Peer-review started: August 2, 2016
First decision: September 28, 2016
Revised: December 23, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: March 28, 2017
Processing time: 235 Days and 20.1 Hours
Radiofrequency ablation (RFA) is currently the most popular and used ablation modality for the treatment of non surgical patients with primary and secondary liver tumors, but in the last years microwave ablation (MWA) is being technically improved and widely rediscovered for clinical use. Laser thermal ablation (LTA) is by far less investigated and used than RFA and MWA, but the available data on its effectiveness and safety are quite good and comparable to those of RFA and MWA. All the three hyperthermia-based ablative techniques, when performed by skilled operators, can successfully treat all liver tumors eligible for thermal ablation, and to date in most centers of interventional oncology or interventional radiology the choice of the technique usually depends on the physician’s preference and experience, or technical availability. However, RFA, MWA, and LTA have peculiar advantages and limitations that can make each of them more suitable than the other ones to treat patients and tumors with different characteristics. When all the three thermal ablation techniques are available, the choice among RFA, MWA, and LTA should be guided by their advantages and disadvantages, number, size, and location of the liver nodules, and cost-saving considerations, in order to give patients the best treatment option.
Core tip: Radiofrequency ablation, microwave ablation, and laser thermal ablation, when performed by skilled operators, can successfully treat all liver tumors eligible for thermal ablation. However, each of them has peculiar advantages and limitations that can make one technique more suitable than the other ones to treat patients and tumors with different characteristics. When all the three techniques are available, the choice should be guided by their advantages and disadvantages, number, size and location of the liver nodules, and cost-saving considerations, in order to give patients the best treatment option.
- Citation: Sartori S, Di Vece F, Ermili F, Tombesi P. Laser ablation of liver tumors: An ancillary technique, or an alternative to radiofrequency and microwave? World J Radiol 2017; 9(3): 91-96
- URL: https://www.wjgnet.com/1949-8470/full/v9/i3/91.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i3.91
Temperatures in excess of 60 °C are known to cause relatively instantaneous cell death, and thermal ablation by heating neoplastic tissue to cytotoxic temperatures is becoming increasingly important for treating primary and secondary liver cancer[1]. Radiofrequency ablation (RFA) is currently the most popular and used ablation modality, but in the last years microwave ablation (MWA) is being technically improved and widely rediscovered for clinical use[2-6]. RFA energy is delivered as an alternating current at a frequency of about 400 MHz, resulting in molecular frictional agitation and heat generation known as the joule effect[1,7]. Tissues nearest to the electrode are heated directly, while more peripheral areas are less effectively heated by thermal conduction[8]. MWA is a special case of dielectric heating where the dielectric material is tissue containing water. MWA induces a high-speed (between 900 and 2450 MHz) alternating electric field, causing the rotation of water molecules and generating heat[1,7,9,10]. In contrast to RFA, energy radiates into the tissue with direct heating of the lesion, and charring and vaporization in the proximity of the needle are not obstacles to the delivery of energy[10,11].
The effectiveness and limits of RFA have widely and extensively been reported worldwide. Due to the physical limitations in energy deposition, the effectiveness of RFA in local tumor control decreases with the increase of tumor size[10]. Local control rates over 90% have been reported for nodules up to 3 cm in diameter, and only 6%-10% for tumors greater than 5 cm[12]. Moreover, tumor location close to large vessels can also influence ablation success, because thermal energy is partially shunted away by the cooler blood (the so-called heat-sink effect)[13,14].
The recent technical developments of MWA technology, such as the introduction of a cooling jacket around the MWA antenna and a miniaturized device for MW confinement into the distal portion of the antenna, have minimized the main limits of the earlier MWA systems, allowing for the reduction of back heating effects, increase of the ablation time, and amount of power that can be safely delivered[2,6]. Due to these technical improvements and the characteristics of heat production and energy delivery[9-11], MWA has recently been reported to achieve larger ablation areas than RFA[3,4,15], and appears to be less susceptible to the heat-sink effect[10,11]. Most studies investigating the effectiveness of MWA were conducted before the introduction into clinical practice of the most recent advancements in MWA technology, and at present the best available evidence suggests similar outcomes for RFA and MWA. Reported three- and 5-year survival rates of Child’s class A patients with single hepatocellular carcinoma (HCC) less than 5 cm, or up to three HCC less than 3 cm, range from 60% to 78%, and from 50% to 64%, respectively, for RFA[16-18], and from 72% to 73%, and 51%-57%, respectively, for MWA[19,20]. The outcomes of RFA and MWA in patients with up to 6 metastases from colorectal cancer with a maximum diameter of 6 cm are also comparable, with 3-year survival rates of 28%-46% and 46%-51%, respectively, and 5-year survival rates of 25%-46% and 17%-32%, respectively[21-24].
However, there is a third hyperthermia-based ablation technique, which uses laser optical fibers to deliver high-energy laser radiation to the tissue. Because of light absorption, temperatures of up to 150 °C are reached, leading to coagulative necrosis[7,9,11]. Neodymium:Yttrium Aluminum Garnet (Nd:YAG, wavelength of 1064 nm) and diode (wavelength of 800-980 nm) lasers are most commonly used, as penetration of light is optimal in the near infrared spectrum. Light is delivered via flexible quartz fibers with a diameter from 300 to 600 μm. Conventional bare-tip fibers provide an almost spherical thermal lesion of 12-15 mm in diameter, and a beam-splitting device or a multi-source device allow for the use of up to four fibers, simultaneously delivering the light into each single fiber[11,25,26]. Moreover, interstitial quartz fibers with flat or cylindrical diffusing tips have been reported to achieve larger ablation areas[9]. Laser-induced interstitial thermotherapy is a special form of laser technique that uses a unique saline-cooled power laser application system to increase the volume of coagulative necrosis while preventing carbonization at the tip of the laser applicator[10]. The device consists of a 9 French catheter with centimetre markings and a 7 French sheathed catheter with irrigated double lumina. Room temperature saline is used as the irrigation fluid, and a pump is integrated with the laser. This permits reliable cooling of the applicator and expansion of the laser-induced necrosis zone, resulting particularly useful for the treatment of liver metastases that require large safety margins to take care of microscopic disease around the lesions[27].
Laser thermal ablation (LTA) is by far less investigated and used than RFA and MWA, but the available data on its effectiveness and safety are quite good. Most of the studies on LTA are focused on the treatment of HCC. Complete response rates ranging from 82% to 97%, and cumulative 3-year survival rates up to 73% were reported in Child’s class A patients with single HCC ≤ 5 cm or up to three nodules ≤ 3 cm treated with multiple bare fibers[28,29]. Moreover, median survival of 3.5 years was achieved in patients with nodules ≤ 5 cm located at high-risk sites by using water-cooled higher power LTA[30]. To date, there are in literature just two randomized trials comparing LTA and RFA in the treatment of HCC, and both of them did not find any significant difference between the two techniques in terms of local tumor control, overall survival, and safety[31,32]. A multicenter study investigating the safety of LTA in five hundred-twenty patients with 647 HCC treated by 1004 LTA sessions reported mortality and major complication rates of 0.8% and 1.5%, respectively[33]. Likewise, also the outcomes of patients with liver metastases from colorectal cancer with diameter up to 5 cm treated with LTA appear comparable to those reported for RFA and MWA, with 3- and 5-year survival rates ranging from 28% to 72.4%, and from 10% to 37%, respectively[9,34-36].
Despite these excellent results, LTA is frequently not considered an effective ablation technique, and the vast majority of reviews, consensus, or position papers dealing with the efficacy or safety of thermal ablation of liver tumors does not even mention LTA among the ablative techniques that are to date available[1,10,37-41].
We do not agree with such an attitude. Although it is true that LTA has been investigated less vigorously than the other ablation techniques, it is also true that the relatively low number of published studies dealing with LTA seems to be due to an unjustified prejudice, rather than to an actual lower efficacy of LTA in comparison with RFA or MWA. All the three hyperthermia-based ablative techniques, when performed by skilled operators, can successfully treat all liver tumors eligible for thermal ablation, and to date in most centers of interventional oncology or interventional radiology the choice of the technique usually depends on the physician’s preference and experience, or technical availability. However, in our opinion RFA, MWA and LTA have peculiar advantages and limitations that can make each of them more suitable than the other ones to treat patients and tumors with different characteristics. For instance, RFA is surely the best established thermal technique, and its efficacy has been largely proven, but lesions larger than 2-2.5 cm require multiple overlapping ablations to create an adequate safety margin, and sub-capsular or high-risk location of the tumors is considered a relative contraindication to RFA, even though some reports documented its feasibility[42,43]. Moreover, tumors strictly close to large vessels can be incompletely treated because of the heat-sink effect. MWA has less sensitivity to the heat-sink effect, deeper penetration of energy and better propagation across the poorly conductive tissue than RFA, and can achieve larger ablation volumes. On the other hand, microwave energy is more difficult to distribute than RF energy, is carried in wavelengths which are more cumbersome than the small wires used to feed energy to RF electrodes, and are prone to heating when carrying large amount of power[11]. Consequently, MWA appears less feasible than RFA in the treatment of high-risk located and sub-capsular nodules. Moreover, the latest versions of MWA devices provided with the most recent technical advances are more expensive than RFA.
As regards LTA, the technique proposed by Pacella et al[28] and improved by Di Costanzo et al[44] uses 300-μm bare optical fibers introduced into the tumor through 21-gauge needles. The diameter of the needles is considerably smaller than RFA electrodes and MWA antennas, making LTA safer and more suitable for ablating lesions in at-risk location or in locations that are difficult to reach[11,45]. Moreover, a multisource device allows to use from one to four fibers at once, enabling to achieve ablation areas from one to 4-5 cm in diameter, and consequently to treat tumors ranging from 5-6 mm to 3 cm in diameter obtaining an acceptable safety margin. Furthermore, in western countries LTA has been reported to be the cheapest ablation technique when up to three fibers are used, and cheaper than MWA when four fibers are used[11]. For these characteristics, LTA has been proposed as a valid alternative to RFA for lesions up to 2 cm[46], and it has been suggested as the technique of choice in presence of multiple small and variably sized liver tumors[45]. On the other hand, the correct placement of the fibers can be challenging, particularly if more than two fibers are needed, and should be performed by very skill operators[11]. Moreover, like RFA, also the efficacy of LTA can be limited by the heat-sink effect.
In the last years, multimodality anti-tumor strategies including surgery, chemotherapy, radiotherapy, ablation techniques, and catheter-based treatments are being more and more advocated, to tailor the best treatment options to patient and tumor characteristics[45,47-49]. Such an approach is often adopted not only to choose the most suitable treatment options, but also to choose the most suitable technique available for each treatment option. For instance, patients candidate to catheter-based treatments can undergo bland embolization, transarterial chemoembolization with lipiodol or with drug-eluting beads, or radioembolization, according to the type of tumor, liver function, and presence or absence of portal venous thrombosis. Likewise, patients candidate to liver surgery can undergo wedge resection, segmentectomy, lobectomy, or transplantation according to the liver function, and number, size, and location of the tumors.
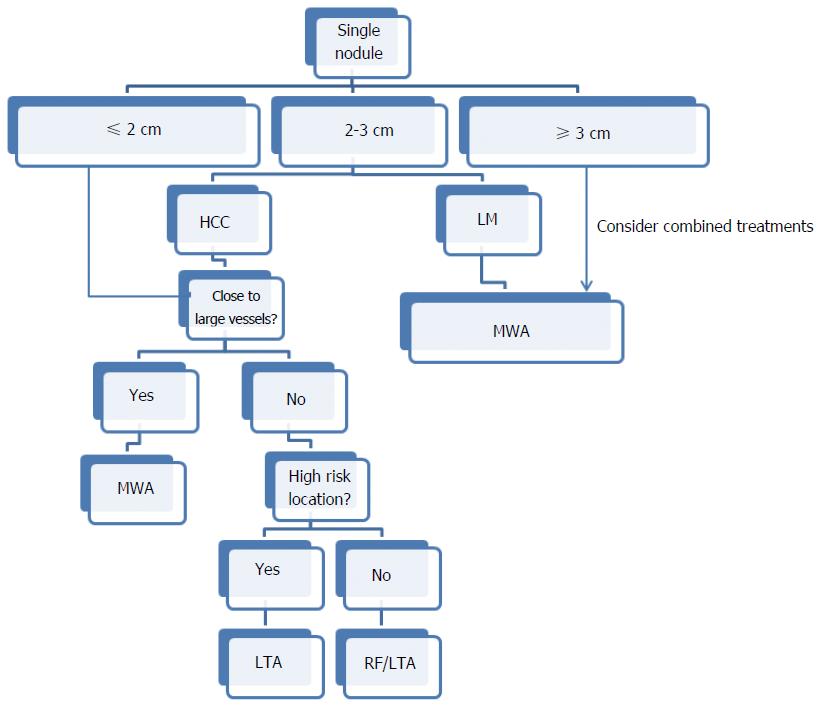
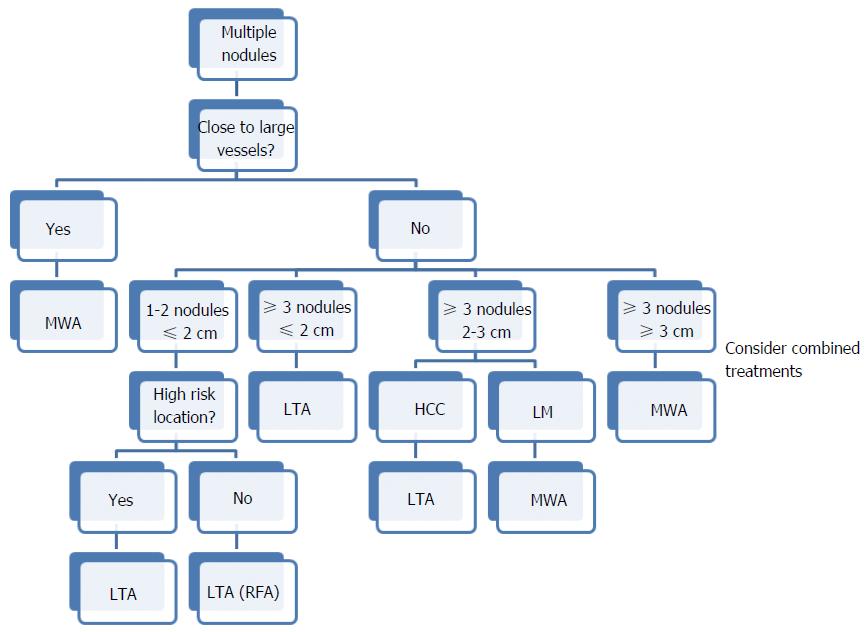
In our opinion, the choice among the thermal ablation techniques should also be based on the same criteria whenever possible. Some authors suggested that the reference centers for thermal ablation should be equipped with all the available techniques so as to be able to use the best and the most suitable one for each type of tumor[26]. Recently, an algorithm has been proposed to tailor thermal ablation on each single patient, according to advantages and disadvantages of RFA, MWA and LTA, number, size, and location of the liver nodules, and cost-saving considerations (Figures 1 and 2)[11]. On the basis of this algorithm, all the three ablation techniques have a preferential role in some specific circumstances. For instance, a single nodule 2 cm or smaller in size can be efficaciously treated using all the thermal modalities, but RFA and LTA are cheaper than MWA and should be preferred. Conversely, MWA should be considered the technique of choice when the tumor is ≥ 3 cm in diameter or is close to large vessels independently of its size, as MWA can achieve larger ablation volumes and is not affected by the heat-sink effect. Multiple small and variably sized lesions should be treated with LTA, and so on (Figures 1 and 2). This algorithm reflects the personal experience and opinion of the authors, and it can surely be modified and improved. However, it is also based on objective considerations that can largely be shared, and in our opinion it could represent the basis for a consensus on the optimal and reasoned use of the thermal ablation modalities.
In conclusion, at present there is no ideal ablation technique that outclasses the other ones. There are ablation techniques that share some main technical aspects and are usually comparable, but each of them has peculiar characteristics that make it the “ideal” technique in some particular settings. We believe we should exploit such peculiarities to give patients the best treatment option.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Oto A, van Beek EJR, Vogl TJ S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38:135-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 472] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 2. | Inokuchi R, Seki T, Ikeda K, Kawamura R, Asayama T, Yanagawa M, Umehara H, Okazaki K. Percutaneous microwave coagulation therapy for hepatocellular carcinoma: increased coagulation diameter using a new electrode and microwave generator. Oncol Rep. 2010;24:621-627. [PubMed] |
| 3. | Qian GJ, Wang N, Shen Q, Sheng YH, Zhao JQ, Kuang M, Liu GJ, Wu MC. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol. 2012;22:1983-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Cavagnaro M, Amabile C, Bernardi P, Pisa S, Tosoratti N. A minimally invasive antenna for microwave ablation therapies: design, performances, and experimental assessment. IEEE Trans Biomed Eng. 2011;58:949-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Goldberg SN. Science to practice: Can we expand focal interventional oncologic ablation treatments into an effective systemic therapy? Radiology. 2013;267:321-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Wang Y, Sun Y, Feng L, Gao Y, Ni X, Liang P. Internally cooled antenna for microwave ablation: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2008;67:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Loffroy R, Estivalet L, Favelier S, Pottecher P, Genson PY, Cercueli JP, Krausé D. Interventional radiology therapies for liver cancer. Hepatoma Res. 2016;2:1-9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Ahmed M, Brace CL, Lee FT, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 562] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 9. | Vogl TJ, Farshid P, Naguib NN, Darvishi A, Bazrafshan B, Mbalisike E, Burkhard T, Zangos S. Thermal ablation of liver metastases from colorectal cancer: radiofrequency, microwave and laser ablation therapies. Radiol Med. 2014;119:451-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Foltz G. Image-guided percutaneous ablation of hepatic malignancies. Semin Intervent Radiol. 2014;31:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Tombesi P, Di Vece F, Sartori S. Radiofrequency, microwave, and laser ablation of liver tumors: time to move toward a tailored ablation technique? Hepatoma Res. 2015;1:52-57. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 741] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 13. | Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 546] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 14. | Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, Sayre J. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 359] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 15. | Di Vece F, Tombesi P, Ermili F, Maraldi C, Sartori S. Coagulation areas produced by cool-tip radiofrequency ablation and microwave ablation using a device to decrease back-heating effects: a prospective pilot study. Cardiovasc Intervent Radiol. 2014;37:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 628] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 17. | Choi D, Lim HK, Rhim H, Kim YS, Yoo BC, Paik SW, Joh JW, Park CK. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14:2319-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | Guglielmi A, Ruzzenente A, Sandri M, Pachera S, Pedrazzani C, Tasselli S, Iacono C. Radio frequency ablation for hepatocellular carcinoma in cirrhotic patients: prognostic factors for survival. J Gastrointest Surg. 2007;11:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, Xiao Q. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. AJR Am J Roentgenol. 2003;180:1547-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Gillams AR, Lees WR. Radiofrequency ablation of colorectal liver metastases. Abdom Imaging. 2005;30:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Hildebrand P, Leibecke T, Kleemann M, Mirow L, Birth M, Bruch HP, Bürk C. Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome. Eur J Surg Oncol. 2006;32:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Liang P, Dong B, Yu X, Yang Y, Yu D, Su L, Xiao Q, Sheng L. Prognostic factors for percutaneous microwave coagulation therapy of hepatic metastases. AJR Am J Roentgenol. 2003;181:1319-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Tanaka K, Shimada H, Nagano Y, Endo I, Sekido H, Togo S. Outcome after hepatic resection versus combined resection and microwave ablation for multiple bilobar colorectal metastases to the liver. Surgery. 2006;139:263-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Francica G, Petrolati A, Di Stasio E, Pacella S, Stasi R, Pacella CM. Effectiveness, safety, and local progression after percutaneous laser ablation for hepatocellular carcinoma nodules up to 4 cm are not affected by tumor location. AJR Am J Roentgenol. 2012;199:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Di Costanzo GG, Francica G, Pacella CM. Laser ablation for small hepatocellular carcinoma: State of the art and future perspectives. World J Hepatol. 2014;6:704-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Vogl TJ, Straub R, Zangos S, Mack MG, Eichler K. MR-guided laser-induced thermotherapy (LITT) of liver tumours: experimental and clinical data. Int J Hyperthermia. 2004;20:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Pacella CM, Bizzarri G, Magnolfi F, Cecconi P, Caspani B, Anelli V, Bianchini A, Valle D, Pacella S, Manenti G. Laser thermal ablation in the treatment of small hepatocellular carcinoma: results in 74 patients. Radiology. 2001;221:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Pacella CM, Francica G, Di Lascio FM, Arienti V, Antico E, Caspani B, Magnolfi F, Megna AS, Pretolani S, Regine R. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: a retrospective analysis. J Clin Oncol. 2009;27:2615-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Eichler K, Zangos S, Gruber-Rouh T, Vogl TJ, Mack MG. Magnetic resonance-guided laser-induced thermotherapy in patients with oligonodular hepatocellular carcinoma: long-term results over a 15-year period. J Clin Gastroenterol. 2012;46:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Ferrari FS, Megliola A, Scorzelli A, Stella A, Vigni F, Drudi FM, Venezia D. Treatment of small HCC through radiofrequency ablation and laser ablation. Comparison of techniques and long-term results. Radiol Med. 2007;112:377-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Di Costanzo GG, Tortora R, D’Adamo G, De Luca M, Lampasi F, Addario L, Galeota Lanza A, Picciotto FP, Tartaglione MT, Cordone G. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma in cirrhosis: a randomized trial. J Gastroenterol Hepatol. 2015;30:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Arienti V, Pretolani S, Pacella CM, Magnolfi F, Caspani B, Francica G, Megna AS, Regine R, Sponza M, Antico E. Complications of laser ablation for hepatocellular carcinoma: a multicenter study. Radiology. 2008;246:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Eickmeyer F, Schwarzmaier HJ, Müller FP, Nakic Z, Yang Q, Fiedler V. [Survival after laser-induced interstitial thermotherapy of colorectal liver metastases--a comparison of first clinical experiences with current therapy results]. Rofo. 2008;180:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Puls R, Langner S, Rosenberg C, Hegenscheid K, Kuehn JP, Noeckler K, Hosten N. Laser ablation of liver metastases from colorectal cancer with MR thermometry: 5-year survival. J Vasc Interv Radiol. 2009;20:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Vogl TJ, Dommermuth A, Heinle B, Nour-Eldin NE, Lehnert T, Eichler K, Zangos S, Bechstein WO, Naguib NN. Colorectal cancer liver metastases: long-term survival and progression-free survival after thermal ablation using magnetic resonance-guided laser-induced interstitial thermotherapy in 594 patients: analysis of prognostic factors. Invest Radiol. 2014;49:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Pepple PT, Gerber DA. Laparoscopic-assisted ablation of hepatic tumors: a review. Semin Intervent Radiol. 2014;31:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Wells SA, Hinshaw JL, Lubner MG, Ziemlewicz TJ, Brace CL, Lee FT. Liver Ablation: Best Practice. Radiol Clin North Am. 2015;53:933-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Callstrom M, Chen MH, Choi BI, de Baere T, Dupuy D. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25:3438-3454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 41. | Meyer J, Toomay S. Update on treatment of liver metastases: focus on ablation therapies. Curr Oncol Rep. 2015;17:420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Sartori S, Tombesi P, Macario F, Nielsen I, Tassinari D, Catellani M, Abbasciano V. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: a prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology. 2008;248:670-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 44. | Di Costanzo GG, D’Adamo G, Tortora R, Zanfardino F, Mattera S, Francica G, Pacella CM. A novel needle guide system to perform percutaneous laser ablation of liver tumors using the multifiber technique. Acta Radiol. 2013;54:876-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Tombesi P, Di Vece F, Sartori S. Laser ablation for hepatic metastases from neuroendocrine tumors. AJR Am J Roentgenol. 2015;204:W732. [PubMed] |
| 46. | Orlacchio A, Bolacchi F, Chegai F, Bergamini A, Costanzo E, Del Giudice C, Angelico M, Simonetti G. Comparative evaluation of percutaneous laser and radiofrequency ablation in patients with HCC smaller than 4 cm. Radiol Med. 2014;119:298-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Oberg K, Astrup L, Eriksson B, Falkmer SE, Falkmer UG, Gustafsen J, Haglund C, Knigge U, Vatn MH, Välimäki M. Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part I-general overview. Acta Oncol. 2004;43:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Govaert KM, van Kessel CS, Lolkema M, Ruers TJ, Borel Rinkes IH. Does Radiofrequency Ablation Add to Chemotherapy for Unresectable Liver Metastases? Curr Colorectal Cancer Rep. 2012;8:130-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Sartori S, Tombesi P, Di Vece F. Thermal ablation in colorectal liver metastases: Lack of evidence or lack of capability to prove the evidence? World J Gastroenterol. 2016;22:3511-3515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |