Published online Jun 28, 2017. doi: 10.4329/wjr.v9.i6.280
Peer-review started: January 7, 2017
First decision: February 17, 2017
Revised: May 9, 2017
Accepted: May 18, 2017
Article in press: May 19, 2017
Published online: June 28, 2017
Processing time: 166 Days and 20.2 Hours
To define the role of cardiac magnetic resonance (CMR) by analyzing a particular group of patients with suspected acute coronary syndrome (ACS) and normal coronary angiogram.
From January 2009 to December 2015, we examined 220 patients with clinical suspicion of ACS, Troponin elevation [the threshold used to define a positive Troponin T test (TnT) was 0.1 ng/mL] and no significant coronary disease at angiography (the patients were considered to have significant angiographic disease only a 50% stenosis was detected in any of their coronary arteries). The role of CMR with the late gadolinium enhancement was evaluated.
CMR was performed to 190 patients (86%) of this group which reveals: Myocarditis in 90 patients (47%); apical ballooning (Tako-Tsubo syndrome) in 32 patients (17%); myocardial infarction (MI) in 40 patients (21%) and no clear diagnosis identified by CMR in 28 patients (15%). A comparison with previous studies was also made. Clinical and echocardiographic follow-ups were performed at 12 ± 2 mo and no major adverse cardiac events were revealed.
There is a group of patients with clinical suspicion of ACS displaying normal coronary angiograms. CMR was demonstrated to be a valuable tool in the differential diagnosis evaluation of myocarditis, apical ballooning and MI.
Core tip: In some patients with suspected acute coronary syndrome and elevated Troponin, the subsequent coronary angiography reveals normal coronaries. These patients represent an obscure and difficult field of diagnosis and investigation. There are several potential causes of this uncertainty, such as myocardial infarction with a recanalized coronary artery, myocarditis, different cardiomyopathies, and other rare conditions. Cardiac magnetic resonance offers a new and more appropriate method in distinguishing between different chest pain etiologies.
- Citation: Camastra GS, Sbarbati S, Danti M, Cacciotti L, Semeraro R, Della Sala SW, Ansalone G. Cardiac magnetic resonance in patients with acute cardiac injury and unobstructed coronary arteries. World J Radiol 2017; 9(6): 280-286
- URL: https://www.wjgnet.com/1949-8470/full/v9/i6/280.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i6.280
Troponin can accurately predict the presence of acute coronary syndrome (ACS) in patients with coronary artery disease (CAD) and is usually used in the risk assessment of patients presenting acute chest pain. In some patients with chest pain and an elevated Troponin, the subsequent Coronary Angiography reveals normal coronaries or some un-obstructed coronary arteries (defined as a < 50% stenosis in the main and side branch arteries). Previous reports have shown a prevalence of patients with suspected ACS and normal coronary arteries of 2.6%-12%[1-3].
These patients represent an obscure and difficult field of diagnosis and investigation[4,5]. There are several potential causes of this uncertainty, such as myocardial infarction (MI) with a recanalized coronary artery[6], myocarditis, different cardiomyopathies, aortic disease, pulmonary embolism, arrhythmias, valvular heart disease, sepsis, and other rare conditions. With the aim of establishing a different cause of the Troponin elevation, cardiac magnetic resonance (CMR) offers a new and more appropriate technique thanks to its ability in identifying areas of inflammation and fibrosis with high spatial resolution. Using a combination of available sequences, CMR was shown to have a good ability in distinguishing between different etiologies of chest pain with elevated levels of Troponin and ECG modifications in cases such as acute infarction, myocarditis and other cardiomyopathies[7]. We hypothesized that CMR offers an incremental diagnostic value in determining the underlying etiology of patients with clinical suspicion of ACS with regards to optimizing the pharmacological dosage and to define the appropriate therapy according to specific etiological diagnosis.
From January 2009 to December 2015, we selected 220 patients with new-onset chest pain, elevated Troponin and normal coronary arteries. The patients presented various ECGs which included ST elevation, ST depression, negative T wave, as well as normal ECGs. We assigned these patients to CMR in order to exclude MI and to provide an alternative diagnosis which would explain the clinical presentation. All CMR scans were performed at admission, and the average interval time between clinical presentation and the administration of CMR were 4 ± 2 d (range 1-14 d). A coronary angiogram was performed on all these patients. Thirty patients were excluded, seven of whom due to severe claustrophobia and twenty-three patients due to their cardiac history in cases such as myocarditis, MI, impaired left-ventricular (LV) function, pulmonary embolism or renal failure. The final number of patients included in our study was 190. Clinical and echocardiograph follow-ups were performed on all patients within 12 ± 2 mo.
CMR was performed by breath-hold using a 1.5-T MRI system (INTERA, Philips Medical Systems, Best, the Netherland). Both cine and contrast-enhanced short-axis MRI images were detected at every 10 mm from base to apex with slice thickness of 8 mm. In plane-resolution 1.2 mm × 1.8 mm. Cine MRI was performed using a steady-state free-precession sequence (SSFP). T2 STIR was performed with the following parameters: repetition times 2 R-R intervals, slice thickness 8 mm. T2-weighted images were considered abnormal if increased signal was observed within the myocardium. Contrast MRI images were also obtained on average 10 to 15 min after a contrast injection, using a segmented Inversion Recovery-Gradient Echo (IR-GE) technique adjusting inversion time to null normal myocardium. The contrast dose [Magnevist (gadoteridol), Shering AG] was 0.1 mmol/kg. Endocardial and epicardial borders were outlined on the short axis cine images. The extension of the late gadolinium enhancement (LGE) was measured on the short-axis contrast images detecting the increased image intensity level ≥ 2SD above the average of normal myocardium enhancement in order to define the abnormal contrast enhancement. The location of LE was classified in accordance with the AHA segmentation. Regional parameters were assessed using a model dividing each short axis into 6 circumferential segments. The extension of contrast enhancement and the left ventricular ejection fraction, were measured using the freely available software segment on the website (http://segment.heiberg.se)[8]. The CMR studies were assessed by two independent observers.
Clinical data including clinical history, physical examination, Troponin levels, ECG recordings, transthoracic echocardiography and coronary angiography, overall were reviewed by a single experienced observer in order to include these patients to our study. The Troponin tests were performed at Vannini Hospital using an internal laboratory with normal range values and measurement units. A “false positive” of rising serum Troponin level, was considered in patients with a single Troponin rise followed by a second normal value within 24 h.
Continuous data were expressed as a mean SD. Categorical variables were respectively expressed as number and percentage. The statistical review of the study was performed by a biomedical statistician
The average age of patients was 50 ± 20 years and the gender prevalence was 52% female (98 patients), 48% male (Table 1). None of the patients had a previous history of MI or myocarditis or renal failure before presenting acute chest pain. About 94% of the cohort had an abnormal ECG at presentation, and ST-segment elevation being the most common abnormality. In particular ECG was: Normal in 11 patients, ST-segment elevation in 130 patients, ST-segment depression in 40 patients, while T-waves were negative in 9 patients. There was a low prevalence of cardiac risk factors. The average interval time between the presentation of chest pain to the CMR was 4 ± 2 d (range 1-14 d).
| Patient: 190 |
| Age (median ± SD) years: 50 ± 20 |
| Gender |
| Male 92 (48%) |
| Female 98 (52%) |
| ECG at presentation |
| Normal in 11 patients (6%) |
| ST-segment elvation in 130 patients (68%) |
| ST-segment depression in 40 patients (21%) |
| Negative T-waves in 9 patients (5%) |
| Mean time interval from presentation to CMR 4 ± 2 d (median ± SD); 1-14 d (range) |
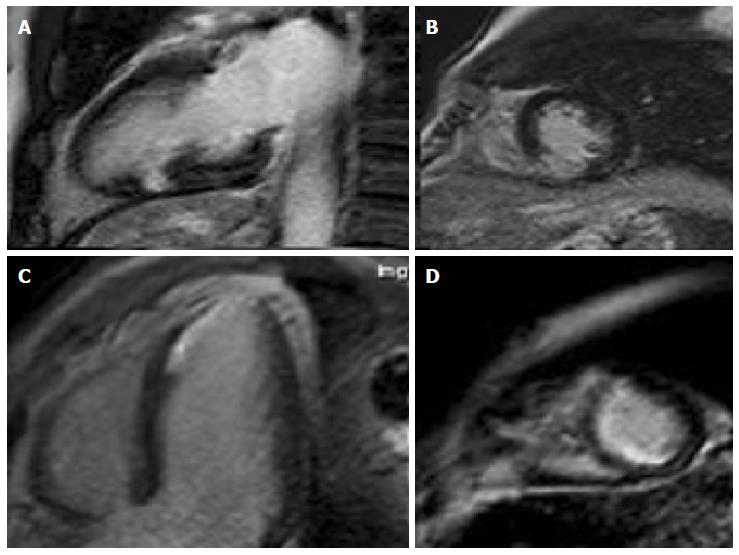
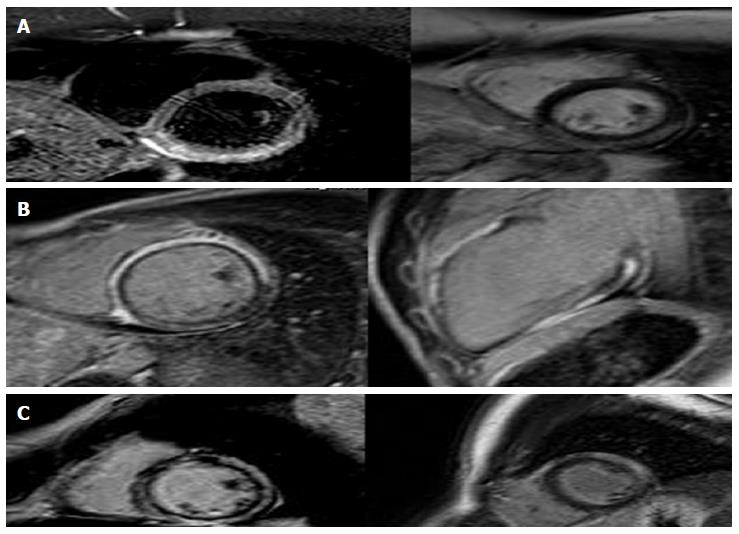
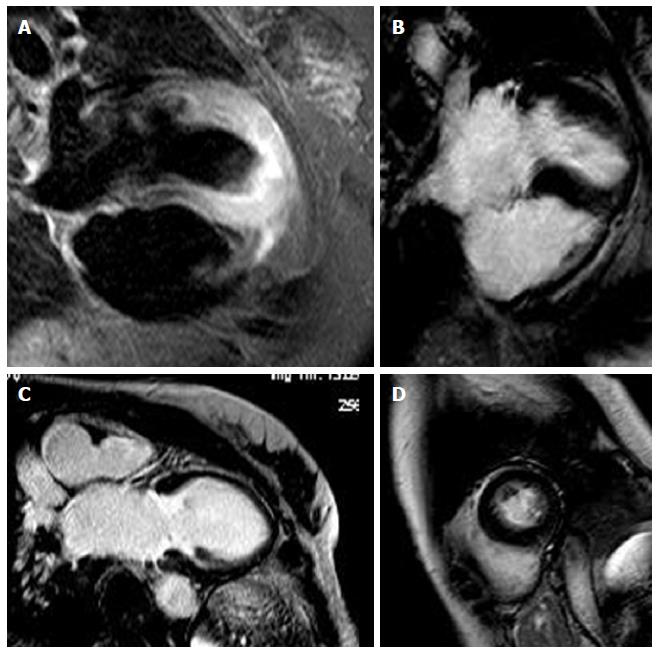
The T2-STIR-weighed images were performed to detect areas of high signal intensity which are compatible with myocardial edema[9]. On the short-axis post-contrast images, the LGE enhancement patterns were classified as subendocardial, midwall, subepicardial, and transmural or any combination of these (Table 2). Areas of edema were detected in the same segments of late enhancement in all the patients. We found intramiocardial or subepicardial LGEs suggestive of myocarditis in 90 patients (47%); absence of LGEs with apical edema suggesting apical ballooning (Tako-Tsubo syndrome) in 32 patients (17%); subendocardial LGEs indicating MI in 40 patients (21%) and an absence of LGEs and edema in 28 patients (15%) with no clear diagnosis identified in the CMR.
| Tako-tsubo: 32 patients (17%) | LGE: Absent |
| Myocarditis: 90 patients (47%) | LGE: Epicardial or intramiocardial |
| Myocardial infarction: 40 patients (21%) | LGE: Subendocardial or transmural |
| No clear diagnosis: 28 patients (15%) | LGE: Absent |
Diagnosis was based on assessment of LV size, regional and global wall motion, and the presence and pattern of LGE. Our aim was to assess and to compare the LGE patterns caused by MI with other myocardial diseases that are not related to ischemic disease. The evidence of edema represents an additional value of LGE imaging in identifying the underlying etiology. In MI we have the subendocardial or transmural LGE. LGE in non-ischemic cardiomyopathy generally does not correspond to any particular coronary artery distribution and is located mostly in the mid-wall to subepicardial layer. The patients who presented areas of subendocardial and transmural LGE had a diagnosis of MI (Figure 1). With regards to cardiac inflammation (Myocarditis), the regional distribution of edema usually does not reflect the coronary perfusion areas and typically appears within a sub-epicardial distribution. In our study we found areas of edema in the same segments of late enhancement in all the patients. The presence of LGE in an area that does not correspond to any particular coronary artery distribution and is located mostly in the mid-wall to subepicardial layer correlates strongly with myocarditis in the correct clinical setting. Myocarditis lesions occur predominantly in the lateral free wall and originate from the epicardial layer of the ventricular wall. The patients who presented areas of subepicardial or intramyocardial LGEs were given a diagnosis of myocarditis (Figure 2)[10-13]. The Tako-tsubo cardiomyopathy was characterized by a global apical and mid ventricular edema matching the distribution of LV dysfunction in the absence of LGE (Figure 3)[14-16]. In these cases CMR provided a new diagnosis in 85% of patients; the remaining patients had no detectable infarction or inflammation and no additional new diagnoses were made. MI was diagnosed in 21% of patients, while Myocarditis was diagnosed in 47%. We observed a peculiar distribution pattern of myocarditis lesions, as they always originate from the epicardial layer of the ventricular walls and moreover they are frequently located at the mid and distal portion of the lateral free wall. Patients with myocarditis are generally young men. In 32 patients with recent stress and minimal ST elevation in the anterior leads at the ECG, CMR revealed severe apical dysfunction with preserved basal LV function and no LGE, so a diagnosis of “Tako-tsubo” cardiomyopathy (apical ballooning) was made. This diagnosis was confirmed in the clinical and echocardiographic follow-ups which revealed a complete normalization of LV function. In 28 patients (15%) CMR did not reveal any structural or myocardial tissue abnormalities. In the follow-up examinations over a 1 year period adverse cardiac events were not detected. Echocardiography revealed complete recovery of contractility in patients with tako-tsubo syndrome and parameters remained unchanged in the remaining patients. We can affirm that an increase in Troponin usually reveals a myocardial necrosis. The great majority of patients presenting acute chest pain, elevated troponin levels and ECG abnormalities were correctly diagnosed and treated for ACS and therefore, coronary angiographies revealed significant coronary stenoses. On the other hand those patients with normal coronary angiography represented this study’s clinical challenge as there are a large number of other reasons for Troponin elevation without obstructive CAD, such as Myocarditis, stress Cardiomyopathy, cardiac contusion, congestive heart failure, infarction and other non-cardiological causes. CMR has a potential role in evaluating the etiology of Troponin elevation, since it is able to provide detailed information on myocardial tissue characteristics, such that it has become the gold standard for in vivo detection of scarring associated with MI and other non-ischemic conditions. The high degree of CMR spatial resolution and contrast allows for the identification of very small areas of infarction. Our study demonstrates that CMR is able to identify the etiology of Troponin elevation in 85% of patients presenting ACS-like symptoms with normal coronary arteries. In this group the most common cause was Myocarditis (47%). Currently, no single clinical or imaging methods confirm a diagnosis of myocarditis with any certainty, thus requiring invasive diagnostic methods such as endo-myocardial biopsy (EMB). However, EMB underestimates the true incidence of myocarditis and is not free of complications. EMB is currently indicated only in severe LV dysfunction or refractory arrhythmia and the patients in our study had no indications for EMB in accordance with therapeutic guidelines. In such patients, CMR appears to be suitable in identifying significant ongoing inflammation. We observed a peculiar distribution pattern of myocarditis lesions, with the lateral wall being the preferred location. The mechanism responsible for LGE has not been fully elucidated. In our series apical ballooning was detected in 17% of patients; it was diagnosed on the basis of cardiac symptoms and the presence of emotional stress. In these patients, the pattern of left ventricular dysfunction is characterized by apical and mid-ventricular contractile abnormalities, absence of LGE, minimal elevation of cardiac enzymes despite the presence of large regions of focal akinesia in the myocardium and finally the absence of coronary stenosis. Edema was present in myocardial segments with the most impaired function. MI was diagnosed in 21% of patients who presented areas of subendocardial and trans-mural LGE. The mechanism responsible for MI in these patients may be due to coronary artery spasm or coronary embolism. In a small percentage (15%) of cases with lower troponin levels it was impossible to evidence or to define a correct diagnosis. In the follow-up examination at 1 year, adverse cardiac events were not detected.
We reviewed the literature beginning from 2007 with the study of Assomull et al[7] regarding the role of CMR in patients with suspected ACS, raised Troponin and normal coronary arteries (Table 3). It is important to emphasize that most of these studies were single-center reports, had a small sample size and variable inclusion criteria. The CMR studies were performed at variable time points after the onset of disease and with non-uniform patient populations (age and gender). Our data are not in line with the previous study of Assomull et al[7] due to the high prevalence of Takotsubo cardiomyopathy (17% vs 1.7%) in our population, this difference is probably due to the gender differences of the two groups of patients (52% females in our group vs 28% females in Assomul’s group). If we compare our results with the study of Laraudogoitia Zaldumbide et al[17] there are some similarities despite the gender differences of the two groups of patients (52% females in our group vs 19% females in Laraudogoitia’s group). The Tako-tsubo cardiomyopathy predominantly affects women, while myocarditis predominantly affects men, thus explaining the variability reflected in the different studies, and the relatively lower frequency indicated in our work. If we correlate this data by gender, the results would be more consistent. We must also consider the average age of different populations (higher in our group compared to the group of Assomul) that can explain the different prevalence of various diseases in our work because myocarditis is most common in young people and Takotsubo cardiomyopathy is more common in older women. In the study conducted by Stensaeth et al[18] MI was not detected, therefore, it is not possible to compare it with other studies. If we compare our results with the study of Gerbaud et al[19] we find a higher percentage of myocarditis (47% vs 26.1%) and a lower percentage of no diagnosis (15% vs 23.1%) in our patients. The review of literature reveals a great variability in the incidence of “no diagnosis” varying from 5.5% (Laraudogoitia) to 35% (Assomul). In our study, we found a average level of no diagnosis (15%).
| Ref. | Year | No. of patients | Age of patients | Time from presentation to CMR (d) | Diagnosis | ||||
| Infarction | Myocarditis | Tako-tsubo | Other | No diagnosis | |||||
| [8] | 2007 | 60 | 44 ± 17 | 1-90 (mean 14.5) | 11.6% | 50% | 1.70% | 1.70% | 35% |
| [18] | 2009 | 80 | 48 | 4 | 15% | 63% | 11% | 5.50% | 5.50% |
| [19] | 2011 | 49 | 45 ± 14 | 1 | 0 | 29% | 10% | 43% | 18% |
| [20] | 2011 | 130 | 54 ± 17 | 6.2 ± 5.3 | 28.5% | 26.1% | 21.50% | 0.80% | 23.10% |
A limiting effect on our study was the small number of patients. It should be considered that CMR presents technical and procedural limitations in particular cases. In patients with irregular breathing patterns or significant arrhythmia, image quality may be reduced. The prognostic value of CMR was not analyzed. Another limitation is the lack of provocative tests for detecting coronary spasms and a lack of EMB although this is currently indicated only in severe LV dysfunction or refractory arrhythmia. None of the patients in our study had these features.
There are non-ischemic disorders that can present a similar clinical picture to ACS such as myocarditis or Tako-tsubo cardiomyopathy. CMR offers an incremental diagnostic value in determining the underlying etiology of patients with suspected ACS, with the aim of optimizing pharmacological therapy as well as in defining the correct therapy in accordance with a specific etiological diagnosis.
There are non-ischemic disorders that can present a similar clinical picture to acute coronary syndrome (ACS). In some patients with chest pain and an elevated Troponin levels the subsequent coronary angiography reveals normal coronaries or some un-obstructed coronary arteries. These patients do not have a defined diagnosis and represent an obscure and difficult field of investigation.
Cardiac magnetic resonance (CMR) with T2-STIR-weighed images can detect areas of myocardial edema and with late gadolinium enhancement (LGE) images can detect areas of myocardial necrosis related to an increase in troponin.
The patients who presented areas of subendocardial and transmural LGE were given a diagnosis of myocardial infarction while the patients who presented areas of subepicardial or intramyocardial LGE were given a diagnosis of myocarditis. In Tako-tsubo cardiomyopathy the pattern of edema was characterized by a global apical and mid ventricular edema matching the distribution of LV dysfunction in the absence of LGE.
CMR offers incremental diagnostic value in determining the underlying etiology of patients with suspected ACS.
CMR: Cardiac magnetic resonance; LGE: Late gadolinium enhancement; ACS: Acute coronary syndrome.
The authors present an interesting study, investigating the differential diagnosis of patients with myocardial infarction and normal coronary arteries by CMR. The study seems to be well designed.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Korosoglou G, Lin GM S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Kang WY, Jeong MH, Ahn YK, Kim JH, Chae SC, Kim YJ, Hur SH, Seong IW, Hong TJ, Choi DH. Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe? Int J Cardiol. 2011;146:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Widimsky P, Stellova B, Groch L, Aschermann M, Branny M, Zelizko M, Stasek J, Formanek P. Prevalence of normal coronary angiography in the acute phase of suspected ST-elevation myocardial infarction: experience from the PRAGUE studies. Can J Cardiol. 2006;22:1147-1152. [PubMed] |
| 3. | Larsen AI, Galbraith PD, Ghali WA, Norris CM, Graham MM, Knudtson ML. Characteristics and outcomes of patients with acute myocardial infarction and angiographically normal coronary arteries. Am J Cardiol. 2005;95:261-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Kardasz I, De Caterina R. Myocardial infarction with normal coronary arteries: a conundrum with multiple aetiologies and variable prognosis: an update. J Intern Med. 2007;261:330-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Korff S, Katus HA, Giannitsis E. Differential diagnosis of elevated troponins. Heart. 2006;92:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Dokainish H, Pillai M, Murphy SA, DiBattiste PM, Schweiger MJ, Lotfi A, Morrow DA, Cannon CP, Braunwald E, Lakkis N. Prognostic implications of elevated troponin in patients with suspected acute coronary syndrome but no critical epicardial coronary disease: a TACTICS-TIMI-18 substudy. J Am Coll Cardiol. 2005;45:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, Pennell DJ, Prasad SK. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J. 2007;28:1242-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 269] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Heiberg E, Sjögren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment--freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 710] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 9. | Wince WB, Kim RJ. Molecular imaging: T2-weighted CMR of the area at risk--a risky business? Nat Rev Cardiol. 2010;7:547-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 350] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 703] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 12. | Camastra GS, Cacciotti L, Marconi F, Sbarbati S, Pironi B, Ansalone G. Late enhancement detected by cardiac magnetic resonance imaging in acute myocarditis mimicking acute myocardial infarction: location patterns and lack of correlation with systolic function. J Cardiovasc Med (Hagerstown). 2007;8:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Danti M, Sbarbati S, Alsadi N, Di Filippo A, Gangitano G, Giglio L, Salvini V, Amoruso M, Camastra GS, Ansalone G. Cardiac magnetic resonance imaging: diagnostic value and utility in the follow-up of patients with acute myocarditis mimicking myocardial infarction. Radiol Med. 2009;114:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Mitchell JH, Hadden TB, Wilson JM, Achari A, Muthupillai R, Flamm SD. Clinical features and usefulness of cardiac magnetic resonance imaging in assessing myocardial viability and prognosis in Takotsubo cardiomyopathy (transient left ventricular apical ballooning syndrome). Am J Cardiol. 2007;100:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Camastra GS, Cacciotti L, Kol A, Ansalone G. Stress cardiomyopathy with apical thrombosis promptly diagnosed with cardiovascular MRI. Cardiology. 2006;105:108-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Cacciotti L, Camastra GS, Beni S, Giannantoni P, Musarò S, Proietti I, De Angelis L, Semeraro R, Ansalone G. A new variant of Tako-tsubo cardiomyopathy: transient mid-ventricular ballooning. J Cardiovasc Med (Hagerstown). 2007;8:1052-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Laraudogoitia Zaldumbide E, Pérez-David E, Larena JA, Velasco del Castillo S, Rumoroso Cuevas JR, Onaindía JJ, Lekuona Goya I, García-Fernández MA. The value of cardiac magnetic resonance in patients with acute coronary syndrome and normal coronary arteries. Rev Esp Cardiol. 2009;62:976-983. [PubMed] |
| 18. | Stensaeth KH, Fossum E, Hoffmann P, Mangschau A, Klow NE. Clinical characteristics and role of early cardiac magnetic resonance imaging in patients with suspected ST-elevation myocardial infarction and normal coronary arteries. Int J Cardiovasc Imaging. 2011;27:355-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Gerbaud E, Harcaut E, Coste P, Erickson M, Lederlin M, Labèque JN, Perron JM, Cochet H, Dos Santos P, Durrieu-Jaïs C. Cardiac magnetic resonance imaging for the diagnosis of patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Int J Cardiovasc Imaging. 2012;28:783-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |