Published online Jun 28, 2017. doi: 10.4329/wjr.v9.i6.269
Peer-review started: February 6, 2017
First decision: March 7, 2017
Revised: March 21, 2017
Accepted: April 18, 2017
Article in press: April 19, 2017
Published online: June 28, 2017
Processing time: 140 Days and 6.8 Hours
The Tumor, Node, Metastasis (TNM) staging system, adopted by the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC), has been recently revised, with the new 8th edition of the staging manual being published in January 2017. This edition has few but important evidence-based changes to the TNM staging system used for lung cancer. Radiologists should be aware of the updated classification system to accurately provide staging information to oncologists and oncosurgeons. In this article, we discuss the rationale, illustrate the changes with relevance to Radiology, and review the clinical implications of the 8th edition of the UICC/AJCC TNM staging system with regards to lung cancer.
Core tip: This article discusses the rationale, illustrates the changes with relevance to Radiology, and reviews the clinical implications of the 8th edition of the Union for International Cancer Control/American Joint Committee on Cancer Tumor, Node, Metastasis staging of lung cancer.
- Citation: Kay FU, Kandathil A, Batra K, Saboo SS, Abbara S, Rajiah P. Revisions to the Tumor, Node, Metastasis staging of lung cancer (8th edition): Rationale, radiologic findings and clinical implications. World J Radiol 2017; 9(6): 269-279
- URL: https://www.wjgnet.com/1949-8470/full/v9/i6/269.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i6.269
Lung cancer is the leading oncologic cause of mortality in both men and women in the United States. Worldwide, Lung cancer, is the leading and the second most common cause of cancer death among men and women respectively[1]. The American Cancer Society estimates about 224390 new cases of lung cancer in 2016, and approximately 158000 deaths[2]. Clinical practice guidelines for lung cancer largely rely on staging models, which are used not only for predicting disease prognosis, but also to guide treatment[3]. The Tumor, Node, Metastasis (TNM) staging system, adopted by both the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC), has been recently revised, and the new edition (8th) of the staging manual was published in January 2017[4] (Table 1). The updated lung cancer chapter introduces few but important evidence-based changes to the prior 7th edition, derived from validation of the TNM system for staging and guiding lung cancer treatment in multidisciplinary centers.
| T | Primary tumor | |
| Tx | Cannot be assessed; Tumor in sputum/bronchial washings not in imaging/bronchoscopy | |
| To | No evidence | |
| Tis | Carcinoma in situ | |
| T1 | ≤ 3 cm surrounded by lung/visceral pleura, not involving main bronchus | |
| T1a(mi) | Minimally invasive adenocarcinoma | |
| T1a | ≤ 1 cm | |
| T1b | > 1 to ≤ 2 cm | |
| T1c | > 2 to ≤ 3 cm | |
| T2 | > 3 to ≤ 5 cm or Involves main bronchus without carina involvement or Visceral pleural invasion or atelectasis/post obstructive pneumonitis extending to hilum | |
| T2a | > 3 to ≤ 4 cm | |
| T2b | > 4 to ≤ 5 cm | |
| T3 | > 5 to ≤ 7 cm or Separate tumor in same lobe or Direct invasion of chest wall (includes parietal pleura and superior sulcus)/parietal pericardium/phrenic nerve | |
| T4 | > 7 cm or | |
| Separate tumor in different lobe of ipsilateral lung or Invasion of heart/ great vessels/diaphragm/mediastinum/trachea/carina/esophagus/ recurrent laryngeal nerve/vertebral body | ||
| N | Regional lymph node | |
| Nx | Cannot be assessed | |
| N0 | No involvement | |
| N1 | Ipsilateral peribronchial and/or hilar nodes and intrapulmonary nodes | |
| N2 | Ipsilateral mediastinal and/or subcarinal nodes | |
| N3 | Contralateral mediastinal or hilar; ipsilateral/contralateral scalene/supraclavicular | |
| M | Distant metastasis | |
| M0 | No distant metastasis | |
| M1 | Distant metastasis is present | |
| M1a | Tumor (s) in contralateral lung; pleural/pericardial nodule/malignant effusion | |
| M1b | Single extrathoracic metastasis | |
| M1c | Multiple extrathoracic metastases, in one/more organs | |
In this article, we discuss the rationale, illustrate the changes with relevance to radiology and review the clinical implications of the 8th edition of the UICC/AJCC TNM staging system.
Developed in France by Pierre Denoix between the years 1943 and 1952, the TNM system has been adopted by the UICC and the AJCC to help clinicians plan treatment, guide prognosis, assist in treatment evaluation, provide a common language for exchange of information, and contribute to the continuing investigation of cancer[5]. Many iterations have led to the preceding 7th UICC/AJCC TNM edition, effective since 2009, which introduced a series of evidence-based advances in comparison with earlier versions. Radiologic reviews of the 7th TNM staging were previously published[6]. The 7th TNM was the result of a combined effort of the International Association for the Study of Lung Cancer (IALSC), the UICC, the AJCC, and the Japanese societies against cancer[7], and reflected the conclusions from a retrospective analysis of 81015 lung cancer patients (67725 with non-small cell lung cancer, NSCLC, and 13290 with small cell lung cancer, SCLC) treated between 1990 and 2000, from Europe (59%), North America (18%), Asia (15%), and Australia (8%). The 7th TNM edition, for the first time, encompassed small cell lung cancer (SCLC) and carcinoid tumors in the scope.
Despite these earlier efforts, some of the 7th TNM descriptors could not be validated given the lack of a tailored methodology, leading the IALSC to commission a task force responsible for gathering and analyzing a comprehensive database, composed of both retrospective and prospective cases, in an attempt to overcome prior limitations[8]. For the first time, the database included information of 4667 patients sent via a specifically designed electronic data capture system, in addition to other sources (consortia, registries, or surgical series), resulting in a final cohort of 77156 lung cancer patients (70967 with NSCLC and 6189 with SCLC) from 35 cancer centers in 16 countries, diagnosed between 1999 and 2010 and treated with surgery or combined modalities[9]. The resultant 8th TNM revision portrays validation of the descriptors and recommendations based on the new findings[10].
The T descriptors in the 8th TNM classification encompass size, invasion into adjacent central/mediastinal structures, and location of an additional tumor nodule in relation to the primary tumor (Table 1). The changes to the T component aim to maintain compatibility with the previous classifications, with an additional benefit of improving prognostic differentiation between the different T categories. The primary tumor is now classified into 7 categories per size (T1a, T1b, T1c, T2a, T2b, T3 and T4) as compared with the 6 categories on 7th TNM (T1a, T1b, T2a, T2b, T3 and T4). The new T classification was validated on 33115 patients with NSCLC and no metastatic disease[9,11]. Robust analysis of the survival data was performed using a log-rank statistic, which confirmed prior 7th TNM size cutoffs, and suggested further subdivision into 1-cm increments. T descriptors were analyzed using Cox regression analysis adjusted for age, sex, histologic type, and geographic region across multiple cohorts, with results showing a clear difference in 5-year survival, and hence prognosis, for each centimeter increase in tumor size between 1-5 cm (Table 2). The 5-cm cutoff remains useful to separate tumors with worse prognosis (T3, > 5 and ≤ 7 cm, and T4, > 7 cm).
| 5-yr survival | |
| T1a | 92% |
| T1b | 83%-86% |
| T1c | 76%-81% |
| T2a | 67%-74% |
| T2b | 60%-65% |
| T3 | 52%-57% |
| T4 | 38%-47% |
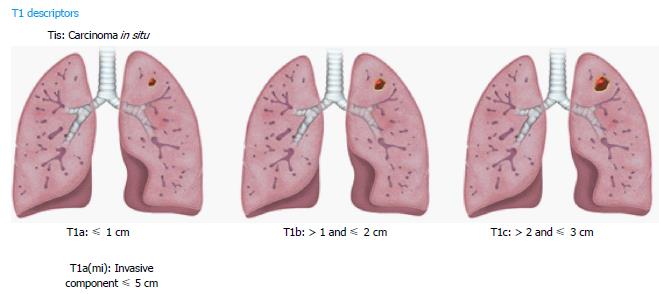
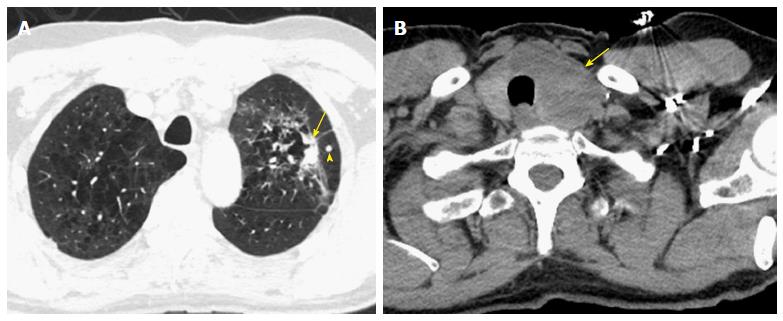
The new classification subdivides T1 lung cancers measuring ≤ 3 cm into T1a, T1b, T1c lesions based on specific size cutoffs (Figure 1). A superficial spreading tumor in central airways is classified as T1a, regardless of its location. Carcinoma in situ is classified as Tis and applies to both adenocarcinoma and squamous cell carcinoma. Minimally invasive adenocarcinomas are classified as T1a (mi) when the invasive component is ≤ 5 mm and the noninvasive lepidic component is ≤ 3 cm (diagnoses can be only made in resected tumors).
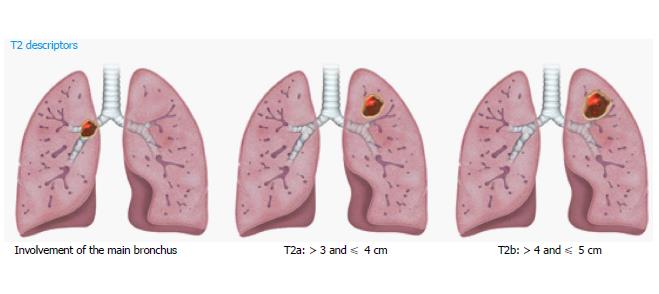
T2 lung cancers measure > 3 and ≤ 5 cm, and are subdivided into T2a and T2b using the 4-cm cutoff (Figure 2). Additional features in the new T2 classification include involvement of any part of the main bronchus, regardless of the distance to (but not involving) the carina. After adjusting for tumor size, partial or complete atelectasis, and pneumonitis secondary to airways invasion, these features still showed better prognosis than T3 tumors with different descriptors. Involvement of lung hilar fat is classified as T2 in 8th TNM, as are tumors involving the visceral pleura. However, involvement of a structure by a tumor that extends from a nodal metastasis (e.g., left recurrent laryngeal nerve involved by an aortopulmonary window node metastasis) is not regarded as T involvement[10].
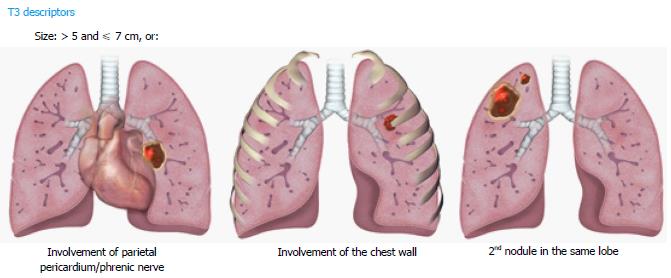
For the T3 category, the new database analysis showed that tumors measuring > 5 and ≤ 7 cm, separate tumor nodules in the same lobe as the primary lesion, and invasion of structures such as the chest wall, phrenic nerve, and parietal pericardium had similar 5-year survival rate. Therefore, these criteria are described in the T3 category (Figure 3). Since the involvement of the parietal pericardium is classified as T3, invasion of the fat overlying the pericardium should be classified T3 rather than T4.
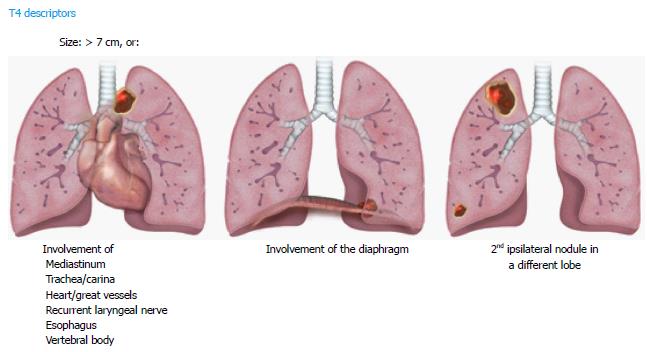
For the T4 descriptors, tumors measuring > 7 cm in longest diameter, and tumor nodules in the same lung but different lobes as the primary lesion are included. Invasion of the diaphragm has also been upstaged to a T4 descriptor (previously T3). This is based on the 5-year survival rate, like other T4 lesions (Figure 4). Invasion of the mediastinal fat (excluding the extrapericardial fat) is classified as T4, in addition to other mediastinal structures like trachea, carina, great vessels, esophagus, recurrent laryngeal nerve, and spine, since they were associated with similar 5-year survival rate and were not changed. An important change has been to eliminate the invasion of the mediastinal pleura as a descriptor in 8th TNM, given the inconsistency of this finding at both clinical and pathologic staging. However, involvement of the visceral pericardium is designated as T4. A superior sulcus tumor with clear involvement of the C8 or higher nerve roots or cords of the brachial plexus, subclavian vessels, and vertebral body, lamina, or spinal canal is also classified as T4, however a tumor is classified as T3 if it involves only thoracic nerve roots (e.g., a tumor involving only T1 and T2 nerve roots)[10].
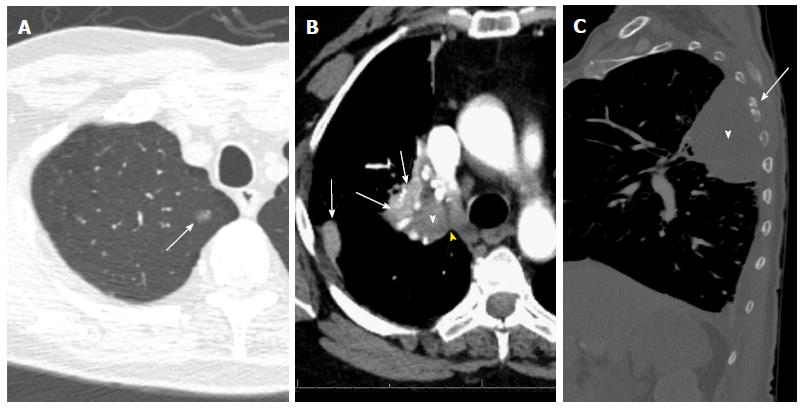
When multiple T descriptors are applicable to a tumor, the highest T category should be used to determine the category. For example, a small tumor with a higher T category by invasion should be classified by the invasion, and a large tumor with a lesser degree of invasion should be categorized by the size (e.g., a 5.3-cm tumor invading the main bronchus should be classified as T3). Some examples on how to apply the new T descriptors are shown in Figure 5.
In patients with NSCLC, lymph nodes measuring more than 1 cm in short axis on CT scans or MRI are abnormal. Reported sensitivity and specificity of CT scans for the detection of pathologic nodes is 51%-64% and 74%-86%, respectively[12]. 18-FDG PET/CT has been shown to have higher accuracy than CT or MRI, with a sensitivity of 58%-94% and specificity of 76%-96% for detection of mediastinal lymph node metastasis[13]. Contrast enhanced CT scan and 18-FDG PET/CT scans are routinely used in the clinical staging (cN) of NSCLC, whereas pathologic stage (pN) is based on histological findings.
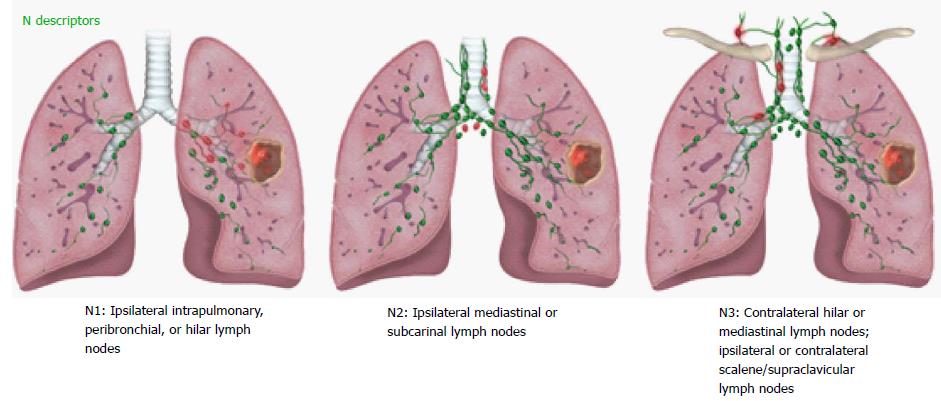
The N staging descriptors for the 8th TNM were validated in 70336 lung cancer patients using descriptors previously used in 6th TNM and 7th TNM. No changes to the N descriptors were proposed in 8th TNM as the four N categories (N0, N1, N2, N3) based on the location of the pathologic nodes have shown to consistently predict distinct prognosis[14] (Table 3). An illustrated scheme with the current N descriptors is provided in Figure 6. Involvement of ipsilateral peribronchial and/or ipsilateral hilar nodes and intrapulmonary nodes, including direct involvement is classified as N1. Involvement of ipsilateral mediastinal nodes and/or subcarinal nodes is classified as N2. Involvement of contralateral mediastinal/hilar nodes or ipsilateral/contralateral supraclavicular/scalene nodes is classified as N3. It has been suggested that addition of the number of involved nodes in N1 and N2 locations and presence or absence of skip metastases can improve the prognostic significance of the anatomic location of involved nodes. The international association for study of lung cancer (IASLC) recommends clinical documentation of these additional parameters in 8th TNM for further testing.
| 5-yr survival | |
| N0 | 60% |
| N1 | 37% |
| N2 | 23% |
| N3 | 9% |
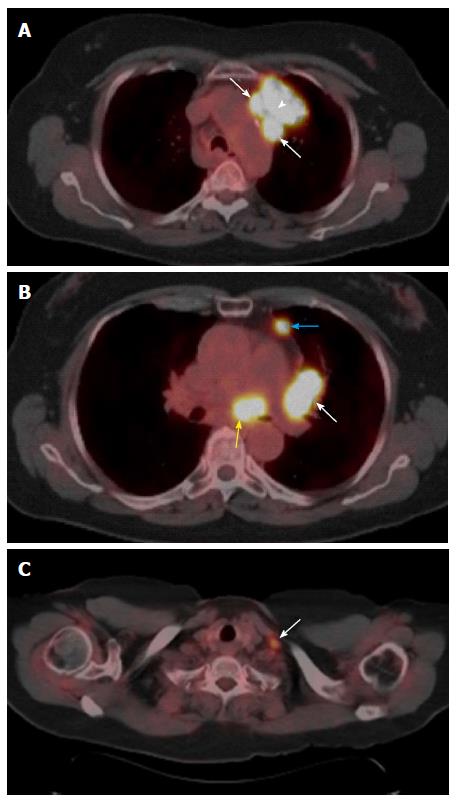
IASLC lymph node map provides detailed anatomic and zonal definitions for all lymph node stations[15,16]. Some examples on how to apply the new N staging descriptors are provided in Figure 7.
Metastatic disease is present in approximately 40% of patients with lung cancer at the time of initial staging with the most common sites of disease being liver, brain, bone, adrenal gland, and contralateral lung. Lung cancer metastases can occur through different routes, including hematogenous, lymphatic, airways and through air spaces. Intrapulmonary metastasis has also been used in the context of two or more malignant pulmonary lesions and no other sites of cancer. Metastatic disease may preclude surgical resection depending on the site and number of metastases. Oligometastatic disease is defined as the limited metastatic disease that may include from 1 to 5 lesions with studies showing that patients with treated oligometastases have better clinical outcomes[17].
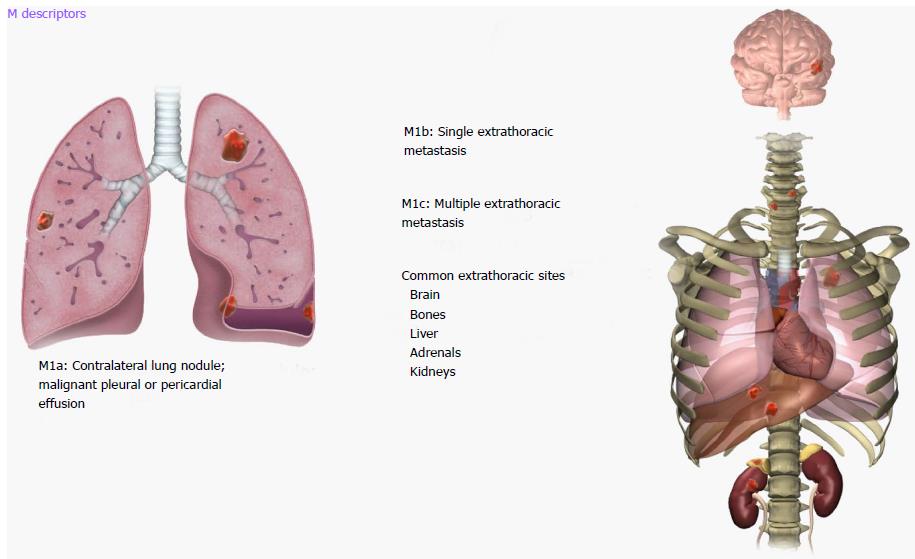
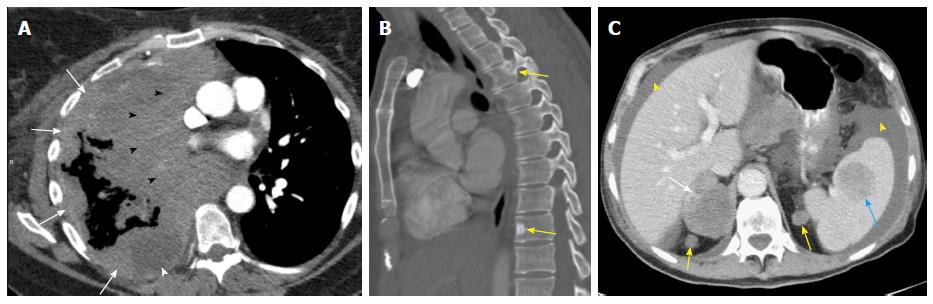
Proposed 8th revisions to the TNM staging system are based on significant differences in patient survival and include significant changes to the M descriptors: M0-M1 (M1 with M1a, M1b and M1c subcategories) and overall stage groupings M descriptors. An illustrated scheme of the updated M descriptors is demonstrated in Figure 8. M1a disease has not changed and comprises of intrathoracic metastases including contralateral pulmonary tumor nodules, pleural/pericardial metastatic effusion/nodules and combination of multiple of these M1a descriptors. However, extrathoracic metastatic disease has been split into M1b and M1c components based on single or multiple metastases due to significant differences in survival amongst these categories (Table 4). A single metastatic lesion involving a single distant organ is now classified as M1b category. Therefore, new M1b descriptor includes single metastatic lesion in various organs like brain, liver, bone, distant lymph node or peritoneum, skin, and adrenal. On the other hand, multiple metastases, irrespective of whether in a single distant organ or multiple distant organs, are now classified as the new M1c category. No differences in survival have been shown among different M1a descriptors or between single and multiple M1a descriptors. M1b metastatic disease is associated with survival that is similar to intrathoracic metastasis (i.e., M1a disease). Averages survival rates are 11.4 mo for M1b and 6.3 mo for M1c disease[11,17].
| Median survival (in months) | |
| M1a | 11.4 |
| M1b | 11.4 |
| M1c | 6.3 |
IASLC recommends reporting the following information during staging for metastatic disease[17]: (1) identification and location of the involved site; (2) differentiation between intra- and extrathoracic metastasis; (3) number and diameter of metastatic lesions and number of involved distant organs; and (4) 18-FDG PET positivity (if available).
Reclassified M descriptors in the 8th revision, besides maintaining the compatibility with the M descriptors of the previous 7th edition, provide better definition of oligometastatic disease, and ability to predict prognosis, thus helping to meet the objectives of the new TNM classification[17]. Some examples of the new M descriptors are provided in Figure 9.
For the validation of the staging groups in 8th TNM, a random training set was selected from the 1999-2000 IALSC database, including 25911 and 599 cases staged M0 and M1, respectively[18]. A tree-based model algorithm for the survival data using log-rank test statistics was used for recursive partitioning and selection of the staging groups. The 8th TNM group candidates were validated by assessing the overall survival per clinical, pathologic, and best stage. Differences in the hazard ratios between adjacent stage groups were analyzed using Cox regression analysis, adjusted for age, performance status, and cell type.
The hazard ratios between adjacent stage groups were significantly different in both 7th TNM and 8th TNM editions, albeit the latter introduces 4 additional levels of discrimination to the older system[18]. Thus, the new stage groups proposed by 8th TNM could determine the 5-year survival with a higher level of detail. The goodness of fit of the survival curves (R2) were slightly better from the 7th to the 8th TNM editions for both pathologic (R2 of 45.7 vs 46.9, respectively) and clinical stage models (R2 of 67.5 vs 68.3, respectively). Tables 5 and 6 demonstrate the new stage groups of 8th TNM and the associated 5-year survival for clinical stages respectively.
| N0 | N1 | N2 | N3 | ||
| T1/M0 | T1a | IA1 | IIB | IIIA | IIIB |
| T1b | IA2 | IIB | IIIA | IIIB | |
| T1c | IA3 | IIB | IIIA | IIIB | |
| T2/M0 | T2a | IB | IIB | IIIA | IIIB |
| T2b | IIA | IIB | IIIA | IIIB | |
| T3/M0 | IIB | IIIA | IIIB | IIIC | |
| T4/M0 | IIIA | IIIA | IIIB | IIIC | |
| TX/M1 | M1a | IVA | IVA | IVA | IVA |
| M1b | IVA | IVA | IVA | IVA | |
| M1c | IVB | IVB | IVB | IVB |
| 5-yr survival | |
| IA1 | 92% |
| IA2 | 83% |
| IA3 | 77% |
| IB | 68% |
| IIA | 60% |
| IIB | 53% |
| IIIA | 36% |
| IIIB | 26% |
| IIIC | 13% |
| IVA | 10% |
| IVB | 0% |
There are a few challenges related to the latest TNM staging system for lung cancer.
A single system has been proposed to stage NSCLC, small-cell lung cancer (SCLC), and bronchial carcinoids since the 7th TNM edition[4]. The 1999-2010 IALSC database was used for validating the TNM system in 5002 cases of SCLC. Interestingly, carcinoid tumors were excluded from the analysis; therefore, validation for this histologic subtype is still lacking despite the current recommendations[9].
Although prognostic information by T and N descriptors matched between SCLC and NSCL, some peculiarities arose when analyzing the M descriptors. M1b patients with SCLC and single-site brain metastasis had better prognoses than those with single-site metastasis elsewhere. Likewise, the presence of pleural effusion in patients with M1b disease conferred an independent worse prognosis[19].
Specific guidelines have been published for addressing cases with involvement of more than one pulmonary site[20]. IASLC recommends that patients with multiple lung lesions be assessed in a multidisciplinary tumor board. The most important question to be answered is the histology of the separate lesions. For different histologic subtypes, a second primary cancer is favored and separate T, N, and M should be provided for each tumor. If the same histologic subtype is found, a single staging should be provided, with T3 descriptor used for lesions in the same lobe, T4 for ipsilateral lesions in different lobes, and M1a for contralateral lesions.
In patients with multiple groundglass lesions (lepidic tumors), the T descriptor is determined by the size and characteristics of the dominant abnormality. In this setting, the T descriptor can be listed with the total number of lesions indicated in parentheses[20]. For example, a patient with three groundglass nodules, the largest measuring 1.4 cm, would be staged as cT1b(3), regardless of the location of the nodules (i.e., same or different lobes). The N/M staging in this context refers to the whole set of lesions[20].
Diffuse pneumonic-type lung cancer is categorized as T3 if in one lobe, T4 if involving multiple same-side lobes, and M1a if involving both lungs with a single N and M category for all areas of involvement.
Specifications on how to measure the lesion size are clearly addressed in the data, although there is no clear mention about the imaging procedures that should be used to achieve this goal[21,22]. In addition, some lesions with necrosis/cavitation, ill-defined margins, and post obstructive pneumonitis or radiation changes may create a challenge to the most experienced reader[22]. Figure 10 illustrates one challenging case for radiologists. Multiple parameters like slice-thickness, reconstruction settings, degree of inspiration, and other scanner parameters are known to affect the observed size, with significant inter and intra-observer variability for size measurements in smaller lesions[23]. Automatic and semiautomatic 3D tools for assessing lesion size are more available, resulting in less variability[24], and may guide future improvements in the TNM system.
For subsolid lesions, the maximum dimension of the solid component on imaging or the invasive component on microscopy is used to assign the T category[21]. However, it is recommended that the maximum dimension of the groundglass or lepidic component be also noted.
The pathologic changes seen in lymphangitis carcinomatosa are characterized by the infiltration of pulmonary lymphatics by malignant cells, usually presenting with symptoms of hypoxemia, with poor prognosis[25] The classic appearance on CT is thickening (smooth, irregular, or nodular) of the peribronchovascular and interlobular interstitium[26]. Despite its potential prognostic information, lymphangitis carcinomatosa has not been added as a descriptor in neither the 7th nor the 8th TNM editions[10,22].
Although the N classification has not changed since the prior 7th edition, a significant limitation of the new IASLC lung cancer database must be mentioned. The anatomical location of lymph node involvement in the new database was defined by either the Naruke (for Japanese data) or Mountain-Dresler modification of the American Thoracic Society MDATS (for non-Japanese data) nodal charts, which are discrepant in the definition of subcarinal nodes. Naruke map considers subcarinal lymph nodes along the inferior border of the main stem bronchus to be station 10 (N1), whereas MDATS map considers them station 7 (N2) nodes. Further validation of the unified nodal chart sponsored by IALSC[15] must be pursued in the future.
In addition to the site analysis, another analysis was performed to determine the prognostic impact of adding the number of involved lymph node stations and presence of skip metastases to the present nodal categories. According to the number of involved lymph node stations (single vs multiple), pN categories were further subdivided: N1 was divided into N1-single (N1a) and N1-multiple (N1b) and N2 was divided into N2-single (N2a) and N2-multiple (N2b). N2a was further subdivided into single skip-metastatic N2 node without N1 involvement (N2a1) and single N2 node with N1 involvement (N2a2). Patients with pN2 metastasis at a single lymph node station without hilar involvement (skip metastasis) had better survival than those with pN1 metastasis at multiple stations. The number of involved nodal stations was found to have prognostic impact on pathologic staging, but was not validated in clinical staging.
There is lack of consensus in how to treat less common nodal sites of metastasis, such as internal thoracic, cardiophrenic, axillary, subpectoral, and extrathoracic chains. Some authors suggest that all chains not included in the N descriptors be considered as M1 disease[16,17], whereas others consider N3 disease for specific sites (e.g., axillary chains)[27]. Further clarification is thus necessary.
Although the new M descriptors evolved to distinguish single-site vs multiple-site metastasis, no detailed distinction is made based on the specific site or metastatic burden[10]. However, IASLC made recommendation for recording ancillary information that may provide basis for future development, including the number of metastatic lesions, diameter of individual metastatic lesions, and number of involved organs. Moreover, recording the histologic confirmation and/or standard uptake values from 18-FDG PET/CT of metastatic sites is also suggested for future analysis[17].
Specific efforts to standardize imaging acquisition and interpretation protocols are necessary for improving efficacy, reproducibility, and communication of radiologic results. Pathways mapped by other radiology-led programs, such as lung cancer screening[28], may provide future directions in lung cancer staging to be followed by radiologic societies.
The 8th edition of the TNM system for lung cancer staging consolidates and expands the base of evidence currently used for predicting prognosis and guiding patient treatment. As new stage groups are shown to demonstrate better precision for determining prognosis, it is expected that improvements in tailoring patient treatment will follow with adoption of the new staging system. It is of utmost importance that radiologists familiarize with the new system to provide accurate communication with referring physicians. Further collection of prospective data and detailed attention to unanswered questions will guide developments for future staging systems.
The authors would like to acknowledge the contribution of Erin Moore, MA with illustrations for this manuscript.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tirumani SH, van Beek EJR S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol. 2016;893:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1259] [Article Influence: 125.9] [Reference Citation Analysis (0)] |
| 2. | SEER NCI. Cancer Stat Facts: Lung and Bronchus Cancer (2016). Available from: https://seer.cancer.gov/statfacts/html/lungb.html. |
| 3. | Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D’Amico TA, Dilling TJ. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 4.2016. J Natl Compr Canc Netw. 2016;14:255-264. [PubMed] |
| 4. | Bierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 8th ed: Wiley 2017; . |
| 5. | Gospodarowicz MK, Miller D, Groome PA, Greene FL, Logan PA, Sobin LH. The process for continuous improvement of the TNM classification. Cancer. 2004;100:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E. The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J Radiol. 2012;4:128-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 155] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (2)] |
| 7. | Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2389] [Cited by in RCA: 2581] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 8. | Giroux DJ, Rami-Porta R, Chansky K, Crowley JJ, Groome PA, Postmus PE, Rusch V, Sculier JP, Shepherd FA, Sobin L. The IASLC Lung Cancer Staging Project: data elements for the prospective project. J Thorac Oncol. 2009;4:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Rami-Porta R, Bolejack V, Giroux DJ, Chansky K, Crowley J, Asamura H, Goldstraw P. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2014;9:1618-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 257] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 10. | Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017;151:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 1113] [Article Influence: 123.7] [Reference Citation Analysis (1)] |
| 11. | Detterbeck FC, Chansky K, Groome P, Bolejack V, Crowley J, Shemanski L, Kennedy C, Krasnik M, Peake M, Rami-Porta R. The IASLC Lung Cancer Staging Project: Methodology and Validation Used in the Development of Proposals for Revision of the Stage Classification of NSCLC in the Forthcoming (Eighth) Edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2016;11:1433-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 12. | Walker CM, Chung JH, Abbott GF, Little BP, El-Sherief AH, Shepard JA, Lanuti M. Mediastinal lymph node staging: from noninvasive to surgical. AJR Am J Roentgenol. 2012;199:W54-W64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Schimmer C, Neukam K, Elert O. Staging of non-small cell lung cancer: clinical value of positron emission tomography and mediastinoscopy. Interact Cardiovasc Thorac Surg. 2006;5:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF, Watanabe H, Wu YL, Zielinski M, Ball D. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2015;10:1675-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 502] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 15. | Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 813] [Article Influence: 50.8] [Reference Citation Analysis (1)] |
| 16. | El-Sherief AH, Lau CT, Wu CC, Drake RL, Abbott GF, Rice TW. International association for the study of lung cancer (IASLC) lymph node map: radiologic review with CT illustration. Radiographics. 2014;34:1680-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Eberhardt WE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A, Goldstraw P, Rami-Porta R. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2015;10:1515-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 18. | Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3190] [Cited by in RCA: 3113] [Article Influence: 345.9] [Reference Citation Analysis (0)] |
| 19. | Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, Eberhardt WE, van Meerbeeck J, Rami-Porta R. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:300-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 283] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 20. | Detterbeck FC, Marom EM, Arenberg DA, Franklin WA, Nicholson AG, Travis WD, Girard N, Mazzone PJ, Donington JS, Tanoue LT. The IASLC Lung Cancer Staging Project: Background Data and Proposals for the Application of TNM Staging Rules to Lung Cancer Presenting as Multiple Nodules with Ground Glass or Lepidic Features or a Pneumonic Type of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J Thorac Oncol. 2016;11:666-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 21. | Travis WD, Asamura H, Bankier AA, Beasley MB, Detterbeck F, Flieder DB, Goo JM, MacMahon H, Naidich D, Nicholson AG. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2016;11:1204-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 523] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 22. | Carter BW, Godoy MC, Wu CC, Erasmus JJ, Truong MT. Current Controversies in Lung Cancer Staging. J Thorac Imaging. 2016;31:201-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Brandman S, Ko JP. Pulmonary nodule detection, characterization, and management with multidetector computed tomography. J Thorac Imaging. 2011;26:90-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Petrick N, Kim HJ, Clunie D, Borradaile K, Ford R, Zeng R, Gavrielides MA, McNitt-Gray MF, Lu ZQ, Fenimore C. Comparison of 1D, 2D, and 3D nodule sizing methods by radiologists for spherical and complex nodules on thoracic CT phantom images. Acad Radiol. 2014;21:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Bruce DM, Heys SD, Eremin O. Lymphangitis carcinomatosa: a literature review. J R Coll Surg Edinb. 1996;41:7-13. [PubMed] |
| 26. | Munk PL, Müller NL, Miller RR, Ostrow DN. Pulmonary lymphangitic carcinomatosis: CT and pathologic findings. Radiology. 1988;166:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 87] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Nair A, Klusmann MJ, Jogeesvaran KH, Grubnic S, Green SJ, Vlahos I. Revisions to the TNM staging of non-small cell lung cancer: rationale, clinicoradiologic implications, and persistent limitations. Radiographics. 2011;31:215-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | McKee BJ, Regis SM, McKee AB, Flacke S, Wald C. Performance of ACR Lung-RADS in a clinical CT lung screening program. J Am Coll Radiol. 2015;12:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |