Peer-review started: August 11, 2016
First decision: September 28, 2016
Revised: October 24, 2016
Accepted: December 7, 2016
Article in press: December 9, 2016
Published online: February 28, 2017
Processing time: 200 Days and 14.7 Hours
To investigated the dose enhancement due to the incorporation of nanoparticles in skin therapy using the kilovoltage (kV) photon and megavoltage (MV) electron beams. Monte Carlo simulations were used to predict the dose enhancement when different types and concentrations of nanoparticles were added to skin target layers of varying thickness.
Clinical kV photon beams (105 and 220 kVp) and MV electron beams (4 and 6 MeV), produced by a Gulmay D3225 orthovoltage unit and a Varian 21 EX linear accelerator, were simulated using the EGSnrc Monte Carlo code. Doses at skin target layers with thicknesses ranging from 0.5 to 5 mm for the photon beams and 0.5 to 10 mm for the electron beams were determined. The skin target layer was added with the Au, Pt, I, Ag and Fe2O3 nanoparticles with concentrations ranging from 3 to 40 mg/mL. The dose enhancement ratio (DER), defined as the dose at the target layer with nanoparticle addition divided by the dose at the layer without nanoparticle addition, was calculated for each nanoparticle type, nanoparticle concentration and target layer thickness.
It was found that among all nanoparticles, Au had the highest DER (5.2-6.3) when irradiated with kV photon beams. Dependence of the DER on the target layer thickness was not significant for the 220 kVp photon beam but it was for 105 kVp beam for Au nanoparticle concentrations higher than 18 mg/mL. For other nanoparticles, the DER was dependent on the atomic number of the nanoparticle and energy spectrum of the photon beams. All nanoparticles showed an increase of DER with nanoparticle concentration during the photon beam irradiations regardless of thickness. For electron beams, the Au nanoparticles were found to have the highest DER (1.01-1.08) when the beam energy was equal to 4 MeV, but this was drastically lower than the DER values found using photon beams. The DER was also found affected by the depth of maximum dose of the electron beam and target thickness. For other nanoparticles with lower atomic number, DERs in the range of 0.99-1.02 were found using the 4 and 6 MeV electron beams.
In nanoparticle-enhanced skin therapy, Au nanoparticle addition can achieve the highest dose enhancement with 105 kVp photon beams. Electron beams, while popular for skin therapy, did not produce as high dose enhancements as kV photon beams. Additionally, the DER is dependent on nanoparticle type, nanoparticle concentration, skin target thickness and energies of the photon and electron beams.
Core tip: This paper investigated the dose enhancement effect due to nanoparticle addition using the kilovoltage (kV) photon and megavoltage (MV) electron beams in skin therapy. Dose enhancements of skin layers with different thicknesses were studied with various nanoparticle types, nanoparticle concentrations, radiation beam types and beam energies using Monte Carlo simulation. From the results, it is found that kV photon beams can achieve much higher dose enhancements at the skin compared to MV electron beams. Moreover, gold nanoparticles, which had the highest atomic number in our study, provided the highest dose enhancement for nanoparticle-enhanced skin therapy.
- Citation: Zheng XJ, Chow JCL. Radiation dose enhancement in skin therapy with nanoparticle addition: A Monte Carlo study on kilovoltage photon and megavoltage electron beams. World J Radiol 2017; 9(2): 63-71
- URL: https://www.wjgnet.com/1949-8470/full/v9/i2/63.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i2.63
In cancer treatment, chemotherapy and radiotherapy are two popular methods to control the tumour cell. Chemotherapy uses anticancer drugs at molecular, cellular and tissue levels through various mechanisms such as enhancing the double-strand break due to the conformation changes in chromatin and DNA, and inhibiting the DNA repair processes leading to the conversion of sublethal DNA damage[1-5]. In radiotherapy, on the other hand, it is necessary to provide conformal dose coverage at the target (tumour) while sparing the surrounding normal tissues. Target dose escalation is therefore desired to increase the tumour control probability, but at the same time decrease normal tissue complication probabilities for organs-at-risk (OARs). Under such circumstances, nanoparticle-enhanced radiotherapy is suggested for providing dose enhancement in the target[6-8]. There are two advantages of accumulating heavy-atom nanoparticles-within the tumour. First, due to the increased compositional atomic number of tumours with nanoparticles, imaging contrast is increased due to the enhancement of photoelectric absorption when using a kilovoltage (kV) photon source (e.g., computed tomography)[9,10]. Such contrast enhancement can help the radiation staff to identify and outline the target during radiation treatment planning. Second, the increase in the photoelectric cross-section due to the addition of nanoparticles increases the dose absorption of the target[11,12]. This results in dose enhancement at the target and improved treatment outcome. Preclinical results of Au nanoparticle enhanced radiotherapy have proven that the addition of 1.9 nm Au nanoparticles to mammary cancer cells of mice can lead to a significant increase in survival rate of 86% compared to 20% with radiotherapy alone and 0% with Au nanoparticle addition alone[13]. For radiotherapy of EGFR-positive cancer, a facile synthetic method of indium-111 to Au nanoparticle was developed with high payload to enhance the delivery of radioactivity to the tumour[14]. Au nanoparticle was also studied as a drug-delivery platform in transient anti-angiogenic therapies to induce tumour vascular normalization and enhance the efficacy of the cytotoxic drugs[15]. Moreover, tumour radiosensitizations for breast and prostate with Au nanoparticle addition were studied both in vitro and in vivo based on the cell-line and small-animal model[16,17].
Since dose enhancement is due to an increase in the photoelectric cross-section by raising the compositional atomic number through heavy-atom nanoparticle addition, such enhancement decreases when using megavoltage (MV) instead of kV range photon beams where Compton interactions dominate. Unlike preclinical models using kV photon beams, human radiotherapy requires MV energies for deep-seated targets. This is due to the considerable differences in size and thickness for humans compared to small animals and therefore higher penetrative MV photon beams are required[18]. Dose enhancement using MV photon beams is lower than kV beams when treating deep-seated tumours in radiotherapy. In skin therapy, however, kV photon beams are used to treat superficial lesions as the target is located on the patient’s surface. Therefore, sufficient dose enhancement at the target can be achieved using kV (e.g., 105 and 220 kVp) photon beams[19,20]. The aim of this study is to investigate the dose enhancement due to nanoparticle addition of various types, concentrations, beam energies for several skin target thicknesses. For dosimetric comparison, similar nanoparticle additions using electron beams were carried out because electron therapy is also popular in skin lesion treatment. In this study, lower electron beam energies of 4 and 6 MeV with relatively short electron paths were focused on as they were clinically used to treat superficial lesions[19].
Monte Carlo simulations using EGSnrc[21] were used to calculate doses of the skin target using the macroscopic approach[22]. Heterogeneous phantoms were used with the clinical kV photon and MV electron beams from the orthovoltage unit and medical linear accelerator in this study, respectively. The dose enhancement ratio (DER), defined here as the ratio of the dose in the skin target with nanoparticle addition to the dose of target without nanoparticle addition, was determined with variations of the nanoparticle type, concentration and skin target thickness using the photon and electron beams.
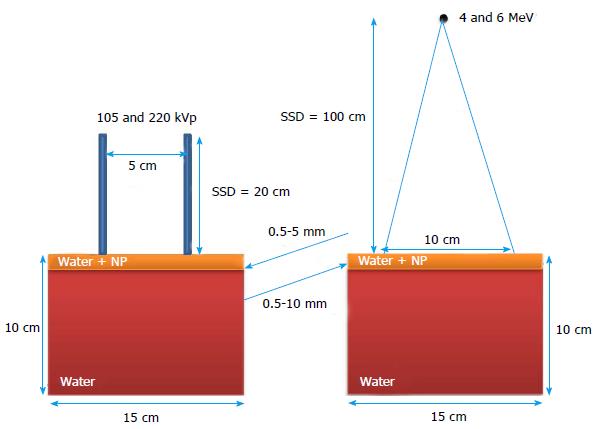
Figure 1 shows the calculation geometry used in the Monte Carlo simulations. A water phantom with dimensions of 15 × 15 × 10 cm3 was used in this study. The top skin target layers with thicknesses ranging from 0.5-5 mm for the photon beams and 0.5-10 mm for the electron beams. While varying the skin target thickness, the height of the phantom was kept constant at 10 cm. For the target layer, different nanoparticles consisting of Au, Pt, I, Ag and Fe2O3 with atomic numbers equal to 79, 78, 53, 47 and 23 were mixed with water for 5 concentrations (3, 7, 18, 30 and 40 mg/mL).
For the kV photon beam irradiations, the 105 and 220 kVp beams produced by a Gulmay D3225 orthovoltage unit were used. The photon beams were conformed by a standard circular applicator of 5 cm diameter with a source-to-surface distance (SSD) equal to 20 cm. In the electron beam irradiations, the 4 and 6 MeV electron beams produced by a Varian 21 EX linear accelerator were used with a 10 × 10 cm2 standard square cutout in the bottom of a 10 × 10 cm2 applicator (not shown in Figure 1). The SSD of the electron beam irradiation was set to 100 cm. It should be noted that both the photon and electron beam irradiations were based on typical clinical geometries for skin therapy.
The EGSnrc code developed by the National Research Council Canada was used in this study[21]. For the kV photon beams, the spectral shape in this code was improved by implementing the electron impact ionization model. In addition, the directional bremsstrahlung splitting approach was used to enhance the efficiency of energy transitions from the electron current to photons[23].
Phase-space files of 105 and 220 kVp photon beams, produced by a Gulmay D3225 orthovoltage machine using a standard open circular applicator with diameter of 5 cm, were generated using the BEAMnrc code[24]. The SSD was set at 20 cm. The treatment head model in simulation included the X-ray tube, primary collimator, filter, ionization chamber and applicator with material and geometry information provided by the manufacturer. Phase-space files containing 36 million particles were generated including information on energy, orientation, type, charge and position of particles crossing the scoring plane at the bottom of the applicator. The phase-space files were verified by comparing the percentage depth doses and beam profiles in water measured by a parallel-plate ionization chamber and water tank elsewhere[19]. For the 4 and 6 MeV electron beams produced by the Varian 21 EX linear accelerator, phase-space files were generated from BEAMnrc using a 10 × 10 cm2 applicator with an SSD of 100 cm. Details of the geometries and materials of the treatment head were provided by the linear accelerator manufacturer, and the parameter reduced electron step transport algorithm II (PRESTA II) was used as the electron-step algorithm[25]. The phase-space files for the electron beams contained 55 million particles, and were verified elsewhere by comparing the percentage depth doses and beam profiles between the Monte Carlo and measurement results using radiographic film, ionization chamber and solid water phantom[19].
The material data sets for different concentrations of nanoparticles were created using the EGSnrc-based PEGS4 code[21]. Data sets regarding particle interaction cross-sections for various concentrations (3, 7, 18, 30 and 40 mg/mL) of Au, Pt, I, Ag and Fe2O3 nanoparticles mixed with water were generated. DOSXYZnrc was used to calculate the dose at the skin target layer irradiated by the photon and electron beams[26]. For the 105 and 220 kVp photon beams, 150 million histories were run for each calculation with the energy cut-off for the electron (ECUT) and photon (PCUT) transport set to 521 keV and 1 keV. The PRESTA II was used for the electron-step algorithm, and the spin effect, bound Compton scattering, Rayleigh scattering, atomic relaxation and electron impact ionization options were all used in the simulation. For the simulation using the electron beams, the ECUT, PCUT and ESTEPE were set to 521 keV, 10 keV and 25%, respectively[27]. Two hundred million histories were simulated in Monte Carlo for the 4 and 6 MeV electron beams. Under these approaches, the relative dose error (statistical uncertainty as a fraction of dose in the voxel) was found to be around 1% according to the Monte Carlo output files[21].
The doses determined from the skin target layer with different thicknesses, nanoparticle concentrations, and types using Monte Carlo simulations were used to calculate the DER, defined in this study as:
DER = Dose with nanoparticle addition in the target layer/Dose without nanoparticle addition in the target layer (1)
It can be seen from Eq. (1) that due to the general dose enhancement effect from nanoparticle addition, the DER is typically close to or larger than one.
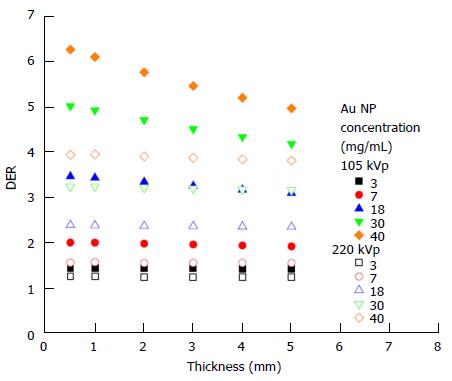
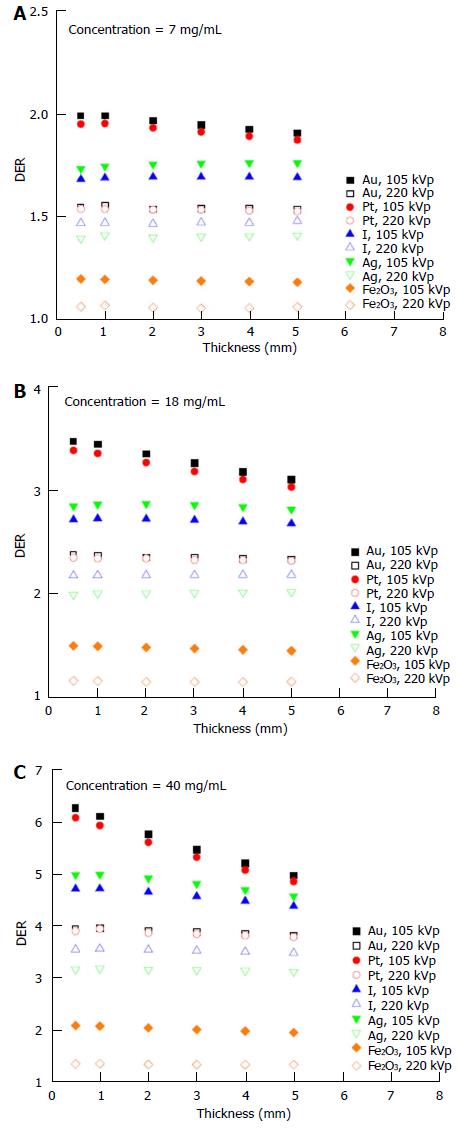
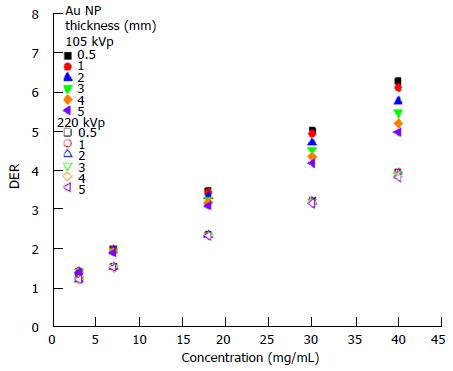
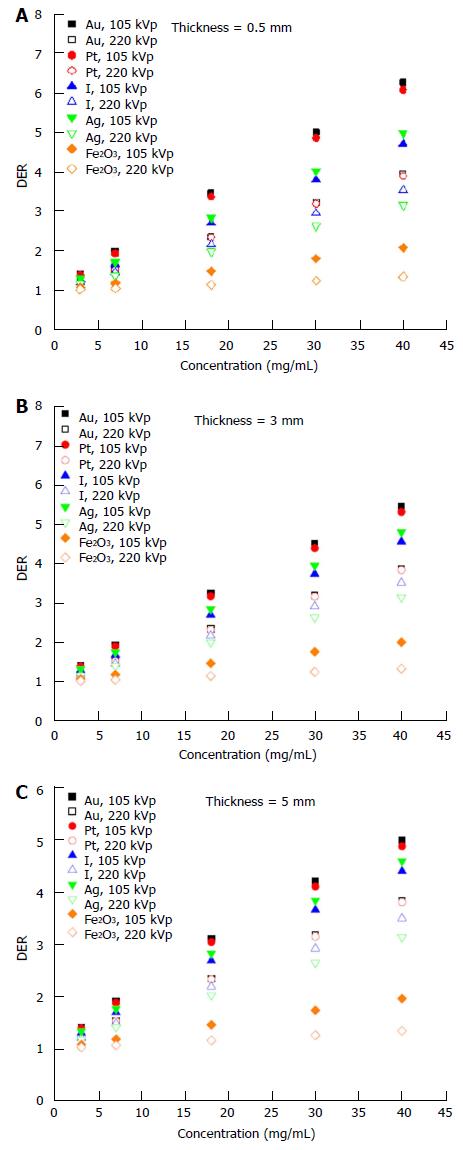
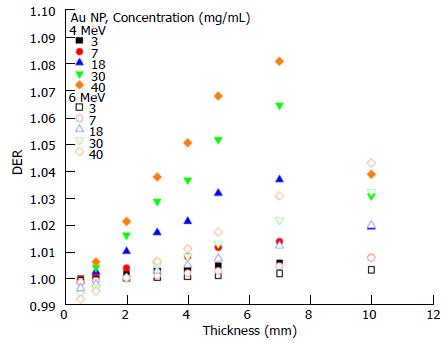
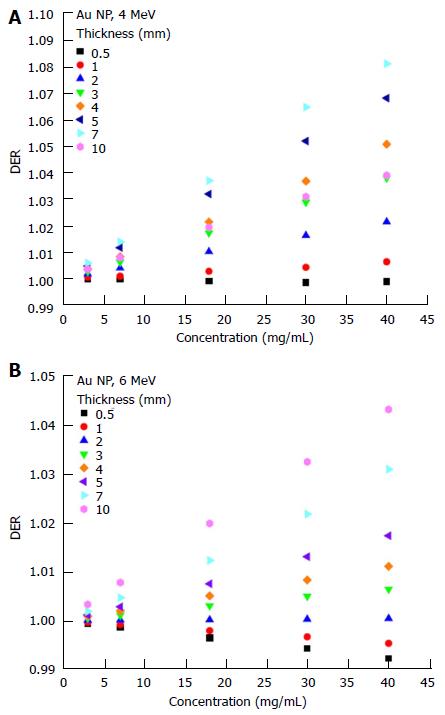
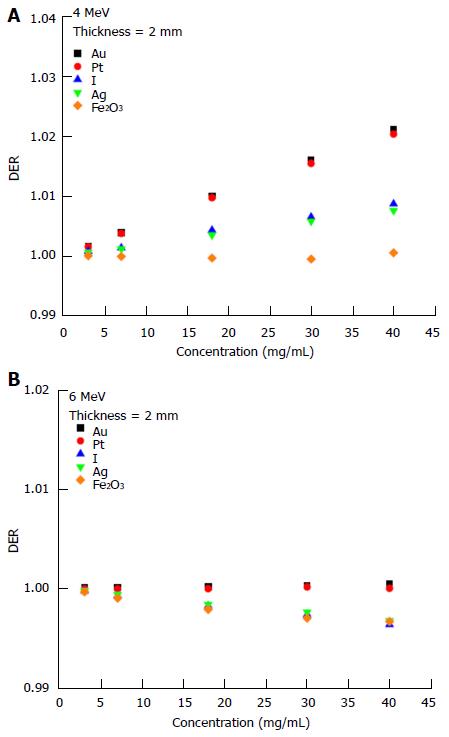
The dependency of the DER on skin target thickness using Au nanoparticles with increasing concentration (3-40 mg/mL) and kV photon beams is shown in Figure 2. The target thickness ranged from 0.5 to 5 mm. For other nanoparticle materials, dependence of the DER on target thickness is shown in Figure 3 for nanoparticle concentrations equal to 7, 18 and 40 mg/mL using the 105 and 220 kVp photon beams. Figure 4 reveals the relationship between the DER and Au nanoparticle concentration for different target thicknesses. In addition, Figure 5 shows relationships between the DER and nanoparticle concentration for different nanoparticle types with target thicknesses equal to 0.5, 3 and 5 mm, respectively. For the 4 and 6 MeV electron beam irradiations, Figure 6 shows the dependence of the DER on the target thickness for the Au nanoparticles with different concentrations. Variations of the DER for the Au nanoparticles with different target thicknesses are shown in Figure 7 using 4 and 6 MeV electron beams, respectively. The relationship between the DER and nanoparticle concentration for different nanoparticle types are shown in Figure 8 with the target thickness equal to 2 mm.
Dependence of the DER on skin target thickness: It can be seen from Figure 2 that the dependence of the DER on the skin target thickness was not significant, when the Au nanoparticle was added with concentrations ranging from 3 to 40 mg/mL using the 220 kVp photon beams. For the 105 kVp photon beams, however, the DER was found increasing with a decrease of target thickness when the nanoparticle concentration was higher than 18 mg/mL. This shows that the dose enhancement effect on the target thickness was more sensitive to lower energy photon beams and higher nanoparticle concentration.
For the dependence of the DER on skin target thickness for other nanoparticles, Figure 3 shows the relationship between the two for nanoparticle concentrations equal to 7, 18 and 40 mg/mL. In Figure 3, it is seen that the dose enhancement was generally affected by the atomic number of the nanoparticle and the quality of the kV photon beams. The DER for the Ag nanoparticles was slightly higher than for I for the 105 kVp photon beams. However, the atomic number of Ag (47) is smaller than I (53). This may be due to the energy spectrum of the polyenergetic 105 kVp photon beam produced by the orthovoltage machine[20]. In Figure 3, the Au nanoparticles are seen to have the highest DERs of 2, 3.5 and 6.3 when the nanoparticle concentration was 7, 18 and 40 mg/mL, respectively. A higher DER was found for higher nanoparticle concentrations and thinner target thicknesses due to the higher depth-dose gradient from the 105 kVp photon beams compared to 220 kVp[28]. Moreover, dependence of the DER on the target thickness was not significant for the Pt, I, Ag and Fe2O3 nanoparticles using 220 kVp photon beams.
Dependence of the DER on nanoparticle concentration: In Figure 4, it can be seen that the DER increased with an increase of Au nanoparticle concentration from 3 to 40 mg/mL using the 105 and 220 kVp photon beams. For the 220 kVp photon beams, the increase of the DER in the nanoparticle concentration did not vary with the target thickness significantly. For the 105 kVp photon beams, however, the rate of change of the DER with nanoparticle concentration was found to increase with a decrease of target thickness. When the Au nanoparticle concentration increased from 3 to 40 mg/mL, the DER was found to increase from 1.4 to 6.3 when using 105 kVp photon beams, respectively.
The degree of DER variation on the nanoparticle concentration was found to be more significant when the atomic number of the nanoparticles increased, with the Au nanoparticles producing the highest DER when using a 105 kVp photon beam. When the target thickness decreased from 5 mm to 0.5 mm (Figure 5), the DER increased for 105 kVp photon beams. In Figure 5, though it can be seen that the DER for the Fe2O3 nanoparticles was only in the range of 1 to 2 in the concentration range of 3-40 mg/mL, DER of higher than 5 can be achieved for the Au and Pt nanoparticles using the 105 kVp photon beams. It is found that dose enhancement was higher when using the lower energy 105 kVp photon beams, whenever the target thickness or nanoparticle concentration varied.
Dependence of the DER on skin target thickness: For the Au nanoparticles, it can be seen in Figure 6 that variation of the DER on the skin target thickness became significant when the electron beam energy decreased from 6 to 4 MeV. However, when the nanoparticle concentration was equal to 40 mg/mL, the DER decreased when varying the target thickness from 7 to 10 mm. Such an effect can also be observed in Figure 7A. Typically, the DER was found to increase as the target thickness increased from 0.5 to 10 mm. Unlike the kV range photon beams, we can see in Figure 6 that the DER only varied between 0.99 and 1.1 for the Au nanoparticle, having the highest atomic number in this study. This is due to the fact that photoelectric effect, which is dominant in the kV photon beam range, does not contribute to the energy absorption of the 4 and 6 MeV electron beams[11].
Dependence of the DER on nanoparticle concentration: Dependence of the DER on nanoparticle concentration was found to vary with the target thickness. For the 4 MeV electron beams, variation of the DER with Au nanoparticle concentration was not significant when the target thickness was small. For a nanoparticle concentration of 40 mg/mL in Figure 7A, it is seen that the DER increased when the target thickness increased from 0.5 mm to 7 mm. A decrease of DER was seen at a target thickness equal to 10 mm (also Figure 6). This is because the depth of maximum dose of the 4 MeV beam (7 mm) was smaller than the target thickness of 10 mm. For the 6 MeV electron beam with a deeper depth of maximum dose (15 mm), the highest DER can be found at the 10 mm target layer as shown in Figure 7B. Moreover, it is interesting to see that the DER was smaller than one when the target thickness was in the range of 0.5-2 mm for the 6 MeV electron beams. This shows that electron beams with higher energy (6 MeV) would not show dose enhancement (i.e., DER ≤ 1) with Au nanoparticle addition when the target thickness was lower than 2 mm.
When the skin target thickness was equal to 2 mm, it can be seen from Figure 8 that only Au, Pt, I and Ag nanoparticles had slight dose enhancement using the 4 MeV electron beams (Figure 8A). The DER was found to be smaller than one when the 6 MeV electron beams were used (Figure 8B) with low atomic number nanoparticles (e.g., Fe2O3) having the smallest DER in the range of nanoparticle concentration between 3 and 40 mg/mL.
In conclusion, different thicknesses of skin target layers with nanoparticle additions were irradiated by clinical kV photon and MV electron beams. The DER was calculated with variations of the target thickness, nanoparticle type, nanoparticle concentration and beam energy. It is found that with kVp photon beams there was a higher DER than MV electron beams, with the Au nanoparticles having the highest DER compared to the other simulated materials (Pt, I, Ag and Fe2O3). For the kV photon beams, the 105 kVp beams showed higher dose enhancement than using a 220 kVp beam. It is therefore concluded that the kV photon beams and Au nanoparticles would be the most appropriate for use in nanoparticle-enhanced skin therapy. Moreover, higher nanoparticle concentration was shown to benefit dose enhancement.
The authors would like to thank Amada Tulk from Gulmay Medical to allow us to share the Monte Carlo input data from Dr T Knoos in the Lund University Hospital for the verification of the kV photon beams. The authors would also like to thank Varti Vartanian and Mitch Spiegel of Varian Medical Systems for providing detailed information about the 21 EX linear accelerator, and Dr F Verhaegen of Maastro Clinic for sharing his BEAMnrc input files for verification of the electron beam simulations. The authors would like to thank Dr D Markel of the Princess Margaret Cancer Centre/University of Toronto for editing this manuscript.
This work studied the dose enhancement due to nanoparticle addition in skin therapy using the clinical kilovoltage photon and megavoltage electron beams.
The Monte Carlo results can guide the radiation staff which combination of nanoparticle type, nanoparticle concentration, beam type and beam energy should be used for different skin lesion thickness.
This Monte Carlo study combined all practical nanoparticle types, nanoparticle concentrations, clinical radiation beams and beam energies in treating skin lesions.
Nanoparticle-enhanced skin radiotherapy.
This is a well-written paper.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cerwenka HR, Loomba RS, Moskowitz SI S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Ali I, Lone MN, Al-Othman ZA, Al-Warthan A, Sanagi MM. Heterocyclic Scaffolds: Centrality in Anticancer Drug Development. Curr Drug Targets. 2015;16:711-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 2. | Ali I, Wani WA, Haque A, Saleem K. Glutamic acid and its derivatives: candidates for rational design of anticancer drugs. Future Med Chem. 2013;5:961-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Ali I, Haque A, Saleem K, Hsieh MF. Curcumin-I Knoevenagel’s condensates and their Schiff’s bases as anticancer agents: synthesis, pharmacological and simulation studies. Bioorg Med Chem. 2013;21:3808-3820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Ali I, Wani WA, Saleem K, Haque A. Platinum compounds: a hope for future cancer chemotherapy. Anticancer Agents Med Chem. 2013;13:296-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 5. | Ali I. Nano anti-cancer drugs: pros and cons and future perspectives. Curr Cancer Drug Targets. 2011;11:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Her S, Jaffray DA, Allen C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv Drug Deliv Rev. 2017;109:84-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 523] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 7. | Coulter JA, Butterworth KT, Jain S. Prostate cancer radiotherapy: potential applications of metal nanoparticles for imaging and therapy. Br J Radiol. 2015;88:20150256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Chow JCL. Characteristic of secondary electrons from irradiated gold nanoparticle in radiotherapy, 2015. Handbook of nanoparticle, Switzerland: Springer 2015; . [DOI] [Full Text] |
| 9. | Cormode DP, Naha PC, Fayad ZA. Nanoparticle contrast agents for computed tomography: a focus on micelles. Contrast Media Mol Imaging. 2014;9:37-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 221] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 10. | Chow JCL. Photon and electron interactions with gold nanoparticles: a Monte Carlo study on gold nanoparticle-enhanced radiotherapy (2016 Nanobiomaterials in medical imaging: applications of nanobiomaterials). Amsterdam: Elsevier 2016; in press. [DOI] [Full Text] |
| 11. | Chow JC, Leung MK, Jaffray DA. Monte Carlo simulation on a gold nanoparticle irradiated by electron beams. Phys Med Biol. 2012;57:3323-3331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Leung MK, Chow JC, Chithrani BD, Lee MJ, Oms B, Jaffray DA. Irradiation of gold nanoparticles by x-rays: Monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Med Phys. 2011;38:624-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49:N309-N315. [PubMed] |
| 14. | Song L, Falzone N, Vallis KA. EGF-coated gold nanoparticles provide an efficient nano-scale delivery system for the molecular radiotherapy of EGFR-positive cancer. Int J Radiat Biol. 2016;92:716-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Li W, Zhao X, Du B, Li X, Liu S, Yang XY, Ding H, Yang W, Pan F, Wu X. Gold Nanoparticle-Mediated Targeted Delivery of Recombinant Human Endostatin Normalizes Tumour Vasculature and Improves Cancer Therapy. Sci Rep. 2016;6:30619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Chattopadhyay N, Cai Z, Kwon YL, Lechtman E, Pignol JP, Reilly RM. Molecularly targeted gold nanoparticles enhance the radiation response of breast cancer cells and tumor xenografts to X-radiation. Breast Cancer Res Treat. 2013;137:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Butterworth KT, Nicol JR, Ghita M, Rosa S, Chaudhary P, McGarry CK, McCarthy HO, Jimenez-Sanchez G, Bazzi R, Roux S. Preclinical evaluation of gold-DTDTPA nanoparticles as theranostic agents in prostate cancer radiotherapy. Nanomedicine (Lond). 2016;11:2035-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Chow JC, Leung MK, Lindsay PE, Jaffray DA. Dosimetric variation due to the photon beam energy in the small-animal irradiation: a Monte Carlo study. Med Phys. 2010;37:5322-5329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Chow JC, Jiang R. Bone and mucosal dosimetry in skin radiation therapy: a Monte Carlo study using kilovoltage photon and megavoltage electron beams. Phys Med Biol. 2012;57:3885-3899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Chow JC, Owrangi AM. Surface dose reduction from bone interface in kilovoltage X-ray radiation therapy: a Monte Carlo study of photon spectra. J Appl Clin Med Phys. 2012;13:3911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Kawrakow I, Rogers DWO. The EGSnrc code system: Monte Carlo simulation of electron and photon transport, technique report PIRS-701. National Research Council of Canada: Ottawa, Canada. |
| 22. | Cho SH. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo study. Phys Med Biol. 2005;50:N163-N173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 284] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 23. | Knoos T, Rosenschold P, Wieslander E. Modelling of an orthovoltage x-ray therapy unit with the EGSnrc Monte Carlo package. J Phys Conf Serv. 2007;74: 012009. [DOI] [Full Text] |
| 24. | Rogers DWO, Walters B, Kawrakow I. BEAMnrc user manual. NRC Report PIRS-0509: Ottawa, Canada: NRCC 2006; . |
| 25. | Bielajew AF, Rogers DWO. PRESTA: The parameter reduced electron-step transport algorithm for electron Monte Carlo transport. Nucl Instrum Methods B. 1986;18:165-171, 174-181. [DOI] [Full Text] |
| 26. | Ma CM, Reckwerdt P, Holmes M, Rogers DWO, Geiser B. DOSXYZ user manual. NRC Report PIRS 509b, Ottawa, Canada: NRCC 1995; . |
| 27. | Chow JC, Owrangi AM. Solid water as phantom material for dosimetry of electron backscatter using low-energy electron beams: a Monte Carlo evaluation. Med Phys. 2009;36:1587-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Chow JC, Grigorov GN. Effect of the bone heterogeneity on the dose prescription in orthovoltage radiotherapy: A Monte Carlo study. Rep Pract Oncol Radiother. 2011;17:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |