Published online Oct 28, 2017. doi: 10.4329/wjr.v9.i10.389
Peer-review started: February 15, 2017
First decision: May 7, 2017
Revised: May 26, 2017
Accepted: July 14, 2017
Article in press: July 17, 2017
Published online: October 28, 2017
Processing time: 259 Days and 0.8 Hours
Accurate nodal staging at the time of diagnosis of prostate cancer is crucial in determining a treatment plan for the patient. Pelvic lymph node dissection is the most reliable method, but is less than perfect and has increased morbidity. Cross sectional imaging with computed tomography (CT) and magnetic resonance imaging (MRI) are non-invasive tools that rely on morphologic characteristics such as shape and size of the lymph nodes. However, lymph nodes harboring metastatic disease may be normal sized and non-metastatic lymph nodes may be enlarged due to reactive hyperplasia. The optimal strategy for preoperative staging remains a topic of ongoing research. Advanced imaging techniques to assess lymph nodes in the setting of prostate cancer utilizing novel MRI contrast agents as well as positron emission tomography (PET) tracers have been developed and continue to be studied. Magnetic resonance lymphography utilizing ultra-small super paramagnetic iron oxide has shown promising results in detection of metastatic lymph nodes. Combining MRL with diffusion-weighted imaging may also improve accuracy. Considerable efforts are being made to develop effective PET radiotracers that are performed using hybrid-imaging systems that combine PET with CT or MRI. PET tracers that will be reviewed in this article include [18F]fluoro-D-glucose, sodium [18F]fluoride, [18F]choline, [11C]choline, prostate specific membrane antigen binding ligands, [11C]acetate, [18F]fluciclovine, gastrin releasing peptide receptor ligands, and androgen binding receptors. This article will review these advanced imaging modalities and ability to detect prostate cancer metastasis to lymph nodes. While more research is needed, these novel techniques to image lymph nodes in the setting of prostate cancer show a promising future in improving initial lymph node staging.
Core tip: Accurate nodal staging at time of prostate cancer diagnosis is crucial in determining a treatment plan for the patient. This review article highlights the newest imaging techniques that have been and are being developed for imaging of lymph nodes in the initial staging of prostate cancer. Magnetic resonance lymphography utilizing ultra-small super paramagnetic iron oxide has shown to detect metastatic disease in normal sized lymph nodes. Considerable efforts are being made in molecular imaging to develop effective positron emission tomography radiotracers that may be combined with computed tomography or magnetic resonance to detect prostate metastasis as well as potential therapeutic applications.
- Citation: Zarzour JG, Galgano S, McConathy J, Thomas JV, Rais-Bahrami S. Lymph node imaging in initial staging of prostate cancer: An overview and update. World J Radiol 2017; 9(10): 389-399
- URL: https://www.wjgnet.com/1949-8470/full/v9/i10/389.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i10.389
Prostate cancer is the most common cancer in American men and is associated with a significant likelihood of cure when patients have organ-confined disease through the use of local definitive therapy such as radical prostatectomy or radiation therapy[1]. However, once prostate cancer spreads beyond the gland to the lymphatic tissues, the opportunity for cure with a local therapy is lost in most cases and significantly diminished in others[1]. Due to the adverse prognostic implications associated with lymph node metastasis, detection of clinically occult lymph node metastasis is of extreme importance[2]. Risk assessment tools are used to predict patients who are at risk for higher pathologic stage and use inputs such as PSA, biopsy Gleason sum, percent positive biopsies, and magnetic resonance imaging (MRI) findings[3-5]. The prostate health index (PHI) test utilizes three forms of PSA (total PSA, free PSA and p2PSA) and the 4K panel (total PSA, free PSA, single chain intact PSA, and human kallikrein 2) have been shown to more accurately predict higher-grade prostate cancer[6,7]. Patients who are deemed low risk, defined as a predicted < 5% (or in some more conservative guidelines ≤ 2%) for lymph node metastasis usually undergo definitive treatment with curative intent without any further radiological imaging or lymph node dissection[1]. Patients determined to be at higher risk for systemic disease need to undergo nodal staging. The most reliable method is pelvic lymph node dissection; however, this is invasive and may be associated with increased morbidity and risk of complications[1]. Furthermore, pelvic lymph node dissection is less than perfect as several studies have reported positive lymph nodes outside the routine dissection template[8-11]. Even extended pelvic lymph node dissections have been shown to miss up to 13% of metastatic lymph nodes[12].
Cross sectional imaging is a non-invasive tool utilized for nodal staging and largely relies on morphologic characteristics such as size and shape. A meta-analysis found a pooled sensitivity of 42% and specificity of 82% for computed tomography (CT) imaging and similar 39% sensitivity and 82% specificity for MR imaging for detection of metastatic lymph nodes[1]. Utilizing CT and MRI, determination of metastatic lymph nodes is determined largely by size. A threshold of 1.0 cm in short axis of oval nodes and 0.8 cm for round nodes are generally used as indicators of likely metastatic disease[13]. However, more than half of lymph nodes involved with metastatic prostate cancer may be less than 1 cm[14]. Moreover, non-metastatic nodes may be enlarged due to reactive hyperplasia. Given the lack of sensitivity of both CT and MRI based on size criteria alone, new techniques of MR lymphography (MRL) have been developed as well as molecular imaging techniques. Herein, we will discuss these modalities for improved prostate cancer lymph node staging.
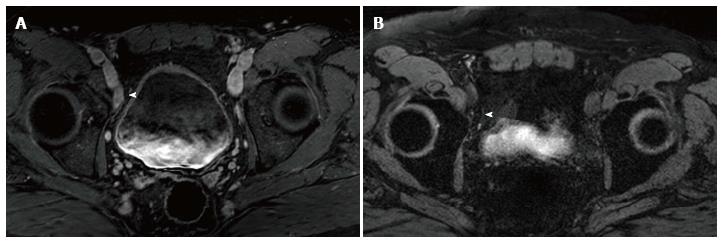
High resolution MRI utilizing ultra-small super paramagnetic iron oxide (USPIO) has been utilized to improve sensitivity for detection of metastatic lymph nodes[15,16]. Lymphotropic superparamagnetic nanoparticles are avidly taken up by lymph nodes where they are internalized by macrophages[17]. Malignant nodes have a relatively paucity of macrophages compared to benign lymph nodes. The intracellular iron-containing particles cause benign lymph nodes to lose signal (appear dark) on T2* images (Figure 1) while lymph nodes affected by metastatic disease do not take up the USPIO as effectively due to the decreased macrophages and hence appear bright[18]. This evaluation of macrophage function does not rely on nodal size to detect metastasis[19]. Moreover, it does not depend on the functional activity of cancer in the lymph nodes as it labels normal macrophages in the lymph nodes[20]. Given the high spatial resolution of MRI, more lymph nodes at smaller sizes can be detected and accurately characterized as benign or malignant with macrophage replacement by metastatic cancer cells[20].
USPIO particles have been used extensively as a lymphotropic contrast agent for detection of metastatic prostate cancer in numerous clinical trials[16,21-26]. In an initial study that utilized USPIO (ferumoxtran-10), nodes were considered malignant when one of the following three criteria are present: (1) A decrease in signal intensity of less than 30 percent on T2-weighted fast spin-echo or gradient-echo sequences after the administration of USPIO; (2) a heterogeneous signal (giving the entire node a mottled appearance), discrete focal defects (isolated islands of high signal intensity), or both on gradient echo imaging; and (3) nodes with a central area of hyperintensity (excluding a fatty hilum) but a peripheral decrease in signal intensity[15]. This initial study utilizing ferumoxtran-10 demonstrated a sensitivity for detection of malignant lymph nodes with short axis diameter of 5-10 mm was increased with use of USPIO compared to MRI alone (96.4% vs 28.5%, respectively)[15]. Other studies have confirmed the ability of MR ferumoxtran-10-enhanced lymphography to detect metastatic disease in non-pathologically enlarged lymph nodes (< 7 mm) with high sensitivity and specificity[20,23,24,27-30].
Meijer et al[31] showed a better prognosis in a subset of patients with MRL positive lymph nodes that were < 8 mm and better outcomes in patients in whom all MRL positive lymph nodes were resected. This highlights a window of opportunity for cure in these patients as those with MRL-detected positive nodes that were entirely removed had a five-year distant metastatic disease free survival of 80% compared to 35% in the patients who did not have all MRL-detected positive nodes removed[31].
Additionally, the potential for MRL to detect metastatic lymph nodes outside of the routine dissection margin has potential for great clinical value including surgical and radiation therapy planning. In series of 269 men with moderate to high risk for nodal metastasis, 41% were found to have lymph node metastasis outside of the routine dissection area that were identified with MR ferumoxtran-10-enhanced lymphography[24]. MRL has been utilized to guide radiation therapy as another study showed that 53% of prostate cancer patients had MRL positive lymph nodes outside of the target volume for pelvic radiation[32]. Salvage radiation therapy is associated with some toxicity[33], and improved selection of patients and detection of nodal targets can decrease morbidity and improve cure rates[25]. However, despite these promising results, ferumoxtran-10 failed to achieve Food and Drug Administration (FDA) approval and production was halted[34].
Ferumoxytol is a newly released USPIO agent that has been more recently utilized in detection of malignant lymph nodes[35]. Ferumoxytol is a compound closely related to ferumoxtran-10 that is FDA approved as iron replacement therapy in patients with chronic kidney failure with the recommended clinical dose of 1020 mg (two doses of 510 mg administered intravenously 3-8 d apart)[35,36]. In phase I and II clinical trials, ferumoxytol was associated with low adverse event rate which the most common events including nausea, dizziness, and diarrhea[37], although more serious reactions such as hypotension and anaphylaxis have been reported[38,39]. This has led to the FDA releasing a safety communication recommending modifications to give ferumoxytol as a dilute infusion[40].
Due to its large size, ferumoxytol remains in a relatively steady concentration within the intravascular space for several hours (circulating half-life is 14-15 h) and then is gradually cleared by macrophages from the blood pool over several days[41]. Following macrophage breakdown, the iron oxide particles are taken up by the reticuloendothelial system[41]. In a recent phase I dose escalation trial, it was shown that the signal intensity of normal lymph nodes drop in a dose dependent manner with the optimal dose determined to be 7.5 mg Fe/kg[35]. A pilot study quantitatively compared the ability of ferumoxytol and ferumoxtran-10 to suppress signal intensity in normal lymph nodes (in patients with high risk prostate cancer) and showed that signal suppression was weaker for ferumoxytol MRL than for ferumoxtran-10 MRL[42]. This study was limited in that only 4 patients received ferumoxytol and the dose was 6.0 mg Fe/kg, but further research is needed to validate the accuracy of ferumoxytol MRL[42]. Ferumoxytol MRL has also been preliminarily evaluated in non-human primates for use as an intraprostatic injection to directly map lymph nodes draining the prostate gland, with a potential of guiding lymphatic drainage patterns for specific gland segments[43].
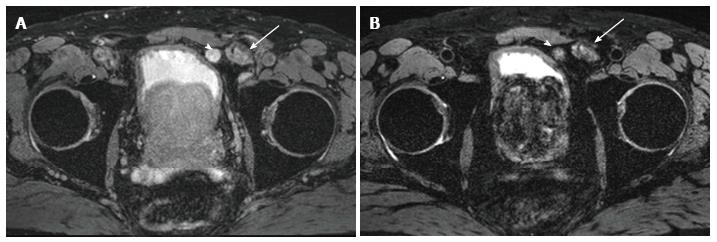
MRL may have false positive results in the setting of reactive nodal hyperplasia or granulomatous diseases in which there are decreased number of macrophages in otherwise benign lymph nodes that may lead to mischaracterization of lymph nodes as malignant suspicion on imaging[44] (Figure 2).
In diffusion-weighted imaging (DWI), the Brownian motion of water molecules within a voxel of tissue is imaged and can be quantitatively expressed using the apparent diffusion coefficient (ADC) value. DWI can be performed quickly without the need of a contrast medium. Malignant tissue tends to have increased cellularity with enlarged nuclei and an abundance of macromolecular proteins, resulting in restricted diffusion[45]. Typical malignant lesions appear hyperintense on images acquired at high b-values (800-1000 s/mm2) and hypointense on the corresponding ADC maps.
Malignant lymph nodes have been shown to have a lower ADC value when compared to benign lymph nodes[46,47], but show less promising results in normal sized lymph nodes with a wide range of ADC values[45,48]. In a study of 29 patients with prostate cancer and a total of 118 lymph nodes evaluated by DWI-MRI, a cut off value of 1.3 × 10-3 mm2/s yielded a sensitivity of 86% and specificity of 85%[46]. ADC values can be elevated in necrotic nodes due to the free diffusion of water, which can lead to misclassification[49]. While some authors have reporting the combination of nodal size and the relative ADC value nodes is more useful in detecting pelvic lymph node metastasis[50], others have found less promising results[45]. Nevertheless, there can be an overlap of ADC values in benign and malignant lymph nodes[51].
In a more recent prospective study utilizing a 3T MRI system and a non-quantitative approach to evaluation DWI and ADC maps, revealed 94% specificity and 41% sensitivity for anatomical region based analysis (mean positive lymph node size was 1.2 cm)[52]. In a prospective evaluation of detection of normal sized metastatic lymph nodes in initial staging of prostate and bladder carcinoma, there was increased detection utilizing DWI when compared to conventional cross-section imaging techniques with detection of metastasis in 64%-79% of patients[51]. Evaluating additional morphologic features at MR imaging such as round shape, irregular border, low T2 signal may improve specificity than relying only on diffusion weighted imaging[51]. Further advances in DWI-MRI technique will require standardization of the technique, image acquisition and sequence parameters of different scanner platforms[53].
Combining DWI-MRI with other techniques such as USPIO or choline show perhaps the most promising results[30,54]. In a study evaluating 2,993 normal sized lymph nodes in patients with prostate or bladder cancer, combining DWI-MRI with USPIO improved sensitivity and specificity (65%-75% and 93%-96%; compared to 55%-65% and 71%-91%, respectively) and decreased imaging interpretation time compared to USPIO alone[30].
Considerable efforts are currently being made to develop effective radiotracers to image prostate cancer in both the setting of primary staging as well as biochemical recurrence. Although not the focus of this paper, there is substantial evidence supporting the use of PET tracers for biochemical recurrence, and two PET tracers [(11C)choline and (18F)fluciclovine] have been approved by the FDA for the detection and staging of biochemical recurrence. However, there has been less investigation regarding the use of PET in the initial nodal staging of prostate cancer. A wide range of PET tracers have been developed and investigated for prostate cancer imaging, and this section summarizes PET tracers that have demonstrated utility for detecting lymph node metastases from prostate cancer. These tracers and their key properties are summarized in Table 1.
| PET tracers | Mechanism of action |
| [18F]FDG | Glucose metabolism |
| Sodium [18F]fluoride | Chemisorption to bone matrix |
| [18F]choline, [11C]choline | Cell membrane metabolism |
| [18F]DCFBC, [68Ga]HBED-CC | PSMA binding |
| [11C]acetate | Fatty acid metabolism |
| [18F]fluciclovine | Amino acid transport |
| [68Ga]DOTA-bombesin | GRPR receptor binding |
| [18F]FDHT | Androgen receptor binding |
Positron emission tomography (PET) is currently performed using hybrid imaging systems that combine PET with CT or MRI for attenuation correction and anatomic localization of the PET findings. In PET imaging, a positron emitting radiotracer is administered to the patient, which then emits 511 keV gamma rays through annihilation of the positron with an electron in the tissue which can be localized through coincidence detection[55]. Compared to conventional planar scintigraphy and single photon computed tomography (SPECT), PET provides higher spatial and temporal resolution. One of the limitations of PET is resolution, with decreased sensitivity of the detection and characterization of PET tracer uptake in lesions less than 8 mm. However, the use of hybrid imaging PET/CT or PET/MRI allows the combination of anatomical imaging from the CT or MRI with molecular information from PET.
PET tracers for prostate cancer imaging have been labeled with several different radionuclides that vary in terms of their physical half-life (t1/2) and their chemistries. PET radionuclides include are carbon-11 (t1/2 = 20 min), fluorine-18 (t1/2 = 110 min), and gallium-68 (t1/2 = 68 min). Carbon-11 and fluorine-18 are produced using cyclotrons while gallium-68 is produced using a generator system. Fluorine-18 is widely available, and its long half-life facilitates production of large batches of PET tracers and distribution to sites that do not have onsite production capabilities.
The most commonly used radiotracer for clinical oncologic imaging is 2-deoxy-2-(18F)fluoro-D-glucose (FDG), but this tracer is of limited utility for the initial staging of prostate cancer due to the low metabolic activity in the early phase of the disease which results in poor sensitivity[56]. Another disadvantage of FDG is urinary excretion, potentially decreasing sensitivity for pelvic lymph nodes[57]. For the evaluation of bony metastases, sodium(18F) fluoride (NaF) has been used for skeletal scintigraphy which takes advantage of higher spatial resolution when compared with conventional planar (99mTc)methyldiphosphinate (MDP). Several studies have demonstrated that cross-sectional skeletal scintigraphy with fluoride-PET/CT or MDP-SPECT/CT have superior sensitivity and specificity for detection of osseous metastases when compared to conventional planar bone scans (96% and 98%, respectively)[58]. The uptake of (18F)fluoride is based on bone turnover with increased binding to newly deposited mineralize bone matrix that occurs in bone metastases, particularly osteoblastic metastases. While (18F)fluoride-PET/CT may be useful in preoperative skeletal staging in prostate cancer, this tracer is not useful for the detection of lymph node metastases.
Choline is a naturally-occurring small molecule that is incorporated into tumor cells after phosphorylation by choline kinase, which is up regulated in prostate cancer[59]. (11C)choline uptake in metastatic lymph nodes occurs in the presence of viable malignant tissue, and (11C)choline has been approved by the FDA for use in the detection and localization of suspected biochemically recurrent prostate cancer. This PET tracer has been used for prostate cancer with a pooled sensitivity of 49.2% and specificity of 95% for detection lymph node metastasis[60]. Results are better for larger nodes, but sensitivity is limited in lymph nodes smaller than 7 mm[20]. Several studies combining (11C)choline PET/CT and diffusion weighted MRI showed that sensitivity remained too low to be clinically useful for initial staging[54,61].
Fluorinated analogues of choline have been developed to take advantage of the longer half-life of fluorine-18 [e.g., (18F)fluorocholine and 2-(18F)fluoroethyl choline]. However, these fluorinated choline analogues have similar limited capability in detecting lymph node metastasis (sensitivity 40% and specificity 96%)[62,63]. Combining 18F-choline PET and MRI may help improve the results of choline PET imaging, but further research is needed[64,65]. The value of choline for the detection of recurrent prostate cancer in patients with low PSA levels (< 2.5 ng/mL) is limited[66,67]. Additionally, 18F-labeled analogues of choline are eliminated via the kidney and urinary tract activity, which is undesirable for pelvic imaging.
Acetate is a naturally occurring metabolic substrate that can enter the fatty acid metabolic pathway which is overexpressed in prostate cancer cells[68]. Most research has been done with (11C)acetate and has shown sensitivity of 68% and specificity of 78% in one study of intermediate and high risk prostate cancer[69], but has the disadvantage of requiring an on onsite cyclotron to due to the short half-life of 20 min.
Prostate specific membrane antigen (PSMA) is a protein expressed by the prostate and overexpressed in prostate cancer[70]. The initial molecular imaging agent targeting PSMA that gained widespread use was (111In)indium capromab pendetide, a radiolabeled monoclonal antibody that targets the intracellular portion of PSMA and is imaged utilizing SPECT/CT. While initial results showed improvement over conventional techniques, there was limited sensitivity and specificity[71,72]. However with the additional of MRI, sensitivity and specificity were increased[73]. The main disadvantage of (111In)indium capromab pendetide is that it targets an intracellular protein making imaging only a possibility upon apoptosis or necrosis and not in viable tissue[55,70,74]. Additionally, the slow kinetics of (111In)indium capromab pendetide requires imaging 5-7 d after injection. More recently, a great deal of research has been focused on small molecule ligands that bind to the extracellular portion of PSMA[75,76].
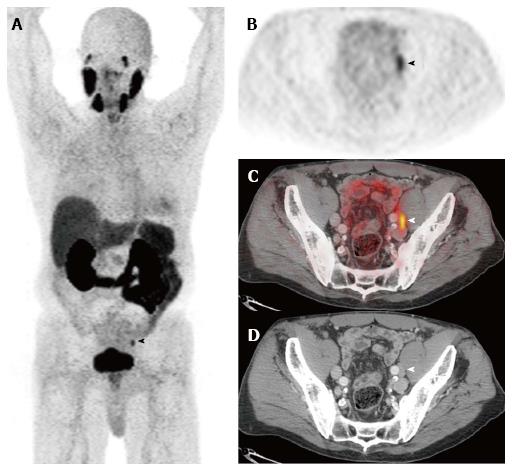
Novel small molecule imaging PSMA ligands have been developed such as N-{N-[(S)-1,3-dicarboxypropyl] carbamoyl}-4-(18F)fluorobenzyl-L-cysteine [(18F)DCFBC], which binds irreversibly to the extracellular component of PSMA and has been shown to improve detection of metastatic prostate cancer[77,78]. The most commonly used PSMA ligand in Europe is 68Ga-N,N’-bis [2-hydroxy-5-(carboxyethyl)benzyl] ethylenediamine-N,N’-diacetic acid (HBED-CC) (Figure 3). In a study utilizing 68Ga-labeled PSMA ligand (HBED-CC), there was improved accuracy of lymph node staging over conventional imaging with 65.9% sensitivity and 98.9% specificity[79]. A retrospective study examining 68Ga-PSMA PET/CT in initial staging of patients with high risk of lymph node metastasis found 33.3% sensitivity and 100% specificity (mean size of true positive nodes was 13.6 cm and of false positive node was 4.3 mm)[80].
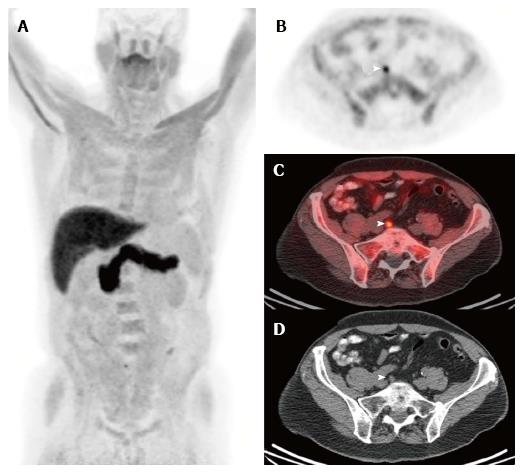
Amino acid radiotracers can accumulate in prostate cancer cells through the upregulation of transmembrane amino acid transport in prostate cancer[81]. The most work with a synthetic amino acid PET radiotracer for prostate cancer has been with (18F)fluciclovine for detection of recurrent disease, and this PET tracer was approved by the FDA for use in biochemically recurrent prostate cancer in 2016 (Figure 4). Preliminary studies have reported results for initial staging[81,82]. In a multicenter phase IIb clinical trial for staging of initial prostate cancer, diagnostic accuracy of (18F)fluciclovine PET/CT was compared to conventional imaging with CT and bone scan[83]. Overall accuracies were similar (85.5% for (18F)fluciclovine PET/CT and 87.3% for conventional imaging); however, (18F)fluciclovine PET/CT was positive in 5-9 mm nodes and skeletal lesions that were not detected by conventional imaging.
An additional molecular target that is currently under investigation is the gastrin-releasing peptide receptor (GRPR) which is overexpressed in prostate but has lower levels in benign prostate tissue including benign prostatic hyperplasia[84]. Initial studies demonstrate GRPR overexpression in 63%-100% of primary prostate carcinomas and 50%-85% of nodal and osseous metastases[85]. A number of peptide based ligands for GRPR have been developed including the 14 amino acid peptide bombesin, as well as analogues of the 27-amino acid gastrin releasing peptide (GRP)[86]. A single human trial utilizing a 68Ga-labeled GRPR antagonist for pre-operative staging has been completed[87]. This trial enrolled 11 patients with primary prostate carcinoma and three patients with evidence of biochemical recurrence, demonstrating a sensitivity of 88% and specificity of 81% for detection of the primary lesion (within a sextant level) and found evidence of biochemical recurrence in lymph nodes and the prostate bed in two out of three patients[88].
Androgen sensitivity and receptor expression remain a mainstay in the diagnosis and treatment of prostate carcinoma. Importantly, androgen receptor expression plays a role in both initial treatment strategies and in the setting for treatment for biochemical recurrence/metastatic disease. Preliminary human imaging studies have been performed using the androgen receptor ligand 16β-(18F)fluoro-5α-dihydrotestosterone (FDHT). The initial human study compared FDHT and FDG, which demonstrated FDHT uptake in 46/59 lesions in seven patients with progressive metastatic prostate cancer (compared to FDG uptake in 57/59)[89]. The role, if any, of (18F)FDHT in the pre-operative evaluation of prostate cancer is not yet defined.
An interesting future in targeted molecular imaging of metastatic prostate carcinoma is the use of PSMA and bombesin agents for targeted therapy with alpha-emitters or beta-emitters (including 90Y and 177Lu). These agents are currently being utilized in clinical trials for patients with biochemical recurrence, but are not currently approved for use in the United States[90,91]. These compounds may play a future role in adjuvant therapy post prostatectomy as the previously described molecular targeted PET agents become established for initial staging.
An exciting development that may increase the impact of molecular imaging for prostate carcinoma is the recent FDA approval of hybrid PET/MRI scanners. These scanners can acquire PET and MRI data simultaneously and combine targeted molecular imaging with PET with the soft tissue contrast and anatomic detail provided by pelvic MRI. The MRI component of PET/MRI can significantly increase detection of lesions compared to the non-contrast CT typically performed with conventional PET/CT. Evaluation of the prostate bed can also be significantly improved utilizing PET/MRI vs PET/CT. Given the established role of MRI for prostate cancer staging and lesion detection, PET/MRI may become the preferred platform for prostate cancer imaging in certain clinical scenarios. However, more data are needed to define the role of PET/MRI in the initial staging of prostate cancer.
Conventional imaging (CT and MRI) cannot depict small metastases in normal sized and normal appearing lymph nodes. The optimal strategy for the preoperative staging of prostate cancer remains a topic of ongoing research. Advanced imaging techniques to assess lymph nodes in the setting of prostate cancer utilizing novel MRI contrast agents as well as PET tracers have been developed and continue to be studied. MRL utilizing USPIO has shown high sensitivity and specificity in detection of normal sized lymph nodes containing metastatic disease and thus a positive finding may alter the treatment course for the patient. Ongoing research is occurring in molecular imaging and continues to show a promising future for detection of prostate metastasis as well as potential therapeutic applications.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arcangeli S, Huang SP S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Hövels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, Severens JL, Barentsz JO. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 690] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 2. | Kothari PS, Scardino PT, Ohori M, Kattan MW, Wheeler TM. Incidence, location, and significance of periprostatic and periseminal vesicle lymph nodes in prostate cancer. Am J Surg Pathol. 2001;25:1429-1432. [PubMed] |
| 3. | Makarov DV, Trock BJ, Humphreys EB, Mangold LA, Walsh PC, Epstein JI, Partin AW. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69:1095-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Schnall M, Tomaszewski JE, Wein A. Combined modality staging of prostate carcinoma and its utility in predicting pathologic stage and postoperative prostate specific antigen failure. Urology. 1997;49:23-30. [PubMed] |
| 5. | D’Amico AV. Combined-modality staging for localized adenocarcinoma of the prostate. Oncology (Williston Park). 2001;15:1049-1059; discussion 1060-106, 1064-1065, 1069-1070, 1073-1075. [PubMed] |
| 6. | Loeb S, Catalona WJ. The Prostate Health Index: a new test for the detection of prostate cancer. Ther Adv Urol. 2014;6:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | McDonald ML, Parsons JK. 4-Kallikrein Test and Kallikrein Markers in Prostate Cancer Screening. Urol Clin North Am. 2016;43:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Allaf ME, Palapattu GS, Trock BJ, Carter HB, Walsh PC. Anatomical extent of lymph node dissection: impact on men with clinically localized prostate cancer. J Urol. 2004;172:1840-1844. [PubMed] |
| 9. | Burkhard FC, Studer UE. The role of lymphadenectomy in prostate cancer. Urol Oncol. 2004;22:198-202; discussion 202-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | McDowell GC 2nd, Johnson JW, Tenney DM, Johnson DE. Pelvic lymphadenectomy for staging clinically localized prostate cancer. Indications, complications, and results in 217 cases. Urology. 1990;35:476-482. [PubMed] |
| 11. | Schuessler WW, Pharand D, Vancaillie TG. Laparoscopic standard pelvic node dissection for carcinoma of the prostate: is it accurate? J Urol. 1993;150:898-901. [PubMed] |
| 12. | Joniau S, Van den Bergh L, Lerut E, Deroose CM, Haustermans K, Oyen R, Budiharto T, Ameye F, Bogaerts K, Van Poppel H. Mapping of pelvic lymph node metastases in prostate cancer. Eur Urol. 2013;63:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Jager GJ, Barentsz JO, Oosterhof GO, Witjes JA, Ruijs SJ. Pelvic adenopathy in prostatic and urinary bladder carcinoma: MR imaging with a three-dimensional TI-weighted magnetization-prepared-rapid gradient-echo sequence. AJR Am J Roentgenol. 1996;167:1503-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 166] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Davis GL. Sensitivity of frozen section examination of pelvic lymph nodes for metastatic prostate carcinoma. Cancer. 1995;76:661-668. [PubMed] |
| 15. | Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491-2499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1588] [Cited by in RCA: 1333] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 16. | Bellin MF, Roy C, Kinkel K, Thoumas D, Zaim S, Vanel D, Tuchmann C, Richard F, Jacqmin D, Delcourt A. Lymph node metastases: safety and effectiveness of MR imaging with ultrasmall superparamagnetic iron oxide particles--initial clinical experience. Radiology. 1998;207:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 170] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Weissleder R, Elizondo G, Wittenberg J, Lee AS, Josephson L, Brady TJ. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology. 1990;175:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 317] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Wunderbaldinger P, Josephson L, Bremer C, Moore A, Weissleder R. Detection of lymph node metastases by contrast-enhanced MRI in an experimental model. Magn Reson Med. 2002;47:292-297. [PubMed] |
| 19. | Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007;243:28-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 380] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 20. | Fortuin AS, Deserno WM, Meijer HJ, Jager GJ, Takahashi S, Debats OA, Reske SN, Schick C, Krause BJ, van Oort I. Value of PET/CT and MR lymphography in treatment of prostate cancer patients with lymph node metastases. Int J Radiat Oncol Biol Phys. 2012;84:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Harisinghani MG, Saini S, Slater GJ, Schnall MD, Rifkin MD. MR imaging of pelvic lymph nodes in primary pelvic carcinoma with ultrasmall superparamagnetic iron oxide (Combidex): preliminary observations. J Magn Reson Imaging. 1997;7:161-163. [PubMed] |
| 22. | Harisinghani MG, Saksena MA, Hahn PF, King B, Kim J, Torabi MT, Weissleder R. Ferumoxtran-10-enhanced MR lymphangiography: does contrast-enhanced imaging alone suffice for accurate lymph node characterization? AJR Am J Roentgenol. 2006;186:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Heesakkers RA, Hövels AM, Jager GJ, van den Bosch HC, Witjes JA, Raat HP, Severens JL, Adang EM, van der Kaa CH, Fütterer JJ. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 2008;9:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Heesakkers RA, Jager GJ, Hövels AM, de Hoop B, van den Bosch HC, Raat F, Witjes JA, Mulders PF, van der Kaa CH, Barentsz JO. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imaging. Radiology. 2009;251:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Ross RW, Zietman AL, Xie W, Coen JJ, Dahl DM, Shipley WU, Kaufman DS, Islam T, Guimaraes AR, Weissleder R. Lymphotropic nanoparticle-enhanced magnetic resonance imaging (LNMRI) identifies occult lymph node metastases in prostate cancer patients prior to salvage radiation therapy. Clin Imaging. 2009;33:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Froehlich JM, Triantafyllou M, Fleischmann A, Vermathen P, Thalmann GN, Thoeny HC. Does quantification of USPIO uptake-related signal loss allow differentiation of benign and malignant normal-sized pelvic lymph nodes? Contrast Media Mol Imaging. 2012;7:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Thoeny HC, Triantafyllou M, Birkhaeuser FD, Froehlich JM, Tshering DW, Binser T, Fleischmann A, Vermathen P, Studer UE. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur Urol. 2009;55:761-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 28. | Triantafyllou M, Studer UE, Birkhäuser FD, Fleischmann A, Bains LJ, Petralia G, Christe A, Froehlich JM, Thoeny HC. Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer. Eur J Cancer. 2013;49:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Heesakkers RA, Fütterer JJ, Hövels AM, van den Bosch HC, Scheenen TW, Hoogeveen YL, Barentsz JO. Prostate cancer evaluated with ferumoxtran-10-enhanced T2*-weighted MR Imaging at 1.5 and 3.0 T: early experience. Radiology. 2006;239:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Birkhäuser FD, Studer UE, Froehlich JM, Triantafyllou M, Bains LJ, Petralia G, Vermathen P, Fleischmann A, Thoeny HC. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging facilitates detection of metastases in normal-sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur Urol. 2013;64:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 31. | Meijer HJ, Debats OA, van Lin EN, Witjes JA, Kaanders JH, Barentsz JO. A retrospective analysis of the prognosis of prostate cancer patients with lymph node involvement on MR lymphography: who might be cured. Radiat Oncol. 2013;8:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Meijer HJ, Debats OA, Kunze-Busch M, van Kollenburg P, Leer JW, Witjes JA, Kaanders JH, Barentsz JO, van Lin EN. Magnetic resonance lymphography-guided selective high-dose lymph node irradiation in prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Pisansky TM, Kozelsky TF, Myers RP, Hillman DW, Blute ML, Buskirk SJ, Cheville JC, Ferrigni RG, Schild SE. Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol. 2000;163:845-850. [PubMed] |
| 34. | George AK, Turkbey B, Valayil SG, Muthigi A, Mertan F, Kongnyuy M, Pinto PA. A urologist’s perspective on prostate cancer imaging: past, present, and future. Abdom Radiol (NY). 2016;41:805-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Turkbey B, Agarwal HK, Shih J, Bernardo M, McKinney YL, Daar D, Griffiths GL, Sankineni S, Johnson L, Grant KB. A Phase I Dosing Study of Ferumoxytol for MR Lymphography at 3 T in Patients With Prostate Cancer. AJR Am J Roentgenol. 2015;205:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Vasanawala SS, Nguyen KL, Hope MD, Bridges MD, Hope TA, Reeder SB, Bashir MR. Safety and technique of ferumoxytol administration for MRI. Magn Reson Med. 2016;75:2107-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 37. | Pai AB, Garba AO. Ferumoxytol: a silver lining in the treatment of anemia of chronic kidney disease or another dark cloud? J Blood Med. 2012;3:77-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Bailie GR. Comparison of rates of reported adverse events associated with i.v. iron products in the United States. Am J Health Syst Pharm. 2012;69:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Santosh S, Podaralla P, Miller B. Anaphylaxis with elevated serum tryptase after administration of intravenous ferumoxytol. NDT Plus. 2010;3:341-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | FDA Drug Safety Communication: FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme. 2015, accessed December 5 2016. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm440138.htm. |
| 41. | Bashir MR, Bhatti L, Marin D, Nelson RC. Emerging applications for ferumoxytol as a contrast agent in MRI. J Magn Reson Imaging. 2015;41:884-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 268] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 42. | Debats OA, Fortuin AS, Meijer HJ, Hambrock T, Litjens GJ, Barentsz JO, Huisman HJ. Intranodal signal suppression in pelvic MR lymphography of prostate cancer patients: a quantitative comparison of ferumoxtran-10 and ferumoxytol. PeerJ. 2016;4:e2471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Sankineni S, Smedley J, Bernardo M, Brown AM, Johnson L, Muller B, Griffiths GL, Kobayashi H, Rais-Bahrami S, Pinto PA. Ferumoxytol as an intraprostatic MR contrast agent for lymph node mapping of the prostate: a feasibility study in non-human primates. Acta Radiol. 2016;57:1396-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Saokar A, Braschi M, Harisinghani M. Lymphotrophic nanoparticle enhanced MR imaging (LNMRI) for lymph node imaging. Abdom Imaging. 2006;31:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Roy C, Bierry G, Matau A, Bazille G, Pasquali R. Value of diffusion-weighted imaging to detect small malignant pelvic lymph nodes at 3 T. Eur Radiol. 2010;20:1803-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Eiber M, Beer AJ, Holzapfel K, Tauber R, Ganter C, Weirich G, Krause BJ, Rummeny EJ, Gaa J. Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Invest Radiol. 2010;45:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 47. | Akduman EI, Momtahen AJ, Balci NC, Mahajann N, Havlioglu N, Wolverson MK. Comparison between malignant and benign abdominal lymph nodes on diffusion-weighted imaging. Acad Radiol. 2008;15:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Budiharto T, Joniau S, Lerut E, Van den Bergh L, Mottaghy F, Deroose CM, Oyen R, Ameye F, Bogaerts K, Haustermans K. Prospective evaluation of 11C-choline positron emission tomography/computed tomography and diffusion-weighted magnetic resonance imaging for the nodal staging of prostate cancer with a high risk of lymph node metastases. Eur Urol. 2011;60:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 49. | Sumi M, Van Cauteren M, Nakamura T. MR microimaging of benign and malignant nodes in the neck. AJR Am J Roentgenol. 2006;186:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Choi EK, Kim JK, Choi HJ, Park SH, Park BW, Kim N, Kim JS, Im KC, Cho G, Cho KS. Node-by-node correlation between MR and PET/CT in patients with uterine cervical cancer: diffusion-weighted imaging vs size-based criteria on T2WI. Eur Radiol. 2009;19:2024-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Thoeny HC, Froehlich JM, Triantafyllou M, Huesler J, Bains LJ, Vermathen P, Fleischmann A, Studer UE. Metastases in normal-sized pelvic lymph nodes: detection with diffusion-weighted MR imaging. Radiology. 2014;273:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 52. | von Below C, Daouacher G, Wassberg C, Grzegorek R, Gestblom C, Sörensen J, Ahlström H, Waldén M. Validation of 3 T MRI including diffusion-weighted imaging for nodal staging of newly diagnosed intermediate- and high-risk prostate cancer. Clin Radiol. 2016;71:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Giannarini G, Petralia G, Thoeny HC. Potential and limitations of diffusion-weighted magnetic resonance imaging in kidney, prostate, and bladder cancer including pelvic lymph node staging: a critical analysis of the literature. Eur Urol. 2012;61:326-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 54. | Heck MM, Souvatzoglou M, Retz M, Nawroth R, Kübler H, Maurer T, Thalgott M, Gramer BM, Weirich G, Rondak IC. Prospective comparison of computed tomography, diffusion-weighted magnetic resonance imaging and [11C]choline positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2014;41:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Fortuin A, Rooij Md, Zamecnik P, Haberkorn U, Barentsz J. Molecular and functional imaging for detection of lymph node metastases in prostate cancer. Int J Mol Sci. 2013;14:13842-13875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Ghanem N, Uhl M, Brink I, Schäfer O, Kelly T, Moser E, Langer M. Diagnostic value of MRI in comparison to scintigraphy, PET, MS-CT and PET/CT for the detection of metastases of bone. Eur J Radiol. 2005;55:41-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 57. | Shreve PD, Grossman HB, Gross MD, Wahl RL. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology. 1996;199:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 222] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 58. | Tateishi U, Morita S, Taguri M, Shizukuishi K, Minamimoto R, Kawaguchi M, Murano T, Terauchi T, Inoue T, Kim EE. A meta-analysis of (18)F-Fluoride positron emission tomography for assessment of metastatic bone tumor. Ann Nucl Med. 2010;24:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Ackerstaff E, Glunde K, Bhujwalla ZM. Choline phospholipid metabolism: a target in cancer cells? J Cell Biochem. 2003;90:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 247] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 60. | Evangelista L, Guttilla A, Zattoni F, Muzzio PC, Zattoni F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur Urol. 2013;63:1040-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 61. | Van den Bergh L, Lerut E, Haustermans K, Deroose CM, Oyen R, Isebaert S, Budiharto T, Ameye F, Mottaghy FM, Bogaerts K. Final analysis of a prospective trial on functional imaging for nodal staging in patients with prostate cancer at high risk for lymph node involvement. Urol Oncol. 2015;33:109.e23-109.e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 62. | Tilki D, Reich O, Graser A, Hacker M, Silchinger J, Becker AJ, Khoder W, Bartenstein P, Stief CG, Loidl W. 18F-Fluoroethylcholine PET/CT identifies lymph node metastasis in patients with prostate-specific antigen failure after radical prostatectomy but underestimates its extent. Eur Urol. 2013;63:792-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Vali R, Loidl W, Pirich C, Langesteger W, Beheshti M. Imaging of prostate cancer with PET/CT using (18)F-Fluorocholine. Am J Nucl Med Mol Imaging. 2015;5:96-108. [PubMed] |
| 64. | Wetter A, Lipponer C, Nensa F, Beiderwellen K, Olbricht T, Rübben H, Bockisch A, Schlosser T, Heusner TA, Lauenstein TC. Simultaneous 18F choline positron emission tomography/magnetic resonance imaging of the prostate: initial results. Invest Radiol. 2013;48:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Pinaquy JB, De Clermont-Galleran H, Pasticier G, Rigou G, Alberti N, Hindie E, Mokrane Y, Fernandez P. Comparative effectiveness of [(18) F]-fluorocholine PET-CT and pelvic MRI with diffusion-weighted imaging for staging in patients with high-risk prostate cancer. Prostate. 2015;75:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Rinnab L, Simon J, Hautmann RE, Cronauer MV, Hohl K, Buck AK, Reske SN, Mottaghy FM. [(11)C]choline PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy. World J Urol. 2009;27:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 67. | Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, Cozzarini C, Di Muzio N, Rigatti P, Fazio F. Predictive factors of [(11)C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010;37:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 68. | Vāvere AL, Kridel SJ, Wheeler FB, Lewis JS. 1-11C-acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. J Nucl Med. 2008;49:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | Haseebuddin M, Dehdashti F, Siegel BA, Liu J, Roth EB, Nepple KG, Siegel CL, Fischer KC, Kibel AS, Andriole GL. 11C-acetate PET/CT before radical prostatectomy: nodal staging and treatment failure prediction. J Nucl Med. 2013;54:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 70. | Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 644] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 71. | Rosenthal SA, Haseman MK, Polascik TJ. Utility of capromab pendetide (ProstaScint) imaging in the management of prostate cancer. Tech Urol. 2001;7:27-37. [PubMed] |
| 72. | Ponsky LE, Cherullo EE, Starkey R, Nelson D, Neumann D, Zippe CD. Evaluation of preoperative ProstaScint scans in the prediction of nodal disease. Prostate Cancer Prostatic Dis. 2002;5:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 73. | Hardie AD, Rieter WJ, Bradshaw ML, Gordon LL, Young MA, Keane TE. Improved performance of SPECT-CT In-111 capromab pendetide by correlation with diffusion-weighted magnetic resonance imaging for identifying metastatic pelvic lymphadenopathy in prostate cancer. World J Urol. 2013;31:1327-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Troyer JK, Beckett ML, Wright GL Jr. Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate. 1997;30:232-242. [PubMed] |
| 75. | Osborne JR, Akhtar NH, Vallabhajosula S, Anand A, Deh K, Tagawa ST. Prostate-specific membrane antigen-based imaging. Urol Oncol. 2013;31:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 76. | Rowe SP, Gorin MA, Allaf ME, Pienta KJ, Tran PT, Pomper MG, Ross AE, Cho SY. PET imaging of prostate-specific membrane antigen in prostate cancer: current state of the art and future challenges. Prostate Cancer Prostatic Dis. 2016;19:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 77. | Rowe SP, Macura KJ, Ciarallo A, Mena E, Blackford A, Nadal R, Antonarakis ES, Eisenberger MA, Carducci MA, Ross AE. Comparison of Prostate-Specific Membrane Antigen-Based 18F-DCFBC PET/CT to Conventional Imaging Modalities for Detection of Hormone-Naïve and Castration-Resistant Metastatic Prostate Cancer. J Nucl Med. 2016;57:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 78. | Eder M, Eisenhut M, Babich J, Haberkorn U. PSMA as a target for radiolabelled small molecules. Eur J Nucl Med Mol Imaging. 2013;40:819-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 79. | Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, Wester HJ, Heck M, Kübler H, Beer AJ. Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J Urol. 2016;195:1436-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 574] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 80. | Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, Graefen M, Steuber T, Rosenbaum C. Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol. 2016;69:393-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 334] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 81. | Schuster DM, Nanni C, Fanti S. Evaluation of Prostate Cancer with Radiolabeled Amino Acid Analogs. J Nucl Med. 2016;57:61S-66S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Odewole OA, Tade FI, Nieh PT, Savir-Baruch B, Jani AB, Master VA, Rossi PJ, Halkar RK, Osunkoya AO, Akin-Akintayo O. Recurrent prostate cancer detection with anti-3-[(18)F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging. 2016;43:1773-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 83. | Suzuki H, Inoue Y, Fujimoto H, Yonese J, Tanabe K, Fukasawa S, Inoue T, Saito S, Ueno M, Otaka A. Diagnostic performance and safety of NMK36 (trans-1-amino-3-[18F] fluorocyclobutanecarboxylic acid)-PET/CT in primary prostate pancer: multicenter Phase IIb clinical trial. Jpn J Clin Oncol. 2017;47:283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Wibmer AG, Burger IA, Sala E, Hricak H, Weber WA, Vargas HA. Molecular Imaging of Prostate Cancer. Radiographics. 2016;36:142-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 85. | Mansi R, Fleischmann A, Mäcke HR, Reubi JC. Targeting GRPR in urological cancers--from basic research to clinical application. Nat Rev Urol. 2013;10:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 86. | Dijkgraaf I, Franssen GM, McBride WJ, D’Souza CA, Laverman P, Smith CJ, Goldenberg DM, Oyen WJ, Boerman OC. PET of tumors expressing gastrin-releasing peptide receptor with an 18F-labeled bombesin analog. J Nucl Med. 2012;53:947-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Zhang H, Abiraj K, Thorek DL, Waser B, Smith-Jones PM, Honer M, Reubi JC, Maecke HR. Evolution of bombesin conjugates for targeted PET imaging of tumors. PLoS One. 2012;7:e44046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Kähkönen E, Jambor I, Kemppainen J, Lehtiö K, Grönroos TJ, Kuisma A, Luoto P, Sipilä HJ, Tolvanen T, Alanen K. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin Cancer Res. 2013;19:5434-5443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 89. | Larson SM, Morris M, Gunther I, Beattie B, Humm JL, Akhurst TA, Finn RD, Erdi Y, Pentlow K, Dyke J. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone vs 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366-373. [PubMed] |
| 90. | Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, Baum RP, Kulkarni HR, Schmidt M, Drzezga A. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J Nucl Med. 2017;58:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 622] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 91. | Chatalic KL, Konijnenberg M, Nonnekens J, de Blois E, Hoeben S, de Ridder C, Brunel L, Fehrentz JA, Martinez J, van Gent DC. In Vivo Stabilization of a Gastrin-Releasing Peptide Receptor Antagonist Enhances PET Imaging and Radionuclide Therapy of Prostate Cancer in Preclinical Studies. Theranostics. 2016;6:104-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |