Peer-review started: May 4, 2016
First decision: July 20, 2016
Revised: October 22, 2016
Accepted: December 13, 2016
Article in press: December 14, 2016
Published online: February 28, 2017
Processing time: 303 Days and 12.8 Hours
Crohn’s disease (CD) is a chronic inflammatory disease of the gastrointestinal tract, with unpredictable clinical course by phases of relapses alternating with other of quiescence. The etiology is multifactorial and is still not completely known; globally the westernization of lifestyle is causing an increasing incidence of CD, with peak age of 20-30 years. The diagnostic workup begins with the evaluation of the clinical history, physical examination and laboratory tests. However, the clinical assessment is subjected interobserver variability and, occasionally, the symptoms of acute and chronic inflammation may be indistinguishable. In this regards, the role of magnetic resonance (MR) enterography is crucial to determine the extension, the disease activity and the presence of any complications without ionizing radiations, making this method very suitable for young population affected by CD. The purpose of this review article is to illustrate the MR enterography technique and the most relevant imaging findings of CD, allowing the detection of small bowel involvement and the assessment of disease activity.
Core tip: Magnetic resonance (MR) enterography represents a non-invasive technique for Crohn’s disease (CD) diagnosis, allowing morphological and functional evaluation of the small bowel loops. For all these reasons, MR enterography is assuming a prominent role as first-choice radiological examination in patients affected by CD. In this setting, the purpose of this review article is to illustrate the MR enterography technique and the most relevant imaging findings of CD, in order to discriminate among the various subtypes of CD (active, fistulizing/perforating or chronic subtype) and to assess disease activity.
- Citation: Mantarro A, Scalise P, Guidi E, Neri E. Magnetic resonance enterography in Crohn’s disease: How we do it and common imaging findings. World J Radiol 2017; 9(2): 46-54
- URL: https://www.wjgnet.com/1949-8470/full/v9/i2/46.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i2.46
Crohn’s disease (CD) is a type of chronic inflammatory bowel disease, characterized by typical fluctuating course with relapses alternating with periods of remission[1]. The incidence of CD is increasing worldwide, but the highest has been reported in Northern Europe, United Kingdom, and North America, occurring 20%-30% more frequently in women, with age of onset during young adulthood and, in a small subset of patients, between the 60 and 80 years of age[2]. Nowadays, the etiopathogenesis is not completely known; however the development of CD depends on several factors (immunological, genetic, and environmental, such as diet or smoking), which lead to a dysregulated immune response to commensal flora or common antigens, in genetically susceptible hosts[3,4]. Usually, CD may manifest with increased frequency of bowel movements, diarrhoea, abdominal pain and weight loss; while symptoms like asthenia, anorexia, nausea, vomiting, fever and extra-intestinal manifestations (i.e., arthritis, uveitis, episcleritis, skin rashes, erythema nodosum, pyoderma gangrenosum) occur in about a quarter of patients[5].
Moreover, CD may affect any portion of the gastrointestinal tract from mouth to anus, mainly involving the ileocaecal region (about one half of all cases), following by the ileum and colon (30% and 20% of patients, respectively)[6]. Both the transmural chronic inflammation and the discontinuous involvement (“skip lesions”) of affected bowel loops, alternating inflamed and uninvolved segments, are typical features of CD[7]. During the active phase of inflammatory response, the enteric mucosa appears as irregular due to neutrophils and mononuclear cell infiltration, alternating ulceration and edema (“cobble-stoning” pattern), associated with cryptitis, crypt microabscesses, and sometimes non-caseating granulomas. When the inflammation becomes chronic, the superficial aphtoid ulcers can penetrate into the bowel wall resulting in deep ulcerations, sinus tracts or fistula formation, thus extending into mesentery, lymph nodes and adjacent structures (i.e., other bowel loops, bladder, uterus, vagina or skin).
Moreover, the chronic inflammatory response promotes smooth muscle cell proliferation, collagen accumulation, wall thickening, stenosis and fibrosis of the affected bowel segments with mesenteric fibro-fatty proliferation[8,9].
Given this context, the management and staging of patients with CD requires the correct determination of inflammatory lesion location, extension, activity and severity, in order to choose appropriate therapeutic strategies[10,11]. Since the clinical presentation of acute and chronic inflammation may be overlapped, the cross-sectional imaging techniques are useful for distinguishing them and preventing the development of potential complications. Among imaging modalities, magnetic resonance (MR) enterography provides the advantages of high-tissue-contrast evaluation with optimal detection of fluid and submucosal edema, multiplanar capability, multiparametric assessment and functional informations (motility, perfusion, diffusion) without ionizing radiations, making this method very suitable for young population affected by CD[12].
The purpose of this article is to review MR enterography technique and the most relevant imaging findings of CD, in order to provide an overview of the current state-of-art of MR imaging in CD, highlighting the recent MR innovations that allow a better evaluation of disease activity.
MR enterography requires fast imaging techniques, luminal distension and 6-h fasting before the procedure[11]. In this regard, an adequate colonic distension is mandatory to better identify wall thickening and parietal enhancement on the post-contrastographic images. The oral contrast agents used to obtain a well-distended lumen are classified based on their effects on T1 and T2-weighted images. The positive contrast agents (diluted gadolinium, some fruit juices or milk) yield the advantage of high intraluminal signal due to their high T2, but they may interfere with the detection of mucosal enhancement in T1-weighted sequences after gadolinium administration due to T1 shortening effect. By contrast, the use of negative contrast agents, as superparamagnetic iron oxide, determines a low intraluminal signal (low T2 and T1) and allows a better evaluation of the bowel walls[13]. However the widest accepted contrast agents are the biphasic ones (methylcellulose, mannitol, polyethylene glycol), characterized by hyperosmolar effect, that promotes luminal distention. Furthermore, they permit the assessment of wall thickening thanks to high signal intensity on T2-weighted sequences (positive effect), in which the bowel walls appear as hypointense, while the lumen is hyperintense. On T1-weighted images after gadolinium administration, these biphasic contrast agents maximize the depiction of wall enhancement by means of low intraluminal signal (negative effect)[14]. As above mentioned the biphasic contrast agent are more frequently preferred for MR enterography in CD. The patients are instructed to ingest about 1.5-2 L of water solution with biphasic contrast agent 45 min preceding the procedure[15]. The patient is positioned in prone decubitus in order to increase bowel loop separation and decrease both the peristalsis and the acquired abdominal volume in MR sequences, consequently reducing blurring and bowel motility artifacts. The supine position is required for noncompliant patients with abdominal stomas or entero-cutaneous fistulas. Moreover, before T2-weighted sequences and contrast medium injection, endovenous administration of 20 mg of hyoscine butylbromide is recommended to further reduce bowel peristalsis[16]. MR enterography is performed using phased-array coils to improve signal-to-noise ratio and spatial resolution, simultaneously minimizing the acquisition time with faster sequences and parallel imaging.
The MR study protocol consists of the following sequences.
It is a very fast sequence thanks to a short repetition and echo time, providing high-contrast MR images dependent on T2*/T1 ratio. It allows motion-free images, ensuring a good visualization of the small bowel, mesentery, vascularization and lymphadenopathy in coronal and axial view. Furthermore, this sequence may provide cine assessment of the bowel loops, facilitating the discrimination between fibrotic stricture and functional stenosis. However, the images suffer from magnetic susceptibility artifacts, caused by the presence of gas or ferromagnetic materials, and chemical shift artifacts resulting in a “black boundary”effect around structures, which may hamper a correct definition of bowel wall thickening[17].
It allows to obtain high-contrast resolution MR images in coronal and axial planes for the depiction of wall thickening, fold pattern changes, ulceration, intramural bowel edema and extraluminal fluid collections (particularly with fat-suppressed images). It is a fast sequence with a long echo train, which utilizes the partial Fourier encoding of K-space data for reducing the acquisition time, nevertheless decreasing signal-to-noise ratio of MR images. It is not sensitive to magnetic susceptibility or chemical shift artifacts, allowing an optimal evaluation of wall thickening. However, this sequence is sensitive to intraluminal flow voids, blurring and bowel motility artifacts; in this regard, the endovenous administration of spasmolytic agent is recommended to reduce bowel peristalsis[13].
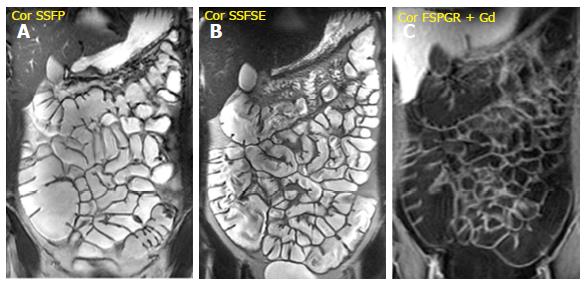
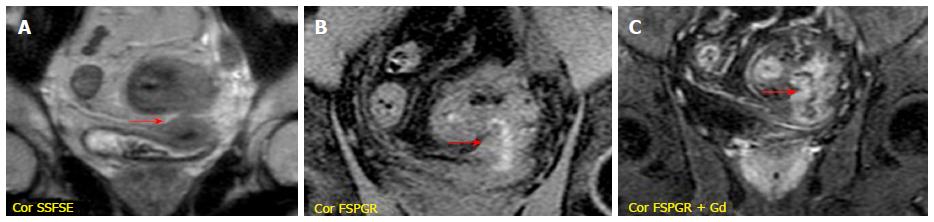
It is performed after the intravenous injection of 0.1-0.2 mmol/kg of gadopentetate dimeglumine (Gd-DTPA) with a delay time of 40-80 s; the acquisition of arterial phase after 25 s is optional. It is very useful to evaluate bowel wall enhancement, which is highlighted by endoluminal low-signal intensity caused by positive contrast agent administration. Moreover, it provides relevant information about vasculature, lymph nodes, fistulas or abscesses. Frequently the sequence is performed in the coronal plane; axial acquisitions may be useful for evaluating the pathological and thickened bowel loops[18] (Figure 1).
Diffusion-weighted imaging sequence in the axial plane has been proposed to better identify the inflamed bowel loops in active phase[16].
Several studies have compared the accuracy of different non-invasive diagnostic methods in the assessment of CD.
In this setting, MR enterography is more effective than ultrasound (US), particularly in the evaluation of the entire gastrointestinal tract, perianal region and complications; altough US permits a rapid and accurate examination of the terminal ileum[19].
Moreover, Lee et al[20] have demonstrated that the effectiveness of MR enterography is comparable to that of CT enterography, with the advantage of not using ionizing radiations, making this modality ideal in imaging the youth.
The clinical indications of MR enterography include the following conditions: (1) assessement of small bowel anatomy, disease localization and segmental extension; (2) morphological evaluation (bowel wall, mesentery, vascular supply and lymph nodes); (3) dynamic evaluation (disease activity and neoangiogenesis); (4) classification of CD into three subtypes based on inflammatory activity, including active inflammatory, fistulizing/perforating and fibrostenosing categories; (5) follow-up of patients with diagnosed CD; (6) exclusion of CD diagnosis in symptomatic patients; (7) suspected disease relapse, stricturing disease and/or extraluminal complications; (8) monitoring therapeutic response or failure; and (9) planning of surgical intervention[17].
The assessment of CD subtypes is required by clinicians for the therapeutic planning, through the detection of linear and aphthoid ulcers, wall edema, skip lesions, fistulas, abscess or strictures; and their correlation with clinical data[21]. Frequently, acute and chronic changes may coexist in the same bowel segment with a wide variety of intestinal and extra-intestinal abnormalities. In this context, the bowel wall lesions characterizing active disease are managed medically, whereas fibrotic strictures with bowel obstruction are frequently treated with surgical intervention[22].
The typical pathological findings of active CD comprehend: Aphthoid and deep ulceration, wall thickening (greater than 4 mm), intramural and mesenteric edema, stratified enhancement pattern of bowel wall, increased mesenteric vascularity, reactive lymphadenopathy[13].
Ulcers: The apthoid ulcers, typical findings of CD in the early stages, can only be detected through an adequate luminal distension and high-resolution MR imaging, they appear as a nidus of high signal intensity in T2-weigthed images, surrounded by a rim of moderate signal intensity[23]. Advanced inflammation may produce other changes, such as deep and transmural (linear) ulcerations. The typical “cobblestone” mucosal appearance result from confluent (longitudinal and transverse) ulcerations combined with bulging of the edematous mucosa[12].
Fold thickening: The SSFSE sequence allows to identify fold thickening and distortion caused by mucosal ulceration[24].
Wall thickening: It is easily identified on steady-state free precession (SSFP), T2-wegthed (SSFSE) and fat-suppressed 3D spoiled gradient-echo (FSPGR) (after Gd-DTPA administration) sequences, previous adequate distension of the small bowel loops. Nevertheless, the SSFP sequence hampers the correct definition of wall thickening, because of the chemical shift artefact. For this reason, the measurement of wall thickness should be performed in SSFSE images. The degree of wall thickening correlates with both the presence of inflammation and the degree of disease activity. Particularly, a wall thickening greater than 3 mm is indicative of inflammation, while a thickening ranging from 5 to 10 mm is suggestive of CD[25].
Intramural edema: This is a typical sign of active inflammation resulting in submucosal thickening, which appears as hyperintensity on T2-weighted (SSFSE) images with fat saturation[26].
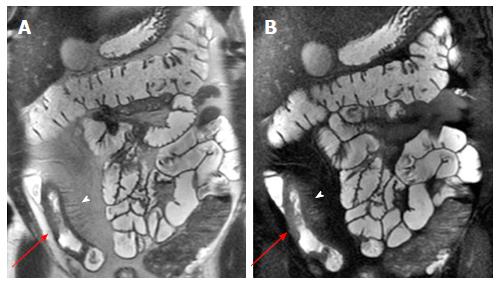
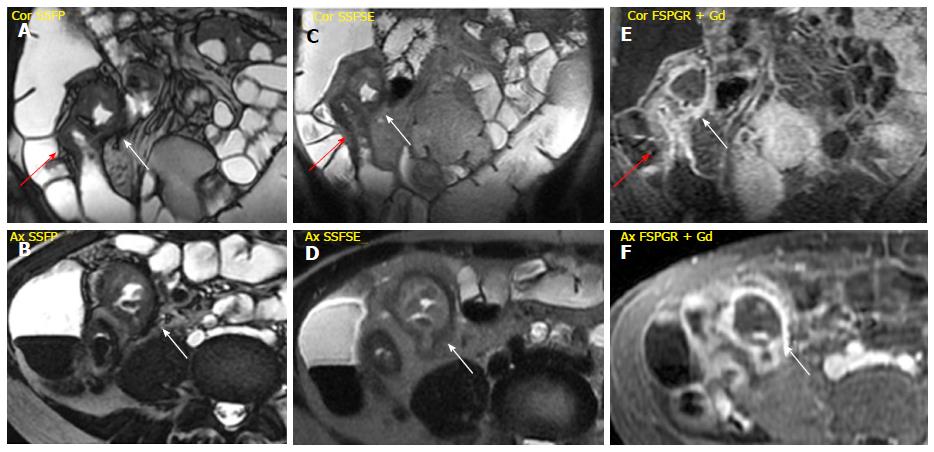
Mesenteric changes: In some cases of advanced inflammation, the mesentery may be edematous around the inflamed intestinal loops. Typically, the mesenteric edema is associated with both submucosal edema and stratified enhancement of the bowel wall; all these findings are suggestive of active inflammation. The mesenteric fibro-fatty proliferation (or fat-wrapping) represents another sign of advanced CD with consolidated transmural inflammation. It can be defined as an increase of mesenteric fat, which can determine mass effect with consequent anatomical displacement of the mesenteric vessels or the adjacent abdominal viscera, increasing the separation among the bowel loops. Moreover, the vascular engorgement produces an increased mesenteric vascularity (“comb sign”), resulting in hyperenhancement of mesenteric vessels supplying inflamed bowel loops[16,21] (Figure 2).
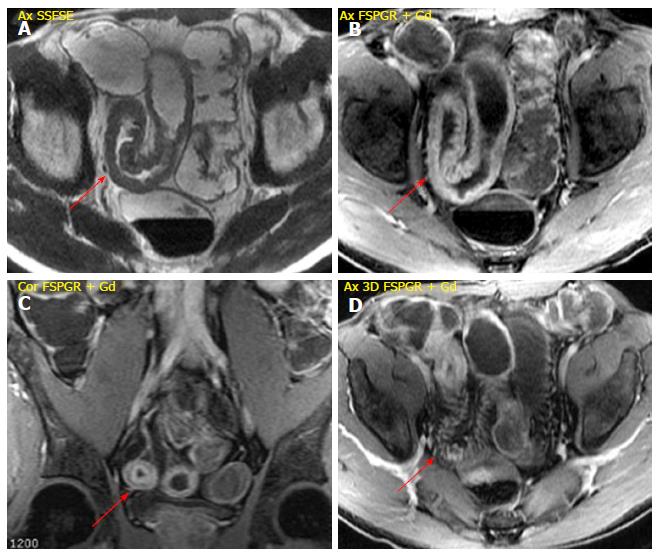
Stratified enhancement pattern: The bowel wall enhancement, evaluated after Gd-DTPA intravenous administration, represents the parameter that most closely correlates to both the degree of inflammation and clinical indices of disease activity. The mucosal hyperemia of the affected loops is represented by hyper-enhancement, which is significantly higher than normal loops; it decreases decrease in response to therapy. The typical stratified enhancement pattern (“target sign”) is produced by mucosal and muscle/serosa increased enhancement with intermediate hypointensity of edematous submucosa[27] (Figure 3).
Reactive mesenteric lymphadenopathy: Hyperenhancement, enlargement, and edema of lymph nodes can be present in active disease, but they are not specific of CD. These findings are easily identified with SSFP and FSPGR (after Gd-DTPA adminitrastion) sequences[28].
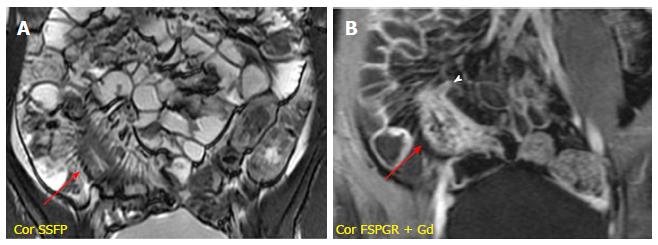
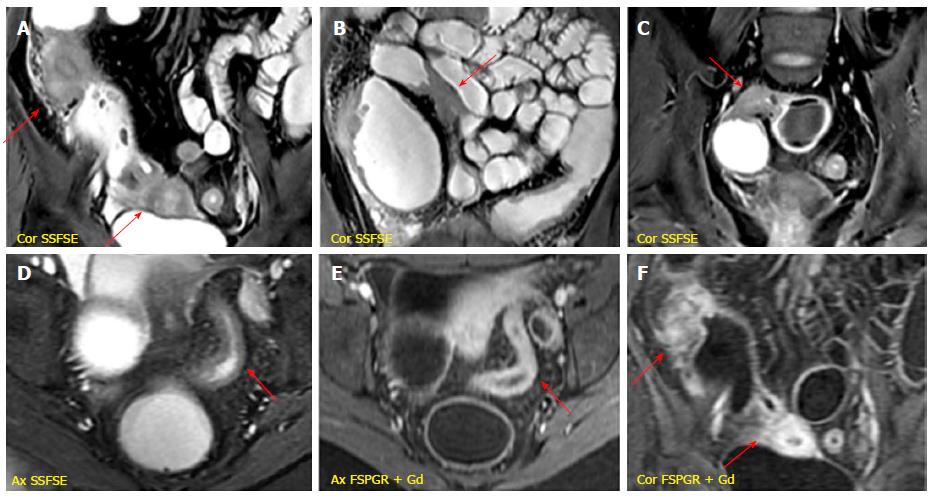
It is characterized by the presence of deep penetrating ulcers, which can lead to the creation of sinus tract, fistulas and/or abscesses. The sinus tracts appear as hyperintense blind-ending tracts on T2-weighted images, that arise from bowel wall without ever reaching the surface of another structure (Figure 4). By contrast, fistulas originate from deep transmural ulcers, which communicate with adjacent epithelial surfaces (bowel loops or other organs), appearing as hyperintense transmural lines on FSPGR (after Gd-DTPA administration) sequences[29,30]. The fistulas can penetrate into contiguous bowel loops (enteroenteric or enterocolic fistulas) or into other structures (i.e., bladder, uterus, vagina or skin); however, their identification in the earliest phase is very difficult due to low spatial resolution and partial volume averaging of MR images (Figure 5). An accurate high-resolution MR study associated with the use of multiplanar imaging can help to reveal the presence of fistulas. The desmoplastic reaction in the mesenteric tissue contributes to produce stellate appearance of fistulas, with spiculated margins[31].
Other locoregional complications of CD comprehend the development of phlegmon and abscesses. The penetrating process may lead to a localized inflammatory reaction resulting into phlegmon formation, which is an inflammatory mass with mild/moderate increase signal on T2-weighted and post-gadolinium sequences[32]. An abscess is an encapsulated collection of pus, which has MR characteristics similar to those of fluid collections (hyperintense on T2-weighted and hypointense on T1-weighted images), but with inhomogeneous content because of solid and gaseous components, delimitated by an enhancing peripheral rim[13] (Figure 6).
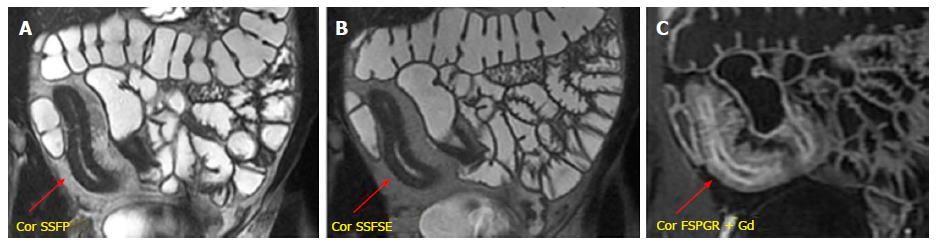
The chronic inflammation of the bowel wall tends to progress towards fibrostenotic and irreversible complications (bowel strictures and obstruction), as consequence of prolonged intestinal injury[33]. During chronic disease, the deposition of submucosal fat is promoted resulting in stratified appearance on T2-weighted images. This finding may be distinguished by submucosal edema on T2-weighted images thanks to fat saturation, which reduces the signal of fat in chronic disease[34]. Moreover, the bowel wall enhancement differs from the stratified pattern, typical of active disease. On this ground, the thickened and fibrotic bowel wall shows diffuse and homogeneous enhancement during subacute transmural inflammation, while the moderate mucosal enhancement with hypointensity of the deep layers suggests fibrotic disease[35] (Figure 7). In chronic disease, fibrosis may lead to stricture formation with high risk of small bowel obstruction of the affected segment, and consequent prestenotic dilatation (Figure 8). The fibrotic stricture appears as fixed luminal narrowing without any high signal intensity on T2-weighted images; by contrast the inflamed stricture, in acute disease, shows submucosal edema with the typical stratified enhancement pattern. It is important to identify the presence of fibrotic strictures because they are not responsive to medical therapy, but require prompt surgical approach to avoid complications such as bowel obstruction[12,36]. Furthermore, MR enterography is useful for detecting asymmetric bowel fibrosis on the mesenteric border with apparent dilatation (pseudodiverticula) on the antimesenteric side, and rare complications such as small bowel adenocarcinoma[37].
The recommended reading strategy for MR enterography examinations should integrate previous morphological evaluation, followed by functional assessment of the small bowel loops. The clinical information received and the specific diagnostic query are crucial for the radiologist, in order to better adapt the examination technique to the specific patient conditions.
According to the RSNA reporting initiative, consisting of a library of report templates, the MR enterography report should include these criteria: (1) clinical indication; (2) description of imaging technique and quality; (3) small bowel findings (i.e., distension, peristalsis, bowel wall thickening, post-contrast findings, fistulas and/or abscess, lymph nodes); (4) collateral findings in abdominal organs; and (5) final impression indicating location and activity of disease, complications and extra-enteric findings[38].
MR enterography is now considered a well-established imaging technique for small bowel evaluation. It plays an increasingly important role as non-invasive and effective method to evaluate the small-bowel involvement and the possible intestinal and extra-intestinal complications, in patients affected by CD.
Nevertheless, MR enterography examination should be tailored both to the patient and diagnostic query, in order to guide the clinical management.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Anzola LK, Giudici F S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL
| 1. | Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1239] [Article Influence: 123.9] [Reference Citation Analysis (0)] |
| 2. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1561] [Article Influence: 111.5] [Reference Citation Analysis (1)] |
| 3. | Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1529] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 4. | Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1277] [Article Influence: 67.2] [Reference Citation Analysis (2)] |
| 5. | Di Sabatino A, Rovedatti L, Vidali F, Macdonald TT, Corazza GR. Recent advances in understanding Crohn’s disease. Intern Emerg Med. 2013;8:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Laass MW, Roggenbuck D, Conrad K. Diagnosis and classification of Crohn’s disease. Autoimmun Rev. 2014;13:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 8. | Alzoghaibi MA. Neutrophil expression and infiltration into Crohn’s intestine. Saudi J Gastroenterol. 2005;11:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Randall CW, Vizuete JA, Martinez N, Alvarez JJ, Garapati KV, Malakouti M, Taboada CM. From historical perspectives to modern therapy: a review of current and future biological treatments for Crohn’s disease. Therap Adv Gastroenterol. 2015;8:143-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Wilkins T, Jarvis K, Patel J. Diagnosis and management of Crohn’s disease. Am Fam Physician. 2011;84:1365-1375. [PubMed] |
| 11. | Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor SA, Baumgart DC, Danese S, Halligan S, Marincek B, Matos C. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 476] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 12. | Leyendecker JR, Bloomfeld RS, DiSantis DJ, Waters GS, Mott R, Bechtold RE. MR enterography in the management of patients with Crohn disease. Radiographics. 2009;29:1827-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Tolan DJ, Greenhalgh R, Zealley IA, Halligan S, Taylor SA. MR enterographic manifestations of small bowel Crohn disease. Radiographics. 2010;30:367-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Laghi A, Paolantonio P, Iafrate F, Borrelli O, Dito L, Tomei E, Cucchiara S, Passariello R. MR of the small bowel with a biphasic oral contrast agent (polyethylene glycol): technical aspects and findings in patients affected by Crohn’s disease. Radiol Med. 2003;106:18-27. [PubMed] |
| 15. | Kuehle CA, Ajaj W, Ladd SC, Massing S, Barkhausen J, Lauenstein TC. Hydro-MRI of the small bowel: effect of contrast volume, timing of contrast administration, and data acquisition on bowel distention. AJR Am J Roentgenol. 2006;187:W375-W385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Griffin N, Grant LA, Anderson S, Irving P, Sanderson J. Small bowel MR enterography: problem solving in Crohn’s disease. Insights Imaging. 2012;3:251-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Furukawa A, Saotome T, Yamasaki M, Maeda K, Nitta N, Takahashi M, Tsujikawa T, Fujiyama Y, Murata K, Sakamoto T. Cross-sectional imaging in Crohn disease. Radiographics. 2004;24:689-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, Maris T, Prassopoulos P. MR enteroclysis protocol optimization: comparison between 3D FLASH with fat saturation after intravenous gadolinium injection and true FISP sequences. Eur Radiol. 2001;11:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Stasi C, Falchini M, Milani S. Imaging modalities for the noninvasive assessment of fibrosis in Crohn’s disease. ScientificWorldJournal. 2012;2012:450151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Lee SS, Kim AY, Yang SK, Chung JW, Kim SY, Park SH, Ha HK. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 21. | Fidler JL, Guimaraes L, Einstein DM. MR imaging of the small bowel. Radiographics. 2009;29:1811-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Maglinte DD, Gourtsoyiannis N, Rex D, Howard TJ, Kelvin FM. Classification of small bowel Crohn’s subtypes based on multimodality imaging. Radiol Clin North Am. 2003;41:285-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Sinha R, Rajiah P, Murphy P, Hawker P, Sanders S. Utility of high-resolution MR imaging in demonstrating transmural pathologic changes in Crohn disease. Radiographics. 2009;29:1847-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Sinha R, Verma R, Verma S, Rajesh A. MR enterography of Crohn disease: part 2, imaging and pathologic findings. AJR Am J Roentgenol. 2011;197:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Sempere GA, Martinez Sanjuan V, Medina Chulia E, Benages A, Tome Toyosato A, Canelles P, Bulto A, Quiles F, Puchades I, Cuquerella J. MRI evaluation of inflammatory activity in Crohn’s disease. AJR Am J Roentgenol. 2005;184:1829-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Yoon K, Chang KT, Lee HJ. MRI for Crohn’s Disease: Present and Future. Biomed Res Int. 2015;2015:786802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Del Vescovo R, Sansoni I, Caviglia R, Ribolsi M, Perrone G, Leoncini E, Grasso RF, Cicala M, Zobel BB. Dynamic contrast enhanced magnetic resonance imaging of the terminal ileum: differentiation of activity of Crohn’s disease. Abdom Imaging. 2008;33:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Prassopoulos P, Papanikolaou N, Grammatikakis J, Rousomoustakaki M, Maris T, Gourtsoyiannis N. MR enteroclysis imaging of Crohn disease. Radiographics. 2001;21 Spec No:S161-S172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Scharl M, Rogler G. Pathophysiology of fistula formation in Crohn’s disease. World J Gastrointest Pathophysiol. 2014;5:205-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Maccioni F, Bruni A, Viscido A, Colaiacomo MC, Cocco A, Montesani C, Caprilli R, Marini M. MR imaging in patients with Crohn disease: value of T2- versus T1-weighted gadolinium-enhanced MR sequences with use of an oral superparamagnetic contrast agent. Radiology. 2006;238:517-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Herrmann KA, Michaely HJ, Zech CJ, Seiderer J, Reiser MF, Schoenberg SO. Internal fistulas in Crohn disease: magnetic resonance enteroclysis. Abdom Imaging. 2006;31:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Cullen G, Vaughn B, Ahmed A, Peppercorn MA, Smith MP, Moss AC, Cheifetz AS. Abdominal phlegmons in Crohn’s disease: outcomes following antitumor necrosis factor therapy. Inflamm Bowel Dis. 2012;18:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Latella G, Di Gregorio J, Flati V, Rieder F, Lawrance IC. Mechanisms of initiation and progression of intestinal fibrosis in IBD. Scand J Gastroenterol. 2015;50:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 34. | Maccioni F, Viscido A, Broglia L, Marrollo M, Masciangelo R, Caprilli R, Rossi P. Evaluation of Crohn disease activity with magnetic resonance imaging. Abdom Imaging. 2000;25:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 186] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E, Bloom S, Cohen R, Windsor A, Obichere A, Hansmann A. Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology. 2009;252:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 36. | Rimola J, Planell N, Rodríguez S, Delgado S, Ordás I, Ramírez-Morros A, Ayuso C, Aceituno M, Ricart E, Jauregui-Amezaga A. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 37. | Cahill C, Gordon PH, Petrucci A, Boutros M. Small bowel adenocarcinoma and Crohn’s disease: any further ahead than 50 years ago? World J Gastroenterol. 2014;20:11486-11495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | MR Enterography Template. [accessed 2016 Apr 23]. Available from: http://www.radreport.org/template/0000051. |