six translation and rotation parameters obtained from the motion correction were deregressed from the BOLD signal.
The T1-weighted scan was parcellated into 82 cortical and subcortical brain regions using FreeSurfer v5.1.0 (The General Hospital Corporation, Boston MA, United States). Subsequently, a connectivity matrix was created by calculating the Pearson’s correlation coefficient between the average (deregressed) BOLD time signal of each combination of two regions. Negative correlations were set to zero. The correlation values were thresholded, based on the average connectivity matrix, to obtain connectivity matrices with only the strongest connections. The number of included connections was varied, with sparsity levels ranging from 0 to 0.9 (0 is fully connected, whereas 1 indicates no connections).
The Brain Connectivity Toolbox[14] was employed to compute graph measures for each individual connectivity matrix. The clustering coefficient and the characteristic path length are commonly used to characterize the functional segregation and integration, respectively. The clustering coefficient quantifies the fraction of a node’s neighbor that is also connected to each other. The characteristic path length is defined as the average shortest distance (the inverse correlation coefficient) between all pairs of nodes. As, in sparse networks, a single weak connection can result in a large, or even infinite average path lengths, global efficiency was computed instead of characteristic path length, which avoids this effect by using inverse path lengths[27]. One hundred null models of the connectivity matrices were computed by randomizing the connections of the original matrices, while preserving the degree and weight distribution[28]. The graph measures were divided by the mean global efficiency and clustering coefficient of these null models, providing a normalized global efficiency (Eg) and clustering coefficient (γ).
To test whether the clustering coefficient and global efficiency differed between the risk categories, an analysis of covariance (ANCOVA) was applied with the graph measures as outcome, cognitive risk category as fixed factor and age as covariate. Associations with cognition were assessed with linear regression analysis, with CVST time as outcome, and Eg or γ, age, and the percentage corrects answers in the Raven test as independent variables. To assess whether these results were affected by confounders, these analyses were repeated with gender, epilepsy severity score, or drug load (ratio of prescribed daily dose to defined daily dose[29]) added to the regression analyses as additional covariates. All statistical analyses were performed in MATLAB (version R2012b). P values lower than 0.05 were considered significant.
In total, 58 patients were included in this study. Three of these patients did not finish the procedures due to claustrophobia, resulting in 16 patients taking AEDs from the low risk category, 34 taking AEDs from the intermediate risk category, and 5 taking high risk AEDs. The age and drug load were significantly higher in the intermediate risk category than in the low risk category (Table 1). Also the number of patients on polytherapy was significantly higher in the intermediate risk category compared with the low risk category, while the high and low risk categories significantly differed in number of patients on polytherapy. The risk categories did not differ in gender distribution, educational level, or epilepsy severity.
| Low risk (n = 16) | Intermediate risk (n = 34) | High risk (n = 5) | |
| General | |||
| Male/female | 5/11 (31%/69%) | 16/18 (47%/53%) | 0/5 (0%/100%) |
| Age (yr)2 | 39.5 ± 13.4 | 50.7 ± 12.5a | 42.4 ± 15.8 |
| Educational level3 | 5 (range 2-6) | 5 (range 2-7) | 5 (range 4-6) |
| Epilepsy-related | |||
| Symptomatic/non-symptomatic epilepsy | 2/14 (13/88%) | 15/19 (44/56%) | 0/5 |
| Seizure frequency | |||
| Weekly | 0 | 1 (3%) | 0 |
| Monthly | 4 (25%) | 3 (9%) | 0 |
| Yearly | 2 (13%) | 6 (18%) | 2 (40%) |
| Seizure free | 10 (63%) | 24 (71%) | 3 (60%) |
| Years since epilepsy onset2 | 22.7 ± 11.7 | 30.4 ± 13.4 | 26.8 ± 23.3 |
| Epilepsy severity score2 | 1.4 ± 0.8 | 1.2 ± 1.0 | 1.0 ± 0.7 |
| AED-related | |||
| Mono-/polytherapy | 16/0 | 8/26 (24/77%)a | 3/2 (60/40%)b |
| Medication type | |||
| CBZ | 0 | 17 (50%) | 1 (20%) |
| LEV | 7 (44%) | 6 (18%) | 0 |
| LTG | 9 (56%) | 10 (29%) | 1 (20%) |
| OXC | 0 | 4 (12%) | 0 |
| PHT | 0 | 16 (47%) | 0 |
| TPM | 0 | 0 | 5 (100%) |
| VPA | 0 | 7 (21%) | 1 (20%) |
| Drug load2,4 | 1.3 ± 0.6 | 1.8 ± 0.7a | 1.2 ± 1.0 |
The results of the CVST and the Raven task are summarized in Table 2. The CVST reaction time was slower than the normal range (range: 7.3 to 30.8 s, while the mean ± SD was 10.3 ± 4.1 s in normal population[31]). A significant effect of risk category on CVST reaction time was observed, which remained significant when controlling for age, gender, and global cognitive level (P = 0.009, ANCOVA). Post-hoc tests showed significant differences in CVST between the low and intermediate risk category (P = 0.035, estimated adjusted mean difference 3.5 s), and between the low and high risk category (P = 0.004, adjusted mean difference 7.8 s). No significant differences were found between the percentage correct answers Raven scores of the different risk categories.
Of the 55 included patients, seven were excluded from further analysis: One patient was excluded because of excessive head motion (maximum head movement of 8.0 mm, while the maximum head movement was below 1.5 mm in all other patients), one because of a deeper large lesion mass, and five patients were excluded because of a failure to automatically parcellate the cortex, due to cortical abnormalities. The analysis was therefore performed on 48 patients: 15 patients taking AEDs from the low-risk category, 29 patients taking AEDs from the intermediate risk category and 4 patients taking the high risk medication. The maximum head displacement did not differ between the three risk categories.
The functional networks were fully connected and showed small-world characteristics within the sparsity range 0.32-0.66 (which was defined as γ/λ significantly larger than one, with γ the normalized clustering coefficient, and λ the normalized characteristic path length). Only the sparsity levels within this range were considered for further analyses. The ANCOVA test revealed significant effects of risk category on Eg at most sparsities within this sparsity range (Figure 1). Post-hoc tests showed a significantly higher Eg for patients from the low category (n = 14) compared with the high risk category (n = 4), and for patients from the intermediate category (n = 29) compared with the high risk category (n = 4). Eg or γ did not differ significantly between patients from the low and intermediate risk categories (P > 0.2 at all sparsity levels), and no significant associations were observed between γ or Eg and CVST time (P > 0.15 at all sparsity levels). Gender, epilepsy severity score, or drug load were not significantly associated with the γ, Eg, or CVST reaction time, and the results of these adjusted analyses were consistent with the results of the analyses without these additional covariates (< 10% change in effect size of the variable of interest).
The current study investigated whether patients taking AEDs with a different risk for cognitive side-effects have different functional brain topologies. To this end, we included epilepsy patients with chronic AED treatment with different risk profiles, i.e., a low risk category, intermediate-risk, and high risk category. Furthermore, we assessed whether cognitive problems, in terms of a decreased central information processing speed, could be associated with the functional brain organization.
A higher global efficiency was shown in patients taking TPM (n = 4, the high risk category), compared with patients taking the low (n = 14) and intermediate risk AEDs (n = 29). The directionality of this difference is strikingly, as this result seems to contradict the cognitive side effects of TPM. The global efficiency is suggested to be particularly important for more complex cognitive tasks, for which different brain areas are involved[32]. The “better” global efficiency in TPM users might however be interpreted as a compensatory mechanism, or could be explained by a “survivor effect”. As patients with side effects are more likely to switch to other AEDs, it is likely that these patients are less vulnerable for cognitive problems. The higher global efficiency in the high-risk group might therefore reflect a lower susceptibility for cognitive side effects of these patients[33,34]. However, these patients did have a lower processing speed compared with the other patients, which argues against this explanation and in favor of a compensatory mechanism.
No differences in graph measures were observed between the patients groups taking AEDs from the low and from the intermediate risk category. It is possible that the effects of TPM on brain organization are more pronounced compared with effects of other AEDs, but TPM can also have distinctive effects on brain organization. TPM is suggested to have a unique cognitive profile, with specific effects on verbal fluency. Moreover, it has multiple mechanisms of action, and both these mechanisms and its chemical structure differ from other AEDs[35].
Furthermore, no associations were found between processing speed and graph measures, in contrast to a previous study that showed not only associations between intellectual decline and a lowered clustering coefficient in patients with epilepsy, but also with increasing drug load[17]. The latter suggests that the intellectual decline (which was based on intelligence tests) was a side effect of the AED treatment, but this could also result from differences in epilepsy characteristics. That study included more patients with a high drug load (15% of the patients had a drug load higher than 3) than the current study (no drug loads higher than 3 in the included patients), thus it is possible that the effects on graph measures are only measureable in patients with higher drug loads or AEDs with high risks on cognitive complaints.
The measured information processing speed covered the whole range from normal to a clearly affected processing speed, and patients taking AEDs known to induce cognitive side effects, showed lower processing speeds than patients with lower risk AEDs. These results could therefore not explain the lack of associations between graph measures and information processing speed, or the lack of differences in graph measures between the low and intermediate risk category. Also no trends were shown, while the total number of participants (48), and the number of patients in the low (16) and intermediate risk categories (34) were relatively large, making it unlikely that this lack of findings were due to limited power.
All included patients in the current study were diagnosed with localization-related epilepsy. Epilepsy is associated with a decreased global efficiency and increased clustering coefficient, although some studies showed a decreased clustering coefficient in patients with epilepsy[36]. It is therefore plausible that the functional brain networks of all three groups of patients in this study were already altered compared with healthy participants, irrespective of AED treatment.
This study has several limitations. Although we tried to include comparable patient groups, the risk categories differed in age and drug load, suggesting that our study population is biased. Therefore, the analyses were corrected for these characteristics by including age and drug load as covariates. Besides these characteristics, also other factors could have confounded our results, such as the location of the epileptic focus or effects of AEDs on the neurovascular coupling, which should be assessed in separate studies[37]. Finally, no information is available about changes over time and causality due to the cross-sectional design.
No differences in functional network graph measures could be detected between patients with epilepsy after chronic use of AEDs with a different risks on cognitive side effects. Only the four patients taking TPM, which has a high risk for developing cognitive side effects, showed a more efficient brain network topology, which might be a compensatory mechanism. Also no associations were found between the graph measures and the measured cognitive impairments, specifically slowing of central information processing. Alterations in functional brain network organization may be only subtle and measureable in patients with more severe cognitive side-effects.
The authors thank Berting R for his assistance with the image acquisition and Geerlings M and Slenter J for continuous hardware and software support.
A persistent problem in antiepileptic drug (AED) treatment is the occurrence of adverse events among which cognitive side effects are commonly seen. The prevalence and severity of the cognitive side effects varies among different AEDs, but a decreased central information processing speed is commonly observed among the different AEDs to some extent.
Functional brain network analysis is being applied to study cognitive processes and cognitive problems.
This is the first study that specifically assessed the relation between functional brain network measures and cognitive problems in patients taking different types of AEDs.
To summarize the practical applications of your research findings, so that readers may understand the perspectives by which this study will affect the field and future research.
AED: Antiepileptic drug; Clustering coefficient: Network measure; indication of segregation of a network; Global efficiency: Network measure; indication of integration of a network; CVST: Computerized Visual Searching Task; measures visual (complex) information processing speed.
The manuscript is well written.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Altamura C, Kilickesmez O, Razek AAKA S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Perucca P, Gilliam FG. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012;11:792-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 478] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 2. | Ijff DM, Aldenkamp AP. Cognitive side-effects of antiepileptic drugs in children. Handb Clin Neurol. 2013;111:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Helmstaedter C, Aldenkamp AP, Baker GA, Mazarati A, Ryvlin P, Sankar R. Disentangling the relationship between epilepsy and its behavioral comorbidities - the need for prospective studies in new-onset epilepsies. Epilepsy Behav. 2014;31:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Bootsma HP, Ricker L, Hekster YA, Hulsman J, Lambrechts D, Majoie M, Schellekens A, de Krom M, Aldenkamp AP. The impact of side effects on long-term retention in three new antiepileptic drugs. Seizure. 2009;18:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Kwan P, Brodie MJ. Neuropsychological effects of epilepsy and antiepileptic drugs. Lancet. 2001;357:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 271] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 811] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 7. | van Veenendaal TM, IJff DM, Aldenkamp AP, Hofman PA, Vlooswijk MC, Rouhl RP, de Louw AJ, Backes WH, Jansen JF. Metabolic and functional MR biomarkers of antiepileptic drug effectiveness: A review. Neurosci Biobehav Rev. 2015;59:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Li X, Ricci R, Large CH, Anderson B, Nahas Z, Bohning DE, George MS. Interleaved transcranial magnetic stimulation and fMRI suggests that lamotrigine and valproic acid have different effects on corticolimbic activity. Psychopharmacology (Berl). 2010;209:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Jokeit H, Okujava M, Woermann FG. Carbamazepine reduces memory induced activation of mesial temporal lobe structures: a pharmacological fMRI-study. BMC Neurol. 2001;1:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Wandschneider B, Stretton J, Sidhu M, Centeno M, Kozák LR, Symms M, Thompson PJ, Duncan JS, Koepp MJ. Levetiracetam reduces abnormal network activations in temporal lobe epilepsy. Neurology. 2014;83:1508-1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Jansen JF, Aldenkamp AP, Marian Majoie HJ, Reijs RP, de Krom MC, Hofman PA, Eline Kooi M, Nicolay K, Backes WH. Functional MRI reveals declined prefrontal cortex activation in patients with epilepsy on topiramate therapy. Epilepsy Behav. 2006;9:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | De Ciantis A, Muti M, Piccolini C, Principi M, Di Renzo A, De Ciantis R, Frondizi D, Iannone G, Ottaviano P, Piccirilli M. A functional MRI study of language disturbances in subjects with migraine headache during treatment with topiramate. Neurol Sci. 2008;29 Suppl 1:S141-S143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Yasuda CL, Centeno M, Vollmar C, Stretton J, Symms M, Cendes F, Mehta MA, Thompson P, Duncan JS, Koepp MJ. The effect of topiramate on cognitive fMRI. Epilepsy Res. 2013;105:250-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6976] [Cited by in RCA: 7452] [Article Influence: 465.8] [Reference Citation Analysis (0)] |
| 15. | van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29:7619-7624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 778] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 16. | Giessing C, Thiel CM, Alexander-Bloch AF, Patel AX, Bullmore ET. Human brain functional network changes associated with enhanced and impaired attentional task performance. J Neurosci. 2013;33:5903-5914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Vlooswijk MC, Vaessen MJ, Jansen JF, de Krom MC, Majoie HJ, Hofman PA, Aldenkamp AP, Backes WH. Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology. 2011;77:938-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Bonilha L, Tabesh A, Dabbs K, Hsu DA, Stafstrom CE, Hermann BP, Lin JJ. Neurodevelopmental alterations of large-scale structural networks in children with new-onset epilepsy. Hum Brain Mapp. 2014;35:3661-3672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Haneef Z, Levin HS, Chiang S. Brain Graph Topology Changes Associated with Anti-Epileptic Drug Use. Brain Connect. 2015;5:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | van Veenendaal TM, IJff DM, Aldenkamp AP, Lazeron RH, Puts NA, Edden RA, Hofman PA, de Louw AJ, Backes WH, Jansen JF. Glutamate concentrations vary with antiepileptic drug use and mental slowing. Epilepsy Behav. 2016;64:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Samarasekera SR, Helmstaedter C, Reuber M. Cognitive impairment in adults with epilepsy: The relationship between subjective and objective assessments of cognition. Epilepsy Behav. 2015;52:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Aldenkamp AP, Arends J, de la Parra NM, Migchelbrink EJ. The cognitive impact of epileptiform EEG discharges and short epileptic seizures: relationship to characteristics of the cognitive tasks. Epilepsy Behav. 2010;17:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Grevers E, Breuer LE, IJff DM, Aldenkamp AP. Mental slowing in relation to epilepsy and antiepileptic medication. Acta Neurol Scand. 2016;134:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Raven J, Raven JC, Court JH. Manual for Raven’s Progressive Matrices and Vocabulary Scales. Section 3: The Standard Progressive Matrices. San Antonio, TX: Harcourt Assessment 2000; . |
| 25. | van Diessen E, Diederen SJ, Braun KP, Jansen FE, Stam CJ. Functional and structural brain networks in epilepsy: what have we learned? Epilepsia. 2013;54:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 26. | Murphy K, Birn RM, Bandettini PA. Resting-state fMRI confounds and cleanup. Neuroimage. 2013;80:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 496] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 27. | Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 777] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 28. | Rubinov M, Sporns O. Weight-conserving characterization of complex functional brain networks. Neuroimage. 2011;56:2068-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 626] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 29. | Lammers MW, Hekster YA, Keyser A, Meinardi H, Renier WO, van Lier H. Monotherapy or polytherapy for epilepsy revisited: a quantitative assessment. Epilepsia. 1995;36:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Verhage F. Intelligentie en leeftijd: Onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar. Assen: Van Gorcum 1964; . |
| 31. | Alpherts W, Aldenkamp A. FePsy: the iron psyche. Heemstede: Instituut voor Epilepsiebestrijding 1994; . |
| 32. | Giessing C, Thiel CM. Pro-cognitive drug effects modulate functional brain network organization. Front Behav Neurosci. 2012;6:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Hahn A, Kranz GS, Sladky R, Ganger S, Windischberger C, Kasper S, Lanzenberger R. Individual diversity of functional brain network economy. Brain Connect. 2015;5:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Santarnecchi E, Rossi S, Rossi A. The smarter, the stronger: intelligence level correlates with brain resilience to systematic insults. Cortex. 2015;64:293-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Mula M. Topiramate and cognitive impairment: evidence and clinical implications. Ther Adv Drug Saf. 2012;3:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | van Diessen E, Zweiphenning WJ, Jansen FE, Stam CJ, Braun KP, Otte WM. Brain Network Organization in Focal Epilepsy: A Systematic Review and Meta-Analysis. PLoS One. 2014;9:e114606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | Kida I, Smith AJ, Blumenfeld H, Behar KL, Hyder F. Lamotrigine suppresses neurophysiological responses to somatosensory stimulation in the rodent. Neuroimage. 2006;29:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |