Published online Apr 28, 2017. doi: 10.4329/wjr.v9.i4.191
Peer-review started: August 8, 2016
First decision: September 28, 2016
Revised: October 25, 2016
Accepted: December 13, 2016
Article in press: December 14, 2016
Published online: April 28, 2017
Processing time: 265 Days and 22.5 Hours
To apply dual-source multidetector computed tomography (DSCT) scanning technology in conjunction with computationally assisted segmentation in order to explore and document skeletal variation that has occurred over the course of evolution.
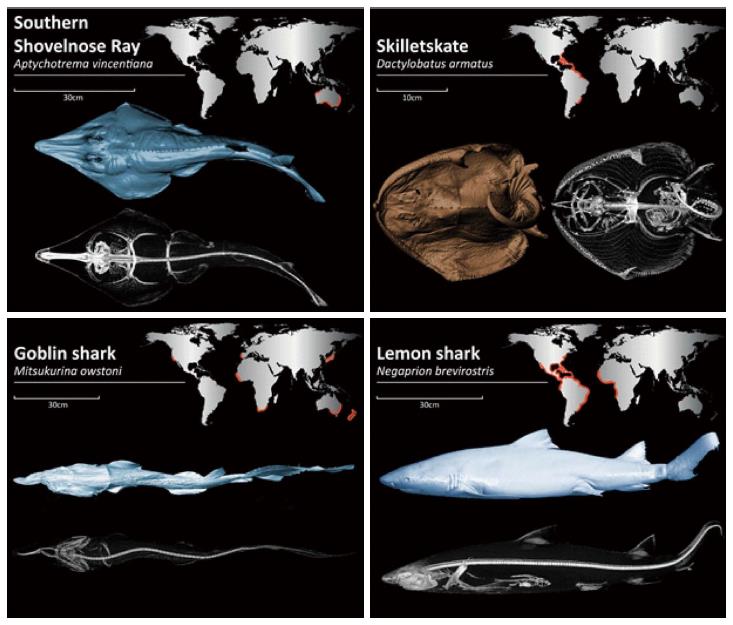
We examined 4 divergent species of elasmobranchs with high-resolution 3rd generation DSCT. The formalin prepared species examined were: Aptychotrema vincentiana, Mitsukurina owstoni, Negaprion brevirostris and Dactylobatus armatus.
All three structures of the hyoid arch (hyomandibular, ceratohyal, and basihyal) were clearly visible whereas in the two batoids, the hyomandibular was the prominent feature, the ceratohyal was not visible and the basihyal was more reduced and closer to the gill arches. The general shape of the puboischiadic bar, or pelvic girdle, illustrated a closer relationship between the two sharks and the two batoids than between the two groups.
In exquisite detail, DSCT imaging revealed important morphological variations in various common structures in the four elasmobranch specimens studied, providing insights into their evolutionary diversification.
Core tip: Computed tomography is a helpful noninvasive imaging tool for comparative biology. The skeletal variations observed through our data will increase our understanding of how the anatomy of these organisms has changed over the course of evolution. The data collected allows for a more comprehensive understanding regarding the evolutionary history of the group.
- Citation: McQuiston AD, Crawford C, Schoepf UJ, Varga-Szemes A, Canstein C, Renker M, De Cecco CN, Baumann S, Naylor GJP. Segmentations of the cartilaginous skeletons of chondrichthyan fishes by the use of state-of-the-art computed tomography. World J Radiol 2017; 9(4): 191-198
- URL: https://www.wjgnet.com/1949-8470/full/v9/i4/191.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i4.191
About 450 million years ago, the evolutionary lineage that lead to sharks, skates, and rays split from the main trunk of the vertebrate tree[1]. One lineage gave rise to bony fishes, tetrapods, amniotes and mammals - including humans - while the other gave rise to modern sharks and rays. The two lineages have been experimenting with different ways of solving environmental challenges for nearly half a billion years. Because they are evolutionarily independent, it is likely that each lineage has developed different solutions to similar challenges and that each harbors architectural attributes and innovations that are lineage specific. Considerable effort has been put into studying the adaptations that have made the lineage leading to humans so successful, but almost nothing is known about the corresponding innovations and adaptations that have made sharks and rays so successful[2,3].
Comparative biology provides an efficient way to reconstruct evolutionary history and to better understand how lineages have overcome environmental challenges. The approach involves exploring the diversity of traits in a suite of related organisms and then organizing the information into an evolutionary hierarchy that reveals how traits have changed over the course of time[4,5]. Traits can be molecular, cellular, physiological or anatomical.
Computed tomography (CT) is an important 3-dimensional (3D) imaging tool for medical and non-medical purposes. Beyond clinical radiology, this modality has previously been described in literature for use in a variety of different fields, including but not limited to aviation security, forensic minimally invasive autopsy, archaeology, and visualization of sarcophagi and mummies[6-8]. Due to its high spatial resolution and non-destructive nature, CT enables the display of distinct skeletal characteristics of different representatives of shark and ray lineages. Furthermore, the high spatial resolution of modern CT technology renders increased material differentiation possible, which may facilitate the assessment of minute disparities within the cartilaginous structures of sharks, skates and rays.
The work presented is a subset of the Chondrichthyan Tree of Life (CToL) project, a multi-institutional endeavor to create a database of evolutionary information for Chondrichthyan fishes (sharks, skates, rays and chimeras). Chondrichthyes are subdivided into two subclasses: Elasmobranchii (sharks, rays and skates) and Holocephali (chimeras). The goal of the CToL is to reconstruct the evolutionary lineages of all extant sharks, skates, rays and chimeras to better understand how these organisms have addressed environmental challenges by collecting both anatomical and genetic information. Thus, we sought to contribute to this body of knowledge through the application of DSCT and computer assisted segmentation to document variations in preserved specimens of sharks and rays.
DSCT scans of 4 highly divergent elasmobranchs: Aptychotrema vincentiana (CSIRO MUW101), Mitsukurina owstoni (HUMZ 204610), Negaprion brevirostris (GMBL uncatalogued) and Dactylobatus armatus (UF 41302), were collected. The geographical distribution associated with each species can be seen in Figure 1. The specimens were preserved with formalin and kept in 70% Ethanol or 50% Isopropanol, sealed and stored in a dark environment and shipped from museums around the world to our institution. Specimens were loaned from Commonwealth Scientific and Industrial Research Organisation (CSIRO; Clayton South, Australia), Hokkaido University (HUMZ; Sapporo, Hokkaido, Japan), Grice Marine Biological Laboratory (GMBL; Charleston, South Carolina, United States), and the University of Florida (UF; Gainesville, Florida, United States). Table 1 provides an overview of the four species. As this study used preserved museum specimens, it was exempt from IACUC approval.
| Southern Shovelnose ray | Skilletskate | Goblin shark | Lemon shark | |
| Scientific name | Aptychotrema vincentiana | Dactylobatus armatus | Mitsukurina owstoni | Negaprion brevirostris |
| Kingdom | Animalia | Animalia | Animalia | Animalia |
| Phylum | Chordata | Chordata | Chordata | Chordata |
| Class | Chondrichthyes | Chondrichthyes | Chondrichthyes subclass: Elasmobranchii | Chondrichthyes subclass: Elasmobranchii |
| Order | Rajiformes | Rajiformes | Lamniformes | Carcharhiniformes |
| Family | Rhinobatidae | Rajidae | Mitsukurinidae | Carcharhinidae |
| Genus | Aptychotrema | Dactylobatus | Mitsukurina | Negaprion |
| Species authority | Haacke, 1885 | Bean and Weed, 1909 | Jordan, 1898 | Poey, 1868 |
| Distribution | Eastern Indian Ocean: endemic to Australia (20°S - 40°S) | Western Central Atlantic: South Carolina, United States to the Gulf of Mexico and along Central America to Venezuela Also found on middle continental slope off southern Brazil (35°N - 35°S, 30°W - 98°W) | Western Atlantic: Guyana, Suriname and French Guiana Eastern Atlantic: France (Bay of Biscay), Madeira, Portugal, and South Africa Western Indian Ocean: off South Africa. Western Pacific: Japan, Australia (South Australia, New South Wales), New Zealand Eastern Pacific: United States (southern California) (8°N - 55°S, 180°W - 180°E) | Western Atlantic: New Jersey, United States to southern Brazil, including the Gulf of Mexico, the Bahamas, and the Caribbean; also in Gulf of Mexico Northeast Atlantic: Senegal, Côte d'Ivoire and probably wide-ranging off West Africa, but this requires confirmation. Eastern Pacific: southern Baja California, Mexico and the Gulf of California to Ecuador (45°N - 39°S, 114°W - 0°) |
| Environment | Marine; demersal; | Marine; bathydemersal; | Marine; bathydemersal; | Marine; brackish; reef-associated; oceano-dromous |
| Depth range: 0-32 m | Depth range: 300-900 m (usually 300-700 m) | Depth range: 30-1300 m (usually 270-960 m) | Depth range: 0-92 m | |
| Size (cm) | Maximun length: 79.0 cm | Maximun length: 32.0 cm | Maximun length: 617.0 cm | Maximun length: 340.0 cm |
| Presented specimen: 72.9 cm | Presented specimen: 23.5 cm | Presented specimen: 119.5 cm | Presented specimen: 95.6 cm | |
| Red list category | Least concern | Data deficient | Least concern | Near Threatened |
| Threat to humans | Harmless | Harmless | Harmless | Minor Threat |
Endemic to Australia, the Southern Shovelnose ray resides primarily in shallow waters on sandy substrates where they feed on crustaceans, molluscs, worms, and other invertebrates[9]. It is easily distinguished by its long triangular snout. They are usually sandy-colored with scattered dark spots, assisting with camouflage on the ocean floor. Reproduction is ovoviviparous, with litters reaching 14-16 in number[10].
Typically found at depths of up to 1300 m, goblin sharks have a nearly global distribution[10]. This shark is distinguished by its disproportionately long, thin snout overhanging rows of sharp, non-serrated fang-like teeth. The highly protrusive jaws extend anteriorly, enabling the Goblin shark to capture cephalopods, crustaceans and bony fish[10]. Goblin sharks have a relatively small optic tectum, indicating that vision is less important to this species; instead they rely heavily on their electro-sensitive snout. Nevertheless, unlike most deep-sea sharks, they have fully functional irises suggesting that they do use vision to some extent. Although a pregnant female is yet to be captured, these sharks are thought to be ovoviviparous. Little is known about their social behavior[10].
Lemon sharks inhabit coral reefs, enclosed sounds, bays, river mouths, and mangrove fringes of coastal inshore waters of the Western Atlantic and Eastern Pacific oceans. They are large, stocky, and blunt nosed with a pair of similar sized dorsal fins; the first just posterior to the pectoral fins and the second just anterior to the origin of the anal fin. They are commonly found in warm, shallow water at depths usually not exceeding 100 m. This species feeds mostly on bony fish[10], however, intra-specific predation of juvenile lemon sharks by adults has been observed[11]. Group living and social behavior is frequently observed among juveniles and thought to be important to survival, possibly reducing the risk from predation. Some believe lemon sharks exhibit social learning and cooperation, based on a relative brain mass overlapping that of mammals and birds[12]. Lemon sharks gather for reproduction in special nursery areas located in shallow water. A 10-12 mo gestation period follows spring and early summer mating, which yields average litters of 4-17 offspring[13].
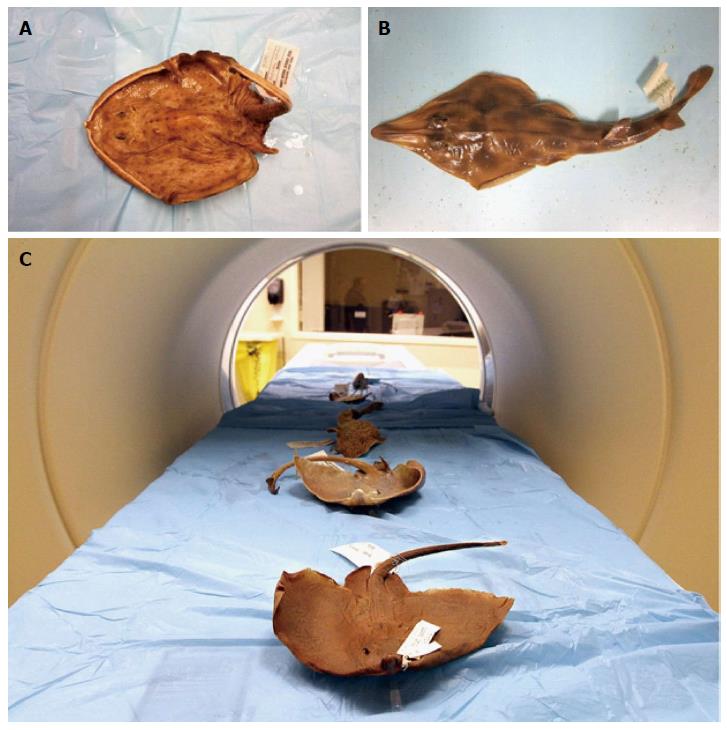
The skilletskate is a deepwater species first discovered in 1905 (Figure 2). It can be found sporadically throughout the western Atlantic on the muddy bottoms of the continental slope, and is occasionally caught by commercial deepwater fisheries. Similar to others in the order Rajiformes, skates are presumed to be oviparous, but little is known about the biology of the skilletskate. The species is presently considered data deficient[14].
All examinations were performed with a high-resolution 3rd generation dual-source multidetector computed tomography (DSCT) scanner (Somatom Force, Siemens, Forchheim, Germany). 3D CT scans of the skeletal anatomy for each species were performed using a high resolution imaging protocol that was optimized for each individual specimen by maximizing the tube current. Acquisitions were performed in dual-energy mode at tube voltages of 80 and 150 kV with the specimen placed along the isocenter Z-axis of the CT gantry (Figure 2). The imaging data were processed with 0.5 mm section thickness, allowing for reconstruction of the 3D skeletal structure and surface anatomy using advanced modelled iterative reconstruction. The cranium was virtually re-sliced in the sagittal, coronal, and frontal planes and movies of rotations of each specimen were generated. Further CT imaging parameters are listed in Table 2.
| Acquisition parameters | Thorax Hd | DE Thorax |
| Section collimation | 192 mm × 0.6 mm | 192 mm × 0.6 mm |
| Slice thickness (mm) | 0.5 | 0.5 |
| Increment (mm) | 0.3 | 0.5 |
| Rotation time (s) | 0.5 | 0.3 |
| Pitch | 0.35 | 0.65 |
| Tube voltage (kV) | 120 | 80 + 150 |
| Effective tube current–time product (mAs) | 37-104 | 1107/615 |
| DLP (mGy/cm) | 2.3-6.23 | 17.55-27.76 |
| Reconstruction kernel | Bf32/Br54 | Qr32/Qr54 |
| Admire 3 | Admire 3 |
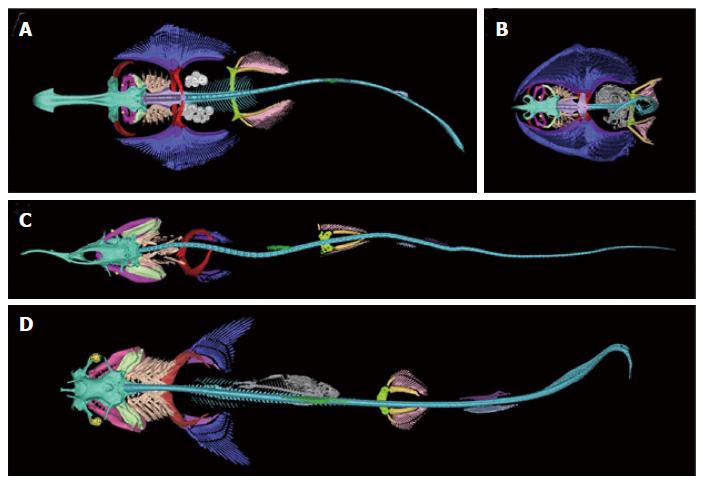
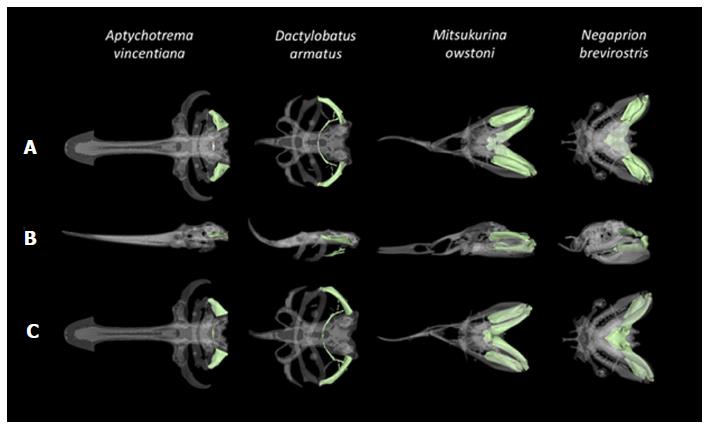
Segmentation and three-dimensional rendering of the cartilaginous skeletons were manually completed with various tools in MIMICS Research version 17.0 64-bit software (Materialise, Leuven, Belgium). Specific cartilaginous elements were color coded to facilitate comparison across species (Figure 3). The 3D images were handled electronically on a workstation, displaying areas of interest in multiple dimensions. The data from the scans will be added to an anatomical database as part of the CToL project which will allow for a more rigorous interpretation of the fossil record.
We were able to acquire high quality images and generate 3D reconstructions without the presence of artifacts in all specimens. Anatomical structures present in all specimens are color coded and shown in Figure 3. Each colored structure corresponds to the structure of the same color in the other panels, illustrating how it has changed in each of the four species over the course of evolutionary history. The following examples demonstrate such structures, which share a common origin but have somewhat diverged in terms of overall structure and function.
Figure 4 shows the structures of the hyoid arch in pale green for each of the specimens in the current study. In the two shark species, all three structures of the hyoid arch (hyomandibular, ceratohyal, and basihyal) were clearly visible whereas in the two batoids, the hyomandibular was a prominent feature while the ceratohyal was not visible and the basihyal was much more reduced and closer to the gill arches.
The pink structure shown in all panels of Figure 3 represents the pelvic fins for each specimen. The general shape of the puboischiadic bar, or pelvic girdle, illustrated a closer relationship between the two sharks and the two batoids than between the two groups. With greater coverage of the families and genera of these two groups of elasmobranchs, the characteristics of the structures can be used to discern relationships in the phylogeny.
About 530 million years ago, the Cambrian explosion gave rise to the first vertebrates. About 80 million years later, the Chondrichthyan lineage of all modern sharks, skates, rays and chimaeras departed from the main vertebrate lineage. This lineage gave rise to all modern elasmobranchs (Kyne, 2007 #174)[15]. The other lineage gave rise to the bony fishes, which in turn gave rise to the well-studied tetrapod, amniotes and mammal diversifications. Both lineages developed jaws prior to diverging, one of the defining features of early vertebrates. However, while most members of the bony fish lineage went on to acquire features more commonly associated with extant vertebrates (e.g., lungs, bone), the elasmobranchs maintained gills and developed a cartilaginous skeleton. Of course, these characteristics should not be thought of as subordinate-rather, they allowed for their extraordinarily lengthy survival over the course of evolutionary history, making these features especially intriguing. It would also be incorrect to think that sharks, skates, and rays have not evolved since they diverged from the main vertebrate trunk. Their main design has remained relatively fixed in comparison with the bony fish lineage, which has diversified incredibly into all extant amphibians, reptiles, birds and mammals. The fact that sharks, skates, and rays are extant today means that they and their ancestors survived at least five global mass extinctions over the last half billion years, one of which (Permian-Triassic extinction) killed 96% of all marine species[16], and are among the oldest groups of surviving vertebrates. To put this into context, ancestors specific to the elasmobranch lineage date back more than 200 million years before the appearance of the first dinosaur. Around 65 million years ago at the end of the Cretaceous period, the most recent mass extinction event occurred, wiping out 75% of extant species during that time, along with all dinosaurs[17]. Some species of elasmobranchs of course survived, giving rise to all modern sharks, skates, and rays.
The superorder Batoidea, including all modern skates and rays, represent a flattened variation of the common ancestor they share with modern sharks, which was likely much more shark-like. Ancient batoid remains as old as 150 million years have been recovered, but they are thought to have first appeared in the late Triassic. By flattening their bodies, batoids adopted an anatomy better suited for occupying the ocean floor. They share many of the same feeding niches with sharks, pursuing krill, crush shelled molluscs, and fish (including sharks). However, the flattened batoid face and resulting ventrally located mouth make the ram feeding mode employed by sharks impossible[18].
Although elasmobranch fossils exist, the fossil record is less complete compared to other vertebrates whose skeletons are better calcified. Teeth, having mineralogical stability, have a significantly increased chance of undergoing fossilization and have provided the majority of the information to biologists studying elasmobranch evolution through fossils. However, elasmobranch teeth cannot paint the whole picture, making other methods for studying their evolution important. Genetic evidence represents the primary alternative to fully articulated fossils. CT imaging constitutes another important method for studying the phylogeny of different species, providing the opportunity to obtain scan data for segmentations of the cartilaginous skeleton of Chondrichthyans. Obtaining CT data and the subsequent segmentation was used to determine the shape, angle, and size of various structures in a noninvasive manner, avoiding the destructive dissections that are generally required to measure these structures. Dissection was not possible for most of the specimens examined due to their frailty and rarity.
Morphology of the hyoid arch, a central component of the jaw protrusion apparatus, has diverged considerably among extant elasmobranchs and those observed in the fossil record[19]. Jaw protrusion is believed to have conferred a distinct advantage for prey capture, and has been well studied[20]. Shape and angle of the hyoid arch may be used to determine whether an elasmobranch is likely to use suction or ram feeding strategies[21], and is often used to infer ancestral feeding mechanisms when vestigial elements remain. We were able to identify the hyoid arch and the shape and angle of its components in each of the four species studied.
The pelvic fins, including the puboischiadic bar, pelvic radials, pelvic propterygium, and pelvic metapterygium have also diverged within the elasmobranchs. In males, this structure also includes the claspers which are often used in species identification. In batoids, the pelvic fins can be used for punting, and sometimes walking as observed in the leg-skates whereas in sharks, the pelvic fins are used more for stabilization, although some sharks have been observed using pelvic fins for walking and punting (Macesic and Kaijura).
Although we did not explore the Micro-CT (MCT) modality in this study due to the fact that the species were too large for the limited field-of-view of MCT; MCT imaging would likely provide significantly more detailed resolution of elasmobranch anatomical structures. The MCT unit at our institution (Siemens Inveon Micro-CT/PET, Siemens Medical Solutions, Knoxville, TN) allows for 30-80 kVp tube potential with a 9 cm × 6 cm maximum field of view. The dual-modality system can produce images with a resolution as low as 15 μm. While size restrictions would severely limit which specimens could be imaged, smaller elasmobranchs such as the cigar shark (Isistius brasiliensis), measuring less than 4 cm in diameter, would fit into the machine’s bore. Furthermore, elasmobranch embryos and some newborn pups would make good candidates for MCT imaging. In some cases, however, embryos and very young individuals are not well calcified, resulting in low-quality images of skeletal structures.
CT-based comparative anatomy of modern elasmobranchs will be used to document anatomical variation among the major elasmobranch lineages for an ongoing CToL project. The skeletal variations observed are expected to increase our understanding of how the anatomy in these organisms has changed over the course of evolution. Completed segmentations will be entered into an online database which will also contain information for the other components of the CToL project.
The work presented is a subset of the chondrichthyan tree of life (CToL) project, a multi-institutional endeavor to create a database of evolutionary information for Chondrichthyan fishes (sharks, skates, rays and chimeras). Chondrichthyes are subdivided into two subclasses: Elasmobranchii (sharks, rays and skates) and Holocephali (chimeras). The goal of the CToL is to reconstruct the evolutionary lineages of all extant sharks, skates, rays, and chimeras to better understand how these organisms have addressed environmental challenges by collecting both anatomical and genetic information.
Computed tomography (CT) is an important 3-dimensional (3D) imaging tool for medical as well as non-medical purposes. Due to its high spatial resolution and non-destructive nature, CT enables the display of distinct skeletal characteristics of different representatives of shark and ray lineages. Furthermore, the high spatial resolution of modern CT technology renders increased material differentiation possible, which may facilitate the assessment of minute disparities within the cartilaginous structures of sharks, skates, and rays.
The skeletal variations observed are expected to increase the understanding of how the anatomy in these organisms has changed over the course of evolution.
CT-based comparative anatomy of modern elasmobranchs will be used to document anatomical variation among the major elasmobranch lineages for an ongoing CToL project. Completed segmentations will be entered into an online database which will also contain information for the other components of the CToL project.
Chondrichthyan Tree of Life (CToL) project: A multi-institutional endeavor to create a database of evolutionary information for Chondrichthyan fishes. Chondrichthyan fishes: Sharks, skates, rays and chimeras. Chondrichthyes are subdivided into two subclasses: Elasmobranchii (sharks, rays and skates) and Holocephali (chimeras).
This is an interesting study on the documentation of skeletal variation in 4 divergent species of elasmobranchs with high-resolution 3rd generation DSCT.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen F, Irurita J, Verlinden O S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Maisey J, Chesek C, Miller D. Discovering fossil fishes. New York, NY: Henry Holt and Company 1996; 223. |
| 2. | Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science. 2007;315:1846-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 439] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 3. | Carrier JC, Musick JA, Heithaus MR. Biology of sharks and their relatives. Boca Raton: CRC Press/Taylor & Francis Group 2012; 3-4. [DOI] [Full Text] |
| 4. | Compagno L. Sharks of the order Carcharhiniformes. Princeton, NJ: Princeton University Press 1988; 12-71, 341-345. |
| 5. | Shirai S. Sqalean phylogeny: a new framework of “squaloid” sharks and related taxa. Sapporo: Hokkaido University Press 1992; 151. |
| 6. | Huppertz A, Wildung D, Kemp BJ, Nentwig T, Asbach P, Rasche FM, Hamm B. Nondestructive insights into composition of the sculpture of Egyptian Queen Nefertiti with CT. Radiology. 2009;251:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Jansen RJ, Poulus M, Kottman J, de Groot T, Huisman DJ, Stoker J. CT: a new nondestructive method for visualizing and characterizing ancient Roman glass fragments in situ in blocks of soil. Radiographics. 2006;26:1837-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Weustink AC, Hunink MG, van Dijke CF, Renken NS, Krestin GP, Oosterhuis JW. Minimally invasive autopsy: an alternative to conventional autopsy? Radiology. 2009;250:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Last P, Stevens JD. Sharks and Rays of Australia. Melbourne, Australia: CSIRO 1994; . |
| 10. | Castro J. The Sharks of North America. Oxford: Oxford University Press 2011; 201-205, 481-485. |
| 11. | Guttridge T, Gruber SH, Franks BR, Kessel ST, Gledhill KS, Uphill J, Krause J, Sims DW. Deep danger: intra-specific predation risk influences habitat use and aggregation formation of juvenile lemon shark Negaprion brevirostris. Marine Ecology Progress Series. 2012;445:279-291. [DOI] [Full Text] |
| 12. | Guttridge TL, van Dijk S, Stamhuis EJ, Krause J, Gruber SH, Brown C. Social learning in juvenile lemon sharks, Negaprion brevirostris. Anim Cogn. 2013;16:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Compagno L. FAO Species Catalogue. In: Ichthyology JSIo, editor Sharks of the World. Rome 1984; . |
| 14. | McEachran J, de Carvalho MR. Batoid Fishes. In: University OD, editor The Living Marine Resources of the Western Central Atlantic. Rome 2002; 508-529. |
| 15. | Kyne P, Simpfendorfer CA. A Collation and Summarization of Available Data on Deepwater Chondrichthyans: Biodiversity, Life History and Fisheries (Marine Conservation Biology Institute, 2007). Available from: http://www.iucnssg.org/uploads/5/4/1/2/54120303/kyne___simpfendorfer_2007.pdf. |
| 16. | Labandeira CC, Sepkoski JJ. Insect diversity in the fossil record. Science. 1993;261:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 239] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Raup DM, Sepkoski JJ. Mass extinctions in the marine fossil record. Science. 1982;215:1501-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1102] [Cited by in RCA: 384] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 18. | Dean MN, Bizzarro JJ, Summers AP. The evolution of cranial design, diet, and feeding mechanisms in batoid fishes. Integr Comp Biol. 2007;47:70-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Wilga C. Evolutionary divergence in the feeding mechanism of fishes. Acta Geologica Polonica. 2008;58:113-120. |
| 20. | Wilga C, Hueter RE, Wainwright PC, Motta PJ. Evolution of the upper jaw protrusion mechanisms in elasmobranchs. American Zoologist. 2002;41:1248-1257. |
| 21. | Tomita T, Sato K, Suda K, Kawauchi J, Nakaya K. Feeding of the megamouth shark (Pisces: Lamniformes: Megachasmidae) predicted by its hyoid arch: a biomechanical approach. J Morphol. 2011;272:513-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |