Published online Apr 28, 2017. doi: 10.4329/wjr.v9.i4.212
Peer-review started: November 8, 2016
First decision: January 16, 2017
Revised: January 31, 2017
Accepted: February 28, 2017
Article in press: March 2, 2017
Published online: April 28, 2017
Processing time: 174 Days and 6.7 Hours
To retrospectively compare the outcomes of catheter drainage, urokinase and ozone in management of empyema.
Retrospective study included 209 patients (111 males and 98 females; age range 19 to 72 years) who were diagnosed with empyema. The patients were divided into 3 groups based on the therapy instituted: catheter drainage only (group I); catheter drainage and urokinase (group II); catheter drainage, urokinase and ozone (group III). Drainage was considered successful if empyema was resolved with closure of cavity, clinical symptoms were resolved, and need for any further surgical procedure was avoided. Success rate, length of stay (LOS), need for further surgery and hospital costs were compared between the three groups using the Kruskall-Wallis nonparametric test, with P < 0.05 considered significant.
Of the 209 patients with empyema, all catheters were placed successfully under CT guidance. Sixty-three patients were treated with catheters alone (group I), 64 with catheters and urokinase (group II), and 82 with catheters, urokinase and ozone (group III). Group I, group II and group III had success rates of 62%, 83% and 95% respectively (P < 0.05). Group I and group II had statistically longer LOS (P < 0.05) and higher hospital costs (P < 0.05) compared to group III. There were statistically significant differences between the three groups when comparing patients who converted into further surgery.
The combination of chest tube drainage, urokinase and ozone is a safe and effective therapeutic modality in thoracic empyema.
Core tip: The use of ozone as an auxiliary antibacterial agent has achieved a relative good result. CT imaging-based guidance offers precise targeting, which is crucial to the success rates of therapeutic treatment. The combination of chest catheter drainage, urokinase and ozone is a safe and effective therapeutic treatment in thoracic empyema.
- Citation: Li B, Liu C, Li Y, Yang HF, Du Y, Zhang C, Zheng HJ, Xu XX. Computed tomography-guided catheter drainage with urokinase and ozone in management of empyema. World J Radiol 2017; 9(4): 212-216
- URL: https://www.wjgnet.com/1949-8470/full/v9/i4/212.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i4.212
Empyema refers to a collection of pus in the pleural cavity that can occur due to lung infection, trauma or surgery, with a mortality rate approaching 20% in adults[1]. Treatment options in the management of empyema include antibiotic therapy, thoracocentesis, drainage using intercostal catheter (ICC) with or without adjunctive fibrinolytic therapy, thoracoscopy, and open thoracotomy and decortications[2].
Image-guided percutaneous catheter drainage (IGPCD) has been shown to be a safer and more effective alternative to ICC, because IGPCD provides direct demonstration of the fluid collection. Thus, considering the advantages of IGPCD, which has less pain and lower postoperative morbidity as well as fewer complications, it has become a reasonable procedure to address various stages of empyema. But there remains much controversy, especially in multiloculated pleural empyema[3-6]. Some studies showed a higher rate of conversions to open decortications during the drainage procedure.
Intrapleural administration of fibrinolytic agents have been used both in pediatric and adult populations without subjection to surgical procedures[7,8]. Ozone has bactericidal, antiviral and antifungal properties and is used empirically for the treatment of chronic wounds, such as trophic ulcers, ischemic ulcers and diabetic wounds[9]. The purpose of this study was to compare the outcomes of simple catheter drainage alone against its treatment with urokinase and the combined treatment of urokinase with ozone for the management of empyema. To our knowledge there has not been a similar study published to date.
Approval was obtained from the Hospital Institutional Review Board to perform a retrospective review of patients between January 2012 and September 2016. The study included 209 patients (111 males, 98 females; mean age: 34.5 years, range: 19-72 years) who were diagnosed with empyema. All patients were at the fibropurulent stages of empyema. Patients who were at organizational stage were excluded. Of the 209 patients, 75 patients had postoperative empyema, and 134 had para-pneumonic empyema. Patients with tuberculosis or diabetes were excluded from this study.
The patients were divided into three groups based on the therapy instituted: Catheter drainage only (group I); catheter drainage with urokinase (group II); and catheter drainage with the combined treatment of urokinase and ozone (group III). CT scans were performed on all patients to define the extent, location, and number of locations.
All catheters were placed by CT-guided procedures and were performed under local anesthesia. By using the Seldinger technique, 8F to 14F pigtail catheters (total 240) were placed into the patients. The choice of catheter depended on the viscosity of the initial aspirate.
In group II, 50000 units of urokinase (Everbright Pharmaceutical Co., Ltd. Shengyang, China) diluted in 20.0 mL normal saline was injected into the pleural space via a pigtail catheter. The catheter was clamped for 4 h following urokinase injection. Then, the catheter was left unclamped for 20 min to allow for open drainage. In group III, in addition to urokinase, 10.0-20.0 mL (according to the size of the empyema cavity) oxygen-ozone gas mixture (ozone concentration 25 μg/mL) was administered through the catheter per day. The oxygen-ozone gas mixture was produced immediately prior to injection by using an ozone generator (Herrmann, Kleinwallstadt, Germany).
Drainage was considered successful when: (1) clinical symptoms were resolved; (2) the empyema cavity was closed; and/or (3) no further surgical procedure was needed. The patients were referred for further surgical management when: (1) the empyema failed to resolve; (2) if the follow-up imaging showed the development of a thick pleural peel with the absence of re-expansion of the lung; or (3) if the patients failed to show clinical improvement.
The difference of patient characteristics among the three groups were evaluated as well as the technical success of catheter placement and length of hospital stay (LOS). Clinical details such as hospital stay, fever (morning oral temperature of > 37.5 °C), conversion into further surgery and average hospital charges were also recorded.
Statistical analysis was used to compare the three groups. Mean and standard deviation (SD) of variables from each group were obtained. Statistical differences among groups were calculated by using the Kruskall-Wallis non-parametric test, with a P value < 0.05 considered significant. Descriptive statistical analysis was also performed. All statistical analyses were conducted with SPSS software (version 19.0; IBM Corporation, Armonk, NY, United States).
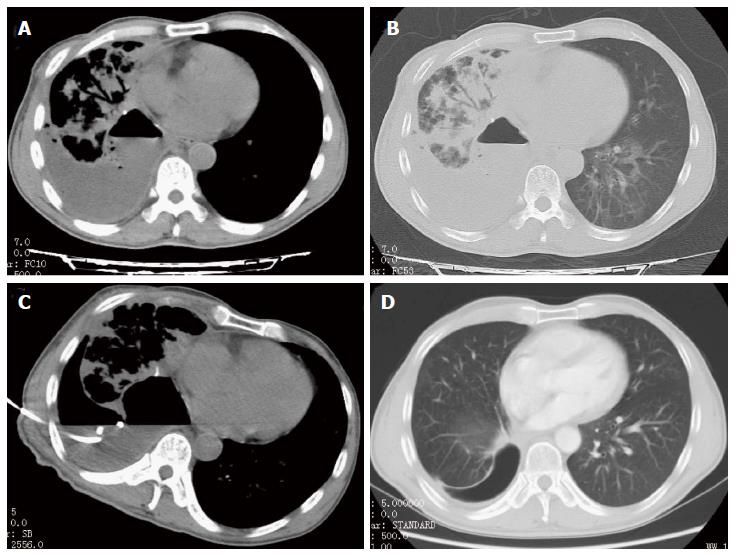
For the 209 patients with empyema, all catheters were successfully placed under CT-guidance (Figure 1). Sixty-three (30.1%) patients were treated with catheters alone (group I), 64 (30.6%) with catheters and urokinase (group II), and 82 (39.3%) patients with catheters, urokinase and ozone (group III). All of the different variables from the three treatment groups are listed in Table 1. The three groups of patients were similar in mean age.
| Variable | Group I | Group II | Group III |
| Patients, n | 63 | 64 | 82 |
| Average age in years | 36.7 (14.3) | 29.6 (12.6) | 38.2 (19.1) |
| Duration of symptoms in days | 12.4 (8.2) | 14.8 (6.3) | 13.7 (5.9) |
| Initial WBC, × 103 | 16.4 (8.8) | 16.9 (7.6) | 17.1 (8.7) |
| Total lymphocytes, × 103 | 13.2 (6.9) | 14.4 (7.4) | 14.7 (7.3) |
Although duration of symptoms, initial white blood cell count, and lymphocytes were different in three groups, the differences were not significant among the three groups. Groups I, II and III demonstrated a success rate of 62%, 83% and 95% respectively (P < 0.05) (Table 2). Moreover, compared to group III, groups I and II had a statistically longer LOS (P < 0.05), longer duration of fever (P < 0.05) and higher hospital costs (P < 0.05). Hospital charges for groups I and II were statistically higher than for group III.
| Variable | Group I | Group II | Group III |
| Technical success of catheter placement | 100% | 100% | 100% |
| Success rate of management | 62% | 83% | 95% |
| Length of hospital stay in days | 21.0 (7.4) | 19.4 (5.7) | 12.8 (5.4) |
| Duration of fever in days | 4.2 (3.0) | 3.7 (2.6) | 2.1 (1.8) |
| Converted into further surgery | 8 (32%) | 8 (25%) | 5 (12%) |
| Average hospital charges in RMB¥ | 25057 | 19814 | 15871 |
There were also statistically significant differences between the three groups when comparing patients who converted into further surgery. There were no statistically significant differences between groups I and II when comparing LOS, duration of fever, and hospital charges. Complications appeared in 4 patients who had a small amount of asymptomatic pneumothorax. No other complications were noted.
Many institutions are now using antibiotics with image-guided catheter drainage and fibrinolytics as first-line therapy[10,11]. However, higher mortality rates (10%-15%) have been reported for drainage and irrigation of empyema even in early stages, whereas decortications in series including chronic cases have produced a mortality rate of 4%-13% only[7]. The study by Maskell et al[12] showed that the intra-pleural instillation of fibrinolysis did not reduce mortality. They associated the failure of conservative treatment with loculated collections; fibrinolysis alone did not produce sufficient clearance of pleural fluid, possibly because the infected pleural fluid was viscous, lumpy, and resistant to tube drainage[12].
To improve the effects of treatment, in the present study, catheter drainage was combined with urokinase and ozone treatment for empyema, and achieved an overall success rate of 95%. This was a relatively higher success rate than reported from previous studies, which had 72%-87% success rates in various smaller series[11,13]. And, as a result, LOS and hospital costs were decreased significantly in our series.
Ozone therapy has been utilized and its benefits studied for more than a century now. Ozone, in the gaseous or aqueous phase, has been shown to be a powerful and reliable anti-microbial agent against bacteria, fungi, protozoa and viruses[14]. It acts by inactivating bacteria, viruses, fungi, yeast and protozoa, which stimulates oxygen metabolism and the immune system[14]. The oxidant potential of ozone induces the destruction of cell walls and the cytoplasmic membranes of bacteria and fungi by acting on glycoproteins, glycolipids and other amino acids, as well as inhibiting the key enzymes of the cells resulting in the increased membrane permeability and death[14].
Because most patients have mixed infections, the use of antibiotics cannot reach adequate results. Also, the longtime use of antibiotics can lead to bacterial resistance[15]. Even then, sometimes the causative organism may not be identified as the patients might have been partially treated before any specimen could be obtained for sensitivity testing. The use of ozone as an auxiliary antibacterial agent has achieved a relatively good result.
In the management of chronic wounds, such as trophic ulcers, ischemic ulcers and diabetic wounds, ozone has been used empirically as a clinical therapeutic agent[16]. The beneficial effects of ozone on wound healing might be assumed to be due to ameliorated impaired dermal wounds healing or increased oxygen tension by ozone exposure in the wound area[16].
Ozone exposure has been associated with activation of transcription factors that are required for regulation of the inflammatory response and the entire process of wound healing[14]. In addition, ozone can be fully diffused into the abscess cavity, causing abscess wall dehydration. It can also split the inflammatory separations and expand drug solution distribution[14]. So, the combined treatment with urokinase and ozone has a synergistic effect in the treatment for empyema.
The present study was performed under CT-guidance. CT imaging-based guidance offers precise targeting, which is crucial to the success rate of therapeutic treatment. By such procedures, a better therapeutic effect was obtained compared to the standard ICC treatment. The use of CT imaging was also advantageous when the presence of air resulted in poorly defined collection under ultrasound guidance or collection volume was small and adjacent to mediastinal structures. CT imaging can display the package of empyema clearly, and provide the best puncture path for the use of the catheter to separate the loculated empyema.
Complications of catheter drainage include hemorrhage from intercostal vessel injury, subcutaneous emphysema, pneumothorax and catheter-related pain[1,3,6]. The patients in this study did not encounter any major complications nor did any of the patients in group II and III have any significant clinical bleeding.
The results presented in this study are retrospective and represent an institutional bias. A larger prospective randomized trial is warranted to further evaluate the role of urokinase with ozone in treating this important clinical scenario.
In conclusion, urokinase and ozone are a useful adjunct in the management of empyema. This technique used early in the exudative and fibropurulent stage of effusion, can decrease the rate of surgical interventions and the length of hospital stay, with minor associated morbidity. It can be concluded that the combined treatment of chest catheter drainage, urokinase and ozone is a safe and effective therapeutic procedure in thoracic empyema, rather than catheter drainage only.
In daily clinical work, the treatment of empyema is not satisfactory. And, according to recent reports, the mortality rates range from approximately 10% to 15% in early stages of drainage and irrigation of empyema. The authors sought to improve the effects of this treatment further. Ozone, in the gaseous or aqueous phase, has been shown to be a powerful and reliable antimicrobial agent against bacteria, fungi, protozoa and viruses.
Now, many institutions are using antibiotics with image-guided catheter drainage and fibrinolytics as first-line therapy of empyema. And, to date, the application of ozone in the treatment of empyema has been infrequently reported.
The use of ozone combined with urokinase in the management of empyema is unusual. The authors compared the outcomes of three groups and found that the combination of chest catheter drainage, urokinase and ozone is a safe and effective therapeutic treatment of thoracic empyema. In addition, CT imaging-based guidance offers precise targeting, which is crucial to the success rate of therapeutic treatment.
Urokinase and ozone are a useful adjunct in the management of empyema, and the patients in this study obtained a preferable outcome.
IGPCD: Image-guided percutaneous catheter drainage; LOS: Length of hospital stay.
The article is good.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Lassandro F, Martusevich AK, Markic D, Sartori S, Schoenhagen P S- Editor: Ji FF L- Editor: Filipodia E- Editor: Wu HL
| 1. | Chung JH, Lee SH, Kim KT, Jung JS, Son HS, Sun K. Optimal timing of thoracoscopic drainage and decortication for empyema. Ann Thorac Surg. 2014;97:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Heffner JE, Klein JS, Hampson C. Interventional management of pleural infections. Chest. 2009;136:1148-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Maier A, Domej W, Anegg U, Woltsche M, Fell B, Pinter H, Smolle-Jüttner FM. Computed tomography or ultrasonically guided pigtail catheter drainage in multiloculated pleural empyema: a recommended procedure? Respirology. 2000;5:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Thourani VH, Brady KM, Mansour KA, Miller JI, Lee RB. Evaluation of treatment modalities for thoracic empyema: a cost-effectiveness analysis. Ann Thorac Surg. 1998;66:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Carr JA, Fales C, Shaikh IA, Foulds K. Computed tomographic modeling before and after treatment for posttraumatic empyema: early decortication is superior to catheter drainage. Ann Thorac Surg. 2011;91:1723-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Yu D, Buchvald F, Brandt B, Nielsen KG. Seventeen-year study shows rise in parapneumonic effusion and empyema with higher treatment failure after chest tube drainage. Acta Paediatr. 2014;103:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Gates RL, Hogan M, Weinstein S, Arca MJ. Drainage, fibrinolytics, or surgery: a comparison of treatment options in pediatric empyema. J Pediatr Surg. 2004;39:1638-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Mahant S, Cohen E, Weinstein M, Wadhwa A. Video-assisted thorascopic surgery vs chest drain with fibrinolytics for the treatment of pleural empyema in children: a systematic review of randomized controlled trials. Arch Pediatr Adolesc Med. 2010;164:201-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Kim HS, Noh SU, Han YW, Kim KM, Kang H, Kim HO, Park YM. Therapeutic effects of topical application of ozone on acute cutaneous wound healing. J Korean Med Sci. 2009;24:368-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Cremonesini D, Thomson AH. How should we manage empyema: antibiotics alone, fibrinolytics, or primary video-assisted thoracoscopic surgery (VATS)? Semin Respir Crit Care Med. 2007;28:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Misthos P, Sepsas E, Konstantinou M, Athanassiadi K, Skottis I, Lioulias A. Early use of intrapleural fibrinolytics in the management of postpneumonic empyema. A prospective study. Eur J Cardiothorac Surg. 2005;28:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Maskell NA, Davies CW, Nunn AJ, Hedley EL, Gleeson FV, Miller R, Gabe R, Rees GL, Peto TE, Woodhead MA. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med. 2005;352:865-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 497] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 13. | Silverman SG, Mueller PR, Saini S, Hahn PF, Simeone JF, Forman BH, Steiner E, Ferrucci JT. Thoracic empyema: management with image-guided catheter drainage. Radiology. 1988;169:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 99] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Elvis AM, Ekta JS. Ozone therapy: A clinical review. J Nat Sci Biol Med. 2011;2:66-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 15. | Kwon YS. Pleural infection and empyema. Tuberc Respir Dis (Seoul). 2014;76:160-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Andreula C. Ozone therapy. Neuroradiology. 2011;53 Suppl 1:S207-S209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |