Published online Oct 28, 2017. doi: 10.4329/wjr.v9.i10.371
Peer-review started: February 12, 2017
First decision: April 17, 2017
Revised: July 18, 2017
Accepted: August 2, 2017
Article in press: August 2, 2017
Published online: October 28, 2017
Processing time: 271 Days and 21.9 Hours
The cerebellum plays a key role in movement control and in cognition and cerebellar involvement is described in several neurodegenerative diseases. While conventional magnetic resonance imaging (MRI) is widely used for brain and cerebellar morphologic evaluation, advanced MRI techniques allow the investigation of cerebellar microstructural and functional characteristics. Volumetry, voxel-based morphometry, diffusion MRI based fiber tractography, resting state and task related functional MRI, perfusion, and proton MR spectroscopy are among the most common techniques applied to the study of cerebellum. In the present review, after providing a brief description of each technique’s advantages and limitations, we focus on their application to the study of cerebellar injury in major neurodegenerative diseases, such as multiple sclerosis, Parkinson’s and Alzheimer’s disease and hereditary ataxia. A brief introduction to the pathological substrate of cerebellar involvement is provided for each disease, followed by the review of MRI studies exploring structural and functional cerebellar abnormalities and by a discussion of the clinical relevance of MRI measures of cerebellar damage in terms of both clinical status and cognitive performance.
Core tip: The cerebellum is involved in movement control and cognition. Conventional and advanced magnetic resonance imaging (MRI) techniques are widely used for the morphologic evaluation and the microstructural and functional investigation of the cerebellum. In this review we show the state of the art of advanced MRI techniques in the investigation of cerebellum alterations, especially in patients affected by neurodegenerative diseases. In particular, we evaluated advantages, limitations and future perspective of these techniques in multiple sclerosis, Parkinson’s disease and Parkinsonisms, Alzheimer’s disease and hereditary ataxia, highlighting how the investigation of cerebellum may play a key role in the assessment of motor performance and clinical status of these diseases.
- Citation: Mormina E, Petracca M, Bommarito G, Piaggio N, Cocozza S, Inglese M. Cerebellum and neurodegenerative diseases: Beyond conventional magnetic resonance imaging. World J Radiol 2017; 9(10): 371-388
- URL: https://www.wjgnet.com/1949-8470/full/v9/i10/371.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i10.371
The cerebellum plays a key role in normal brain function and its structural and functional involvement in several neurological diseases is associated with the impairment of both motor and non-motor functions such as cognition, mood and behavior. Imaging studies have been challenged in the past by the complex cerebellar anatomical structure and by its location in the posterior fossa. The advent of high-field magnets and the development of new algorithms for image acquisition and analysis have, at least in part, improved the study of cerebellar structure and functions. This review provides a brief description of cerebellar macro- and microscopic anatomy and functions and focuses on the imaging methods and segmentation tools for the analysis of the cerebellum with emphasis on each method’s advantages and limitations. Further, the clinical implications of the cerebellar involvement in neurological diseases such as multiple sclerosis, hereditary ataxias, Parkinson’s and Alzheimer’s disease are discussed.
The cerebellum is a large folded structure consisting of two cerebellar hemispheres, united by a central part known as vermis located in the posterior cranial fossa, lying dorsal to the brainstem and inferior occipital lobes. It is separated from the cerebrum by a dura mater layer known as tentorium cerebelli and it is surrounded postero-laterally and infero-medially by venous structures, respectively transverse and sigmoid sinuses. The cerebellar cortex is tightly folded and composed by three layers: Molecular layer, Purkinje cell layer and granular layer. Each ridge or gyrus of gray matter is called folium. Underneath these layer of gray matter there is a central mass of white matter, also called corpus midollare or arbor vitae (tree of life), in which are embedded the three deep gray matter cerebellar nuclei: Fastigial nucleus, interposed nucleus (composed by the emboliform and globose nuclei) and dentate nucleus. Three white matter peduncles (superior, middle and inferior) connect the cerebellum to the brainstem, respectively to the midbrain, pons and medulla oblungata. In addition to the above reported macro- and microscopic description, cerebellar structure can be further characterized from a morphologic, phylogenetic and functional perspective. The morphologic classification describes, without any functional basis, a division into three lobes: Anterior, posterior and flocculonodular lobe, while the phylogenetic classification divides the cerebellum into archicerebellum (the most ancient portion), paleocerebellum (developed after archicerebellum) and neocerebellum (the newest portion). The functional classification divides the cerebellum in three regions: Vestibulocerebellum, spinocerebellum and cerebrocerebellum based on the location of the afferent and efferent neurons[1,2]. The vestibulocerebellum corresponds to the flocculonodular lobe, with afferents neurons arising from vestibular nuclei (and some portion of visual cortex) and efferents neurons going to vestibular nuclei. It modulates gait balance and eye movements. The spinocerebellum is formed by the superior and inferior portion of the vermis (with the exception of the nodule) and by a bilateral paravermian portion, located on both sides of the vermis. The vermian part of the spinocerebellum has its afferent neurons arising from the spinal cord, vestibular, visual and acoustic nuclei and has its efferents neurons going through the fastigial nucleus. It modulates head and neck muscle movement as well as trunk and limb proximal portions. The paravermian part of the spinocerebellum has its afferents neurons arising from the spinal cord and trigeminal sensory nuclei, and its efferents neurons going through the interposed nucleus. It completes movement modulation performed by the vermian part, acting on limb distal portions. The cerebrocerebellum is composed by the two cerebellar hemispheres and receives afferent neurons from most of the neocortex (frontal, parietal, temporal, and occipital lobes) through the pons nuclei, sending its efferent neurons to thalamus and cerebral cortex through the dentate nucleus. Functional specificity is granted by the presence of multiple close-loop circuits between cerebral and cerebellar cortex, in which the same brain area that is the major target of output from the cerebro-cerebellar circuit it is also its major source of input[3,4]. In each loop, a specific cortical area projects through the pontine nuclei to a distinct region of the cerebellar cortex. A specific portion of the dentate nucleus projects then to a specific cortical area through a distinct thalamic region, thus closing the cerebro-cerebellar loop[5,6]. According to studies conducted on primates, the dentate nucleus is topographically organized in a ventral portion, projecting to the prefrontal and posterior parietal cortex, and a dorsal portion, projecting to the motor cortex[7]. Both motor and non-motor domains of the dentate also project to the striatum (input stage of basal ganglia processing), raising the hypothesis that cerebellum could also modulate basal ganglia facilitation of voluntary movements[8]. The cerebrocerebellum is the largest part of the cerebellum and accounts for motor planning and motor learning. It is responsible for the transition from controlled to automatic movement: Once motor memories storage has been achieved in the cerebellar cortex, the execution of movements can be triggered by sparse high-level command from cerebral cortex[9]. The ability of the cerebellum to adjust performance according to context, automatically integrating interoception with perception and internal models, does not apply only to movements controls but also to cognitive function, as proved by the occurrence of the cerebellar cognitive-affective syndrome following acute cerebellar lesions of the posterior lobe[10]. In particular, lesions of the posterolateral hemispheres cause cognitive disturbances, while vermis lesions induce behavioral and affective alterations. Cerebrocerebellum is considered an essential modulator of cognitive abilities, such as language processing and visuospatial perception (respectively lateralized in the right and left cerebellar hemisphere), as well as high order functions as emotions, behaviors and personality. For example, thanks to its connection to the prefrontal cortex, the cerebellum is involved in the execution of abstract rules that govern response selection, regardless of whether they specifically relate to the selection of actions[11,12].
The posterior cranial fossa (PCF) is located between the tentorium cerebelli and foramen magnum and houses the cerebellum and the brainstem. For its peculiar conformation, small dimensions and contained structures, an accurate study by means of computed tomography (CT) and MRI had always represented a great challenge[13-15]. However, several progresses have been made in this field to avoid the artifacts related to the X-ray beam hardening and the partial volume effects (which is non-linear) caused by the thickness and the irregularity of the skull-base bones. Specifically, the introduction of thin-section spiral multidetector CT, which allows the acquisition of isotropic voxel scans and the use of MRI scanners which allow the acquisition of multiplanar and multiparametric images have contributed to minimize PCF artifacts[16-23]. Most of PCF artifacts are related to blood flow pulsation, inflow/outflow of cerebro-spinal fluid (CSF) and to the brain-bone-air interfaces[24-30].
Artifacts related to vessels blood pulsation generate a signal that is displaced from its correct anatomical position causing a wrong/inappropriate image, also called “ghost” artifacts[31]. Transverse sinuses blood-flow related artifact, which often generate a false image projecting onto cerebellar parenchyma, may lead to an inaccurate or inappropriate interpretation of MR images. Artifacts related to the inflow and outflow of CSF inside PCF from the superior or inferior regions can be minimized by the use of fluid attenuated inversion recovery (FLAIR) images where the CSF signal is nulled out by setting a proper inversion pulse. Unfortunately, these artifacts can still be present if the inversion pulse is spatially selective, allowing a partial suppression[30,32]. As shown by Baksi et al[30] and Lavdas et al[33] this type of artifact may mimic or hide a brain parenchymal lesion due to the presence, along with the phase encoding direction, of a redundant CSF signal.
The brain-bone-air interfaces artifact represents one of the magnetic susceptibility artifacts that are often present on gradient-echo sequences[28], especially in regions like the skull base, petrous temporal bone, paranasal sinuses and orbits[34,35].
It should be noted that some MRI sequences are more prone to specific artifacts than others: i.e., gradient-echo for susceptibility artifacts (since this sequence does not use a refocusing 180° pulse and signal dephases fast due to field inhomogeneity), inversion recovery (e.g., short tau inversion recovery and FLAIR) for pulsatile artifacts, or 2D time-of-flight for slow-flow artefactual gaps in non-dominant transverse sinus[13,30,33,36]. Nonetheless, some of these artifacts are routinely exploited for diagnostic purposes: Susceptibility weighted images (and in general T2*w gradient-echo images) thanks to its capability of being susceptible to paramagnetic molecules, is able to recognize small amounts of blood degradation products better than other sequences and to distinguish between parenchymal calcifications and blood products[37].
Although the combined use of MRI and CT is recommended for the diagnosis of PCF pathologies, MR is preferred to CT in order to evaluate soft tissue structures and to determinate their spatial relationship. While MRI provides higher accuracy in detecting bone marrow changes, brain parenchyma, meningeal infiltration, peri-neural and perivascular spread caused by tumors[14,38,39], CT is more sensitive for the evaluation of bone structures, PFC tumoral and non-tumoral conditions[14,40].
The possibility to study the brain at high and ultra-high field strength has become quite common after the United States FDA approval of MR magnets up to 4 Tesla (T) for clinical use. Currently, the term ultra-high field is used for MR scanners with a magnetic field strength higher than 3T. The application of high and ultra-high static magnetic field strength can improve the visualization of brain anatomy and the study of changes in brain structure and function in several neuropsychiatric diseases. For example, the higher signal-to-noise ratio (SNR) of 3T scanners allows not only to perform faster imaging compared to 1.5T scanners (doubling the strength of the magnetic field would theoretically lead to a reduction of acquisition time of a factor of four), but also to acquire images at higher spatial resolution, that turns be very useful in the evaluation of small structures such as the cerebellum and the brainstem. It has been shown that higher spatial resolution is helpful in studying the cerebellar cortex, whose thickness is lower than cerebral thickness (< 0.5 mm vs the 3-4 mm, respectively)[41,42].
In the research field, 7T MR scanners have proven to be of great use for the identification and the study of each cerebellar folia (which are approximatively 260)[43] and for the study of cerebellar cortical layers. Specifically, the granular and molecular cerebellar layers, whose thickness is approximatively 240 μm, are well recognized and morphologically studied at 7T which has the unique advantage to allow an in-plane voxel size of 120 μm not possible at low-field MR scanners[44]. It is important to bear in mind that higher magnetic fields bring the inherit heavy burden of several artifacts and limitations. For instance chemical shift imaging and susceptibility artifacts, are only some of the artifacts that will increase with high magnetic field. These phenomena, which may cause images misinterpretation, can also be exploited, leading for example to a better separation of metabolites in spectroscopy, a better performance in perfusion weighted imaging, or a better blood products detection. Further, when using high and ultra-high magnetic fields in humans, the specific absorption rate (SAR) should also be carefully evaluated, since it will be quadruplicate with the doubling of the field strength, limiting the use of some sequences and making some parameter modulation necessary in order not to exceed the SAR threshold limit given by International Electrotechnical Commission[45].
The remarkable wide range of MR sequences available for the study of the PCF and the cerebellum comes with the difficulty of making the optimal selection based on the clinical or research question. In a standard clinical study the MRI protocol should include a turbo spin-echo (TSE) T1weighted (T1w) sequence to assess shape and dimension of the cerebellum and of the PCF, TSE-T2w and FLAIR-T2w sequences to detect potential white matter (WM) lesions. The choice of an isotropic voxel (≤ 1 mm) should always be preferred when available or when a high-resolution multiplanar evaluation is needed. For example, it has been showed that, in comparison to TSE-T2/proton density sequences, isotropic 3D-FLAIR is more accurate in detecting not only white matter lesions but also cortical and infratentorial lesions in patients with multiple sclerosis (MS)[46]. 3D FLAIR has advantages even when acquired at ultra-high magnetic field, such as in the study of Kilsdonk et al[47] in which cortical gray matter (GM) lesions were better detected at 7T with 3D FLAIR than with GM-Specific 3D double inversion recovery (DIR) or with 2D-T2w and 3D-T1w. Finally, quantitative T1 and proton density (ρ) magnetic resonance imaging at 3T provide a good visualization of deep cerebellar nuclei, like dentate nuclei at 1.5T[48]. Similarly, susceptibility weighted imaging (SWI) allows a better detection of the dentate nuclei at 1.5T while higher magnetic field allows visualization of the dentate wall corrugation, which is the iron-poorer dorsal portion of it[49].
There is an ever-increasing interest in the evaluation of cerebellar volume as a potential correlate of motor and cognitive performance and as a biomarker of progression and/or treatment outcome in neurodegenerative disorders[50].
Several computerized methods with specific advantages and limitations are available to perform cerebellar segmentation, lobule parcellation and thus to assess global cerebellar volume, regional or lobular volumes and GM and WM volume. Although manual volume segmentation is considered the “gold standard”, this option is extremely time-consuming and its reliability may vary with the experience of the raters[51]. Several semi-automatic computerized methods have been developed and validated in order to minimize operator-dependent limitation. We will focus on the methods that have been more extensively applied in clinical studies and will describe each method’s advantages and limitations.
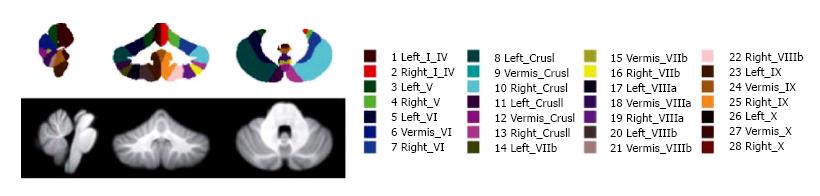
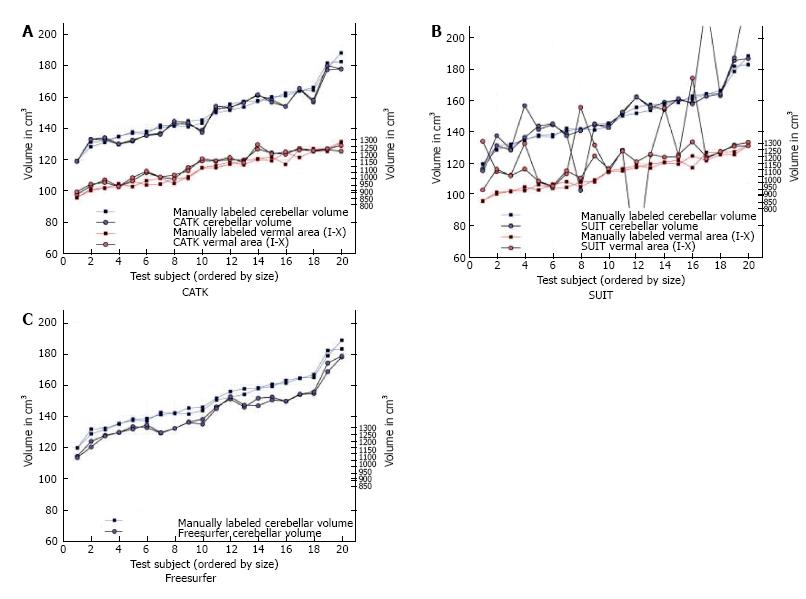
SUIT (spatially unbiased atlas template of the cerebellum and brainstem) is a Statistical Parametric mapping software (SPM) toolbox for MATLAB, based on a nonlinear coregistration of MRI images to a high-resolution cerebellum template obtained from images of healthy controls[52]. This method allows the parcellation of the cerebellum in at least 28 lobules, thus measuring GM volume for each lobule and the global GM volume as the sum of all lobule volumes (Figure 1). SUIT has been applied to the study of several diseases such as multiple sclerosis (MS), autism spectrum disorder, attention deficit hyperactivity disorder, developmental dyslexia and primary craniocervical dystonia, and it has been shown that it has higher sensibility to volume changes than conventional whole-brain voxel-based morphometry (VBM) methods[50,53,54]. The main advantage of SUIT is that it provides an optimal overlap of the cerebellar lobules, preserving anatomical details, and thanks to its cropping step avoids results bias from supratentorial structures[50,53,54]. However, Bogovic et al[51] reported that SUIT accuracy may decrease in patients with severe cerebellar atrophy especially with regard to lobule specific segmentation.
Cerebellar analysis toolkit (CATK) is based on a Bayesian framework of FMRIB’s Integrated Registration and Segmentation Tool[55]. Using hand-delineated examples, active appearance models are created in order to perform cerebellar labeling and segmentation. CATK has shown a high reliability (Intraclass Correlation Coefficients, ICCs, of 0.96 for test-retest) a good manual segmentation agreement (ICC 0.87) and a better performance than other softwares, such as SUIT (v 2.7) and Freesurfer when compared to manual segmentation (gold standard). However, the main limitation when compared to other softwares, is that CATK does not allow cerebellar hemisphere parcellation, although Price et al[55] in their paper stated a pending further improvement which would solve this issue and which would give higher image delineation (Figure 2).
ECCET is a semiautomatic toolkit based on a manually drawn region of interest ROI software. It is able to perform a fast semiautomatic segmentation after a manual outline drawing of few brainstem slices. It allows, when needed, manual editing in a 3D volume rendering mode (https://http://www.eccet.de/projects/neuro_en.html). This method has shown a good interobserver (ICC = 0.98, 95%CI = 0.74-0.99) and test-retest reliability (ICC = 0.99, 95%CI = 0.98-0.99) and the capability of avoiding some segmentation errors such as the inclusion of venous sinuses without the need of manual editing[56].
Unfortunately, to date, all the above-mentioned softwares provide pipelines for one time point evaluation (cross-sectional), but not for longitudinal analysis along time. This gap has been fulfilled by Freesurfer, which is a software that allows a reliable automatic whole brain segmentation, with up to 40 subcortical structures, labelling each voxel in a normalized space of the brain volume[57]. In addition to the cross-sectional analysis, Freesurfer comprises a longitudinal stream where each time-point is co-registered to a subject-specific template by creating an average segmentation along time points, resulting in volume and GM thickness estimation[57]. Although Freesurfer presents this important advantage, it does not allow sub-regional segmentation in cerebellar lobules[55]. Moreover, manual editing of segmentation outputs is needed in order to improve volume evaluation reliability[58]. Although the editing can be performed manually, resulting in a very time-consuming (up to 4h) and operator-dependent approach, Wang et al[58], in a recent paper, proposed an accurate and efficient new machine-learning based method in order to overcome this issue.
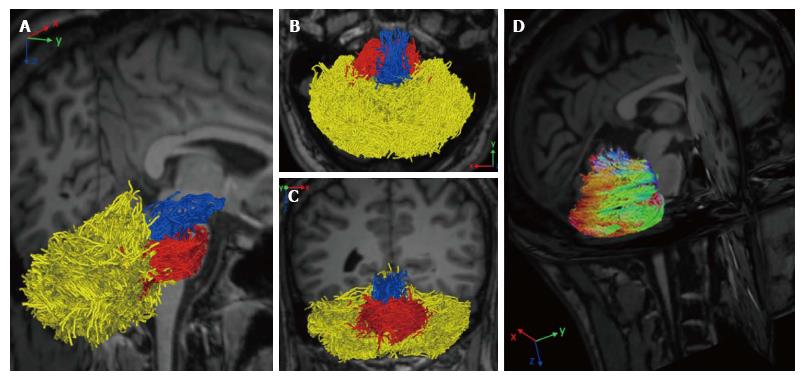
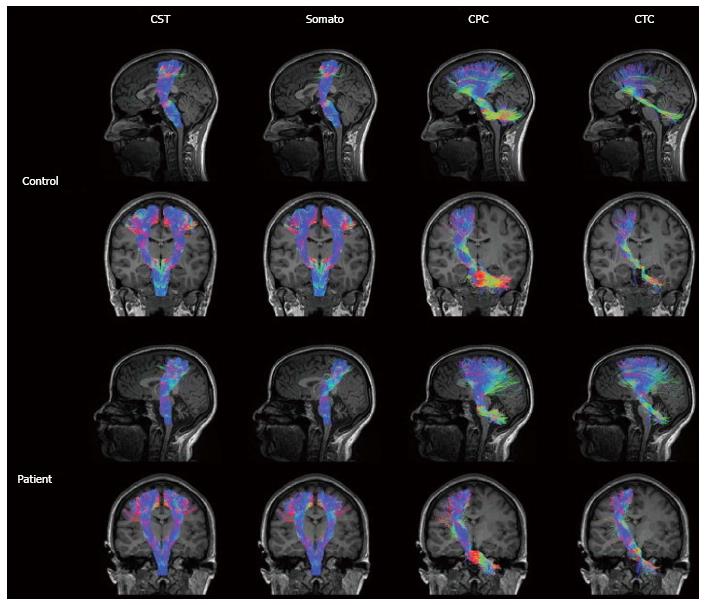
Diffusion magnetic resonance imaging (dMRI) is a MRI technique able to detect water molecules diffusion inside brain tissue. While diffusion of water molecules in GM is isotropic, diffusion in WM is anisotropic, i.e., it occurs in one main direction due to the presence of myelin barriers[59]. The evaluation of diffusion in a three dimensional space and along at least 6 directions, allows the creation of a diffusion tensor matrix, which provides the bases of tractographic reconstruction of brain neural circuitry[60]. Diffusion tensor imaging (DTI) and more advanced approaches (such as diffusion spectrum imaging or constrained spherical deconvolution) have been applied to determine cerebellar pathways and their connection with other supratentorial areas[61-63] (Figure 3). Although conventional DTI techniques are the most used technique to reconstruct cerebellar pathways by means of tractography, they are also well known for their limitations in the study of connectivity. DTI, indeed, is limited by the inability to discriminate different fiber populations with complex configurations at a voxel level (kissing fibers, bridging fibers, merging fibers, crossing fibers), thus leading to artefacts in fiber reconstructions and connectivity evaluations. Methods such as diffusion spectrum imaging and Q-ball imaging have been developed over the last few years to overcome these limitations; however, the more complex hardware set up and the longer acquisition times have limited so far their application in the clinical setting[64]. Other promising techniques, such as diffusion kurtosis imaging (DKI), neurite orientation dispersion and density imaging (NODDI) and constrained spherical deconvolution can be performed within clinically feasible acquisition times[65] (Figure 3). Moreover, all these techniques can be analyzed with both deterministic and probabilistic fiber reconstruction models. While deterministic models reconstruct streamlines (virtual fiber tracts) taking into account the principle eigenvector of fractional anisotropy (FA) in each voxel, probabilistic models generate a connectivity map from a larger numbers of possible pathways, obtaining the probability of each voxel to be connected to another[66]. Regardless of the used approach, tractographic output can be used not only to reconstruct the spatial configuration of fiber tracts but also to measure diffusion parameters (i.e., FA or mean diffusivity) at voxel level. However, it has been proved that, not only parameters’ quantification but also the accuracy of tractographic results may be affected by the poor quality of the acquired data that can lead to the detection of false connections or to miss detection of a real connections between structures[59]. Therefore, a better data quality, optimized in terms of SNR, spatial resolution, number of diffusion directions, and number and values of b values, with the choice of a proper tractography method, may improve the method performance avoiding inaccuracy in structural connectivity analysis[67]. Since cerebellar pathways tracking is particularly difficult due to the presence of sharp turning angles along their crossing, the assessment of cerebro-cerebellar and intra-cerebellar connectivity could especially benefit from data quality improvement[61,63].
Functional MRI (fMRI) is based on the detection of the blood oxygen level-dependent (BOLD) changes that take place as a consequence of neuronal activity. An increase in neural activity leads to an increase in the arterial blood flow, in order to increase the activity itself. To this increase in the amount of oxygenated blood does not correspond a similar increase in oxygen extraction at the level of capillary bed, leading to a relative decrease of deoxyhemoglobin levels, that directly affects the MR signal.
fMRI experiments can be divided in task and rest-related. The first measure brain activity during a task performance, the second evaluate the interaction between different brain regions without the execution of any specific task, in a rest condition (resting-state fMRI - RS-FMRI).
With regard to task-based fMRI studies, two main experimental paradigms are commonly used[68]. The first one is the so-called block design experiment, in which stimuli are presented to the subject in blocks of variable length alternated to blocks of rest, in which the stimulus is removed. MRI signals are then compared between the two conditions, in order to extrapolate the areas that show more activation during the execution of the task. The second is the event-related experimental design, in which the stimuli and the resting blocks are not alternated in a set sequence, but the administration, as well as the duration of the stimulus, are randomized.
RS-fMRI experiments can be analyzed using two major approaches. The first one is the seed-based approach, in which a region of interest is selected and the corresponding time-activity curve is extracted. Then, voxels with similar activation are searched whole brain, and are assumed to be functionally correlated to the chosen seed[69]. The second method is the independent component analysis (ICA), a mathematical algorithm that allows to subdivide a multivariate and noisy signal in its subcomponents[70]. In this approach, no a priori seeds are chosen, but the operator is asked to identify the component of interest, and discard those obtained from noise or physiological signals. ICA analysis has allowed the identification of preferential connections between specific cerebral structures, that take the name of resting state networks[71]. Cerebellar lobules are not only hubs of several of these resting state networks (i.e., lobule IX in the default mode network, crus I and II in the executive control network or lobule VI in the salience network)[72], but also an entire and separate cerebellar network is recognized among the major resting state networks[71].
With this knowledge, it is easy to understand how future improvement is warranted to increase the of functional changes with respect of the cerebellar lobular anatomy. In particular, the possibility of increasing spatial resolution in fMRI experiments is a future challenge for investigating cerebellar functional connectivity due to the characteristic lobular anatomy. The increase in spatial resolution could further help in elucidating the exact functional lobular topography of the cerebellum, with regards to specific motor and cognitive functions[73].
Proton magnetic resonance spectroscopy (1H-MRS) is an analytical method that allows the investigation of brain metabolites. Every metabolite at sufficient concentration level generates a specific peak in function of its resonance frequency[74]. Since metabolic abnormalities occur earlier than structural MRI alterations, 1H-MRS can provide a valid tool for early diagnosis and for monitoring of neurological diseases[75].
The most commonly studied brain metabolites are the N-acetylaspartate, a marker of neuroaxonal integrity, choline containing compounds, a marker of membrane turnovers, creatine/phosphocreatine, a marker of energy metabolism, and myo-inositol, a marker of astroglial activation. From a quantification point of view, metabolites’ levels are expressed as absolute quantifications or as ratios where the denominator is the creatine level which is assumed to be stable in normal as well as in many pathologic states[76].
Although the infratentorial structures are often involved in neurodegenerative processes, strong B0 inhomogeneities due to nearby skull bone, scalp lipids and tissue/air interfaces constitute a technical challenge for the 1H-MRS acquisition. In addition, the small size of cerebellum increases the risk of partial volume effects[77], which can be accounted for by combining spectroscopic data with structural MRI segmentation[78]. Nevertheless, 1H-MRS of the infratentorial fossa is feasible[79] and could provide an early biomarker of neuronal damage in cerebellar diseases, with even increased specificity when used in combination with other techniques[80].
Perfusion weighted imaging (PWI) allows the measurement of blood perfusion in brain tissue and it is categorized as: “minimally invasive” if requiring gadolinium injection (i.e., dynamic susceptibility contrast MRI or DSC-MRI and dynamic contrast enhanced MRI or DCE-MRI) or “non-invasive” if no contrast agent is needed (i.e., arterial spin labelling or ASL-MRI)[81]. Data from either technique above is subsequently processed and normalized to estimate the well-known perfusion values: cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), Ktrans and etc., which are all representable on parametric color maps.
Cerebellar tissue is subject to a great blood supply, and measurement of local variations of blood request/availability is clinically relevant in the assessment of neurodegenerative diseases. Specifically, cerebral blood flow (CBF) alterations (i.e., general or local CBF reduction) appear to precede structural abnormalities (for example: atrophy). Moreover, the cerebellum has been used as a reference region for intensity normalization of “relative” Cerebral Blood Volume (rCBV), based on the assumption that its CBV is not affected in neurocognitive disorders[82]. Although hard to eradicate, this assumption is nowadays obsolete, as alternative reference regions have recently been proposed and validated[83] .
With respect to the study of the cerebellum and the PCF, DSC-MRI provides higher spatial resolution, high sensitivity in transit time and whole-brain coverage in shorter scan times, and it is preferred in all situations where a fast assessment is required. DCE-MRI has the advantage of reducing artifacts especially for the measurement of CBV and Ktrans. ASL-MRI is often preferred in the study of neurodegenerative diseases for its complete lack of invasiveness, vessel selective capability[84] and the best accuracy in absolute tissue perfusion quantification[81]. Particular care in placing the “labeling plane” is essential to avoid artifacts in the lower cerebellar sections[85]. Another limit of ASL-MRI is WM assessment, which is particularly challenging due to the low blood transit and the consequent low SNR[86].
Unfortunately, despite the theoretical indications of each method, in the routine clinical settings, is the availability and practicality of the techniques that forces the choice, rather than its potential performance. This could explain the more frequent use of DSC and DCE imaging, faster, easy to perform and widely installed on most clinical scanners, compared to the use of ASL.
Multiple Sclerosis (MS) is an inflammatory/demyelinating disease of the central nervous system (CNS) characterized by heterogeneous symptoms and signs that can present a relapsing remitting (RR) or a progressive course. In 1877 Jean Martin Charcot first described the disease as a triad of symptoms consisting of nystagmus, dysartria and ataxia[87], thus underlining the dominant role of cerebellar deficits. Not only is the cerebellum frequently involved by the disease pathological processes but the presence of MRI visible infratentorial lesions provides high specificity to the diagnostic criteria for MS[88]. Indeed, 31% of patients with a clinically isolated syndrome (CIS) present with at least one infratentorial lesion and about 20.5% with a cerebellar lesion. The detection of a cerebellar lesion at onset is associated with an increased risk of conversion to MS[89]. In patients with a clinically defined MS, cerebellar lesions have been described in up to 49% of cases and patients with a progressive form have an increased number of PCF lesions when compared to patients with a RR type[90]. Disease pathology involves not only the cerebellar WM but also the GM; in fact, cortical cerebellar lesions are observed in patients with MS, even at the early stages of the disease, and correlate with the cerebellar functional score of the expanded disability status scale (EDSS)[91]. Longitudinal studies have shown cerebellar GM volume loss and an increased number of cortical lesions in both CIS, RR and progressive patients[92,93].
Along with cerebral atrophy, also cerebellar volume loss occurs in patients with MS, at all the disease stages. Edwards and coworkers found reduced global cerebellar volumes in patients with a secondary progressive (SP) form when compared to RR patients, and in both groups when compared to healthy controls[94]. However, in a more recent study, when compared to healthy controls only MS patients with a SP form, but not RR or patients with benign MS, showed lower cerebellar volumes[50]. When cerebellar WM and GM are considered separately, study results are discordant. Ramasamy et al[95] found a reduced cerebellar WM but not GM volume in CIS and MS patients when compared to healthy controls. However, Anderson and coworkers detected a reduced cerebellar GM volume in RR and SP MS patients versus controls and only a trend of significance, when comparing WM volumes, between SP MS and controls[96]. In the latter study, cerebellar GM and WM volumes were related with performance at the nine-hole peg test, highlighting the clinical relevance of measures of cerebellar volumes. A greater loss of GM in patients with a progressive course and its correlation with measures of clinical outcome are findings mirroring the process at the whole brain level.
DTI has been widely used for the study of cerebellar WM, in particular to evaluate the damage of the cerebellar peduncles and its clinical impact. Anderson et al[97] found reduced FA and increased radial diffusivity values in the middle cerebellar peduncle in patients with primary progressive (PP) MS; DTI metrics correlated with clinical impairment both of the upper and lower limbs. A study on a cohort of patients at different stages of the disease revealed greater mean, axial and radial diffusivity and reduced FA in MS patients, when compared to healthy controls, at the level of the middle and superior cerebellar peduncles; moreover, when compared to cerebellar peduncles T2 lesion load or atrophy, diffusivity measures better distinguished between patients with a worse EDSS score[98]. Disability in RR patients also correlated with the FA values of the cerebellar normal appearing WM[99].
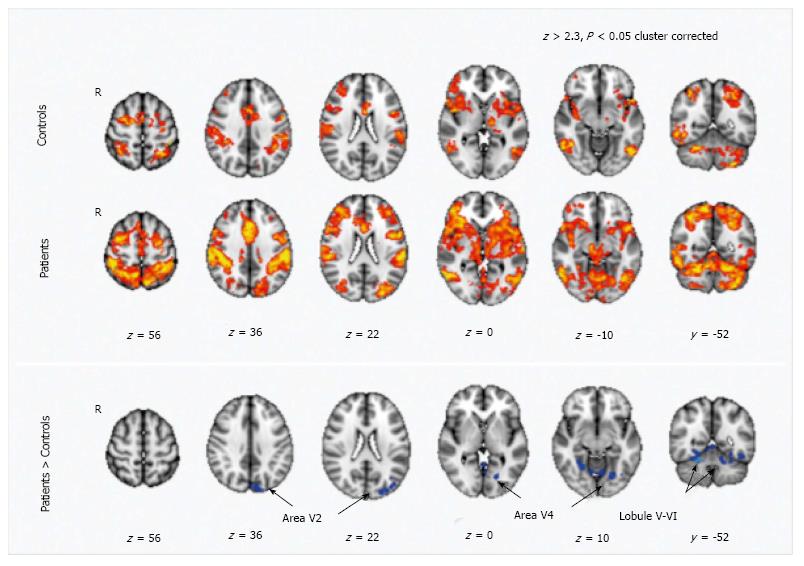
MS is characterized by a reorganization of the functional connectivity. Functional MR studies with motor tasks revealed an increased activation in several cortical areas within the sensorimotor network, including the cerebellum, in patients when compared to healthy controls[100,101] (Figure 4). The altered functional cerebellar connectivity could represent a compensatory mechanism to WM damage; accordingly, in RR MS patients a damage in the dentatorubrothalamic tract, assessed by means of DTI, was related to an increased functional connectivity between right sensorimotor cortex and cerebellum[101]. The cerebellum activation is also increased in patients with greater perceived fatigue where fatigue is conceived as a correlate of an increased resource demand for motor activities[102].
Besides the classical motor clinical features associated with cerebellar dysfunction, lately more attention has been focused on the role of cerebellum in cognitive impairment. MS patients with cerebellar signs perform worse at the symbol digit modalities test (SDMT) and the paced auditory serial addition test (PASAT); moreover, the PASAT execution is predicted by cerebellar lesion volume[103]. In particular the posterior cerebellum has been implicated in cognitive processing; in MS patients, a reduced posterior volume predicted a worse cognitive performance[50]. Information processing speed impairment has been associated with GM atrophy of the posterior lobules, especially at the level of the vermis VI[104]. As with motor tasks, a functional reorganization could also occur in response to the impairment of cognitive processes. Recently, a greater functional connectivity of the dentate nucleus with frontal and parietal cortical areas was detected in MS patients versus controls[105,106] and the increased connectivity was related to a better cognitive performance. Moreover, an increased connectivity between anterior cingulate cortex and cerebellum was also associated with a better performance at PASAT in CIS, RR and SP patients versus controls and authors suggested an adaptive mechanism[107].
The hyperintense signal on T1-weighted images at the level of dentate nucleus has been detected in almost 50% of patients with a secondary progressive phase of the disease, in contrast to what found in the RR or PP form[108]. Although this change in signal could be related to the reduction in the number and axosomatic synapses in the dentate nucleus as documented by pathological studies[109], an association between the nuclei hyperintensity in T1-weighted images and Gadolinium retention has recently received greater attention[110].
Parkinson’s disease (PD) is a neurodegenerative disorder pathologically characterized by the loss of dopaminergic neurons of the pars compacta of the substantia nigra; clinical features include motor, in particular rest tremor, bradykinesia and rigidity, and non-motor symptoms. The role of cerebellum in the physiopathology of this frequent neurodegenerative disorder has received increasing interest. Studies on monkeys discovered direct anatomical connections between cerebellum and basal ganglia, in particular with the subthalamic nuclei and the striatum[8,111]. Such findings have been confirmed with a DTI study in humans[112]. Interestingly, patients receiving deep brain stimulation had a clinical benefit when the electrode was positioned nearby one of these basal ganglia-cerebellum connections, in particular the dentato-thalamic tract.
Cerebellar atrophy has been documented in patients with PD. MRI morphometric studies have shown cerebellar volume loss at the level of the left cerebellum when compared to controls[113], and at the level of the right quadrangular lobe and declive in patients with tremor compared to those without, thus suggesting a possible role of cerebellum in the genesis of rest tremor[114]. A more recent study confirmed cerebellar GM atrophy, which correlated with a reduced connectivity between cerebellum and sensorimotor, dorsal attention and default networks and an increased connectivity with the frontoparietal network[115]. Altogether these results confirm the role of cerebellum in the physiopathology of motor symptoms in PD. Atrophy occurs since cerebellum is involved in the neurodegenerative pathological process of the disease, with the accumulation of α-sinuclein and neuronal loss.
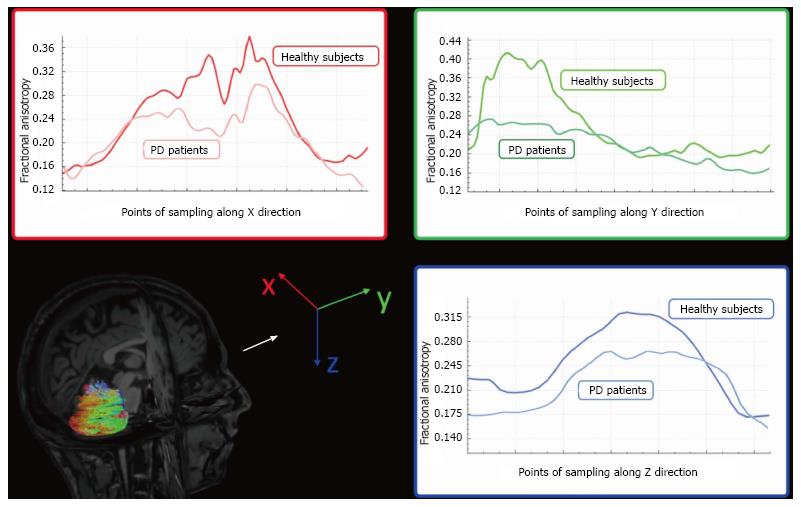
DTI studies have detected decreased FA in the cerebellar hemispheres of patients with PD when compared to healthy controls (Figure 5)[63,116]. Although no differences in DTI parameters in superior and middle cerebellar peduncles have been detected between patients with PD and healthy subjects[63], DTI metrics of the superior cerebellar peduncles could be helpful in differentiating PD and other parkinsonism, such as progressive supranuclear palsy (PSP)[117].
Cerebellar hyperactivation has been showed and confirmed in several studies on patients with PD, both with akynesia-rigidity subtype and with tremor subtype. Whether the contribution of cerebellum is mostly an adaptive mechanism or a primary pathologic change of the disease is still a matter of debate. In a resting-state fMRI study, Wu et al[118] observed an increased activation in the cerebellum in PD patients versus healthy controls; moreover, the altered pattern of activation was normalized after levodopa administration. A substitutive hyperactivity of the cerebello-thalamo-cortical circuit has been proposed as a mechanism to compensate the hypoactivation of the striato-thalamo-cortical circuit in hypokinetic patients; however, for PD tremor subtype, a dysfunction in the cerebello-thalamo-cortical circuit has been advanced as a physiopathologic mechanism for rest tremor[118,119].
Multiple systemic atrophy (MSA) is a movement disorder, with a poor prognosis, which has two clinical phenotypes: one with a prominent akinetic-rigid parkinsonism (parkinsonian variant, MSA-P) and the other with a progressive ataxia (cerebellar variant, MSA-C). It has been suggested that imaging of cerebellum could be useful in the differential diagnosis of MSA. Atrophy of the middle cerebellar peduncles is frequent in patients with MSA and reduced volume of basal ganglia, middle and inferior cerebellar peduncles and pons have been found in the parkinsonian subtype of MSA, when compared with controls and PD patients[120]. Moreover, both MSA-P and MSA-C patients were found to have higher MD values in cerebellar hemispheres when compared to PD and PSP patients[121]. Brainstem and middle cerebellar peduncles atrophy have also been found to be very specific for the cerebellar subtype of MSA when compared to idiopathic late-onset cerebellar ataxia[122].
Dystonia is a disorder characterized by sustained and abnormal spontaneous muscle contractions. It can be classified according to the etiology (inherited or acquired) or the topographic distribution. Cerebellar dysfunction has been implicated in the physiopathology of dystonia. Morphological cerebellar abnormalities have been reported in 14% of patients with cervical or segmental dystonia[123]. Focal cerebellar lesions have been associated with dystonia and cerebellar atrophy has been described in patients with writer’s cramp[124]. Besides morphological studies, several functional studies have shown an increased activation in cerebellum in patients with writer’s cramp[125] and in patients with blepharospasm[126].
Ataxias are an heterogeneous group of conditions characterized by slowly progressive incoordination of gait, often associated with a reduction of coordination of hands, speech, and oculomotor signs[127]. Ataxias can be subdivided, basing on the etiology behind the development of the condition, in three major groups: acquired ataxias, nonhereditary degenerative ataxias and hereditary ataxias (HA), which can be further divided in dominant and recessive[127]. Spinocerebellar ataxia type 3 (SCA3) is the most frequent type of dominant ataxia, followed by SCA2 and SCA6, while Friedreich Ataxia (FRDA) and ataxia-telangiectasia (AT) are respectively the first and the second most common type of recessive ataxias, with FRDA that is, independently from the inheritance, the most frequent ataxia in terms of incidence[128].
Atrophy of the cerebellar cortex is not a distinguish feature of all the HA. Different patterns of atrophy can be identified, depending on the degree of cerebellar atrophy and the involvement of midbrain structures. In particular, SCA3 and SCA2 are characterized by the presence of cortical, cerebellar and pons atrophy[129]. On the other hand, SCA6 shows a pattern of pure cortical cerebellar atrophy, with a relative sparing of midbrain structures[130]. With regard to recessive ataxias, FRDA is characterized by a prominent spinal cord atrophy[131] compared to cerebellar cortical structures[132], with atrophy that, when significant, affects mainly the lateral cerebellar hemispheres[133]. Finally, AT is characterized by superior cerebellar hemispheres atrophy, in particular of the vermis, which appears hypoplastic in its inferior portion[134-137].
Unlike volumetric measures, almost all the major HA show similar microstructural changes affecting infratentorial WM tracts. Indeed, SCA3 patients showed a significant FA reduction in different cerebellar areas, including both anterior and posterior cerebellar lobes, nodule, culmen, dentate, fastigial, lingual, and all three cerebellar peduncles, as well as in pons and midbrain[138]. These abnormalities were correlated with clinical variables, such as scale for the assessment and rating of ataxia (SARA) scores or disease duration[138,139]. In SCA2, significant microstructural changes were present in cerebellar WM, brainstem and cerebellar peduncle[140,141], with changes in the mode of anisotropy that were lower in SCA2 patients compared to healthy controls in a longitudinal evaluation[141]. Unlike in SCA3, no correlation with clinical variables emerged for these infratentorial clusters of microstructural changes in SCA2. In FRDA, microstructural changes were found to affect predominantly cerebellar peduncles[142-145], as well as cerebellar hemispheres and vermis[144] and to be associated with clinical disability[142-144]. Finally, in AT patients a reduction of mean diffusivity was reported within cerebellar peduncle regions[146] (Figure 6).
Similar to structural connectivity, functional connectivity changes are present in almost all HA. However, these modifications are not limited to a somehow expected reduction of FC in HA patients compared to healthy controls, but also increase in FC are reported in some HA, probably due to compensatory phenomena, reflecting the known heterogeneity of this group of conditions. Indeed, a significant increase of activation of the ventral part of the dentate nuclei, but not of the cerebellar cortex, was found during an hand-movement task fMRI experiment in SCA3 patients compared to healthy controls, suggesting a compensatory phenomenon of the cerebrocerebellum to a prominent damage of the spinocerebellum[147]. Moreover, a recent fMRI study, involving a bilateral audiopaced thumb movements paradigm, showed a diminished movement synchronization in SCA3 patients[148] that would suggest the presence of functional reorganization of the motor network and a potential role of fMRI as a tool to monitor the disease[148]. In SCA2, a seed-based fMRI analysis showed a decreased putaminal connection with the pons, together with a decreased connectivity between the rostral sensorimotor area and both cerebellum and pons[149]. Furthermore, a decrease of the functional connectivity of the cerebellar components of the default mode, executive and right fronto-parietal networks was described in SCA2, unrelated to the cortical gray matter volume loss[150], with some authors that showed a significant correlation between both motor and neuropsychological scores and the abnormal cerebellar functional connectivity strength[151]. In SCA6 patients, a significantly higher activation at the level of the lobules V and VI, as well as in the dentate nuclei, was detected compared to controls when performing a hand-movement task fMRI experiment[147]. Likewise, in FRDA patients the same motor task showed a higher activation mainly prominent at the level of lobules V and VI and dentate nuclei, compared to healthy control[147]. Furthermore, a working memory task fMRI experiment showed that FRDA patients had a decrease functional connectivity between cerebellum and prefrontal areas, with a correlation between disease severity and cerebellar dysfunction[152].
Alzheimer’s disease (AD) is the most common cause of dementia and it is preceded by a predementia phase, called mild cognitive impairment (MCI), characterized by a deficit in one or more cognitive domains. The role of cerebellum involvement in AD is controversial. While a volumetric MRI study in patients with AD, MCI and healthy controls found a volume loss at the level of the posterior cerebellar lobes in AD patients compared to healthy controls, no significant differences were showed between patients with MCI and healthy controls[153]. Another study detected a regional GM atrophy in cerebellar lobule VI in patients with AD[154] (Figure 7). These findings were not confirmed[155] in a more recent study, suggesting that the decrease of cerebellar volume could be influenced by the patients’ age. This is supported by the findings of cerebellar atrophy in patients with a late-onset, but not early-onset AD[156] and by the presence of cerebellar atrophy in cognitively preserved old subjects[157].
The cerebellum plays a key role in the control of motor and cognitive functions due to the multiple connections to the forebrain, the thalamus, and the spinal cord. However, the cerebellar complex anatomical structure and its location in the posterior fossa represent a challenge for in vivo structural and cerebellar neuroimaging. The recent advancement in MRI hardware and software and the development of more accurate and robust algorithms for image analysis have improved the structural and functional assessment of the cerebellum. This is of paramount importance due to the frequent and early involvement of the cerebellum in several neurodegenerative diseases. Therefore, MRI measures of cerebellar structure and function could serve as early and sensitive marker of disease progression and help monitor response to current or experimental treatments.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Quattrocchi CC S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Nieuwenhuys R, Voogd J, Huijzen Cv. The human central nervous system. New York: Springer 2008; 807-840. [DOI] [Full Text] |
| 2. | Barahona ML MJ, Querol Pascual R, Alvarez-Linera Prado J, Gañan Presmanes Y, Fernández Gil MÁ. Structural and Functional anatomy of cerebellum. More than a motor conception. Poster No: C-0497 Congress: ECR 2011; Educational Exhibit 2011. [DOI] [Full Text] |
| 3. | Glickstein M, May JG 3rd, Mercier BE. Corticopontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J Comp Neurol. 1985;235:343-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 339] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1135] [Cited by in RCA: 1244] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 6. | Milardi D, Arrigo A, Anastasi G, Cacciola A, Marino S, Mormina E, Calamuneri A, Bruschetta D, Cutroneo G, Trimarchi F. Extensive Direct Subcortical Cerebellum-Basal Ganglia Connections in Human Brain as Revealed by Constrained Spherical Deconvolution Tractography. Front Neuroanat. 2016;10:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89:634-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 485] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 8. | Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 605] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 9. | Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2198] [Cited by in RCA: 2000] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 10. | Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2064] [Cited by in RCA: 2151] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 11. | Balsters JH, Ramnani N. Symbolic representations of action in the human cerebellum. Neuroimage. 2008;43:388-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Balsters JH, Ramnani N. Cerebellar plasticity and the automation of first-order rules. J Neurosci. 2011;31:2305-2312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Raut AA, Naphade PS, Chawla A. Imaging of skull base: Pictorial essay. Indian J Radiol Imaging. 2012;22:305-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Borges A. Imaging of the central skull base. Neuroimaging Clin N Am. 2009;19:669-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Laine FJ, Nadel L, Braun IF. CT and MR imaging of the central skull base. Part 2. Pathologic spectrum. Radiographics. 1990;10:797-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Ertl-Wagner B, Eftimov L, Blume J, Bruening R, Becker C, Cormack J, Brueckmann H, Reiser M. Cranial CT with 64-, 16-, 4- and single-slice CT systems-comparison of image quality and posterior fossa artifacts in routine brain imaging with standard protocols. Eur Radiol. 2008;18:1720-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Jones TR, Kaplan RT, Lane B, Atlas SW, Rubin GD. Single- versus multi-detector row CT of the brain: quality assessment. Radiology. 2001;219:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Kamalian S, Atkinson WL, Florin LA, Pomerantz SR, Lev MH, Romero JM. Emergency department CT screening of patients with nontraumatic neurological symptoms referred to the posterior fossa: comparison of thin versus thick slice images. Emerg Radiol. 2014;21:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Rozeik C, Kotterer O, Preiss J, Schütz M, Dingler W, Deininger HK. Cranial CT artifacts and gantry angulation. J Comput Assist Tomogr. 1991;15:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Levy JM, Hupke R. Composite addition technique: a new method in CT scanning of the posterior fossa. AJNR Am J Neuroradiol. 1991;12:686-688. [PubMed] |
| 21. | Moström U, Ytterbergh C. Artifacts in computed tomography of the posterior fossa: a comparative phantom study. J Comput Assist Tomogr. 1986;10:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Glover GH, Pelc NJ. Nonlinear partial volume artifacts in x-ray computed tomography. Med Phys. 1980;7:238-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 86] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Joseph PM, Spital RD. A method for correcting bone induced artifacts in computed tomography scanners. J Comput Assist Tomogr. 1978;2:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 152] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Morelli JN, Runge VM, Ai F, Attenberger U, Vu L, Schmeets SH, Nitz WR, Kirsch JE. An image-based approach to understanding the physics of MR artifacts. Radiographics. 2011;31:849-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Hwang DY, Silva GS, Furie KL, Greer DM. Comparative sensitivity of computed tomography vs. magnetic resonance imaging for detecting acute posterior fossa infarct. J Emerg Med. 2012;42:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Young IR, Bydder GM, Hall AS, Steiner RE, Worthington BS, Hawkes RC, Holland GN, Moore WS. The role of NMR imaging in the diagnosis and management of acoustic neuroma. AJNR Am J Neuroradiol. 1983;4:223-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 27. | Wilms G, Decrop E, Plets C, Demaerel P, Marchal G, Van Hecke P, Baert AL. Magnetic resonance imaging in acoustic neurinoma. Comparison with CT. J Belge Radiol. 1989;72:151-158. [PubMed] |
| 28. | Wenz F, Hess T, Knopp MV, Weisser G, Blüml S, Schad LR, Hawighorst H, van Kaick G. 3D MPRAGE evaluation of lesions in the posterior cranial fossa. Magn Reson Imaging. 1994;12:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Kallmes DF, Hui FK, Mugler JP 3rd. Suppression of cerebrospinal fluid and blood flow artifacts in FLAIR MR imaging with a single-slab three-dimensional pulse sequence: initial experience. Radiology. 2001;221:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Bakshi R, Caruthers SD, Janardhan V, Wasay M. Intraventricular CSF pulsation artifact on fast fluid-attenuated inversion-recovery MR images: analysis of 100 consecutive normal studies. AJNR Am J Neuroradiol. 2000;21:503-508. [PubMed] |
| 31. | Winkelmann R, Börnert P, Dössel O. Ghost artifact removal using a parallel imaging approach. Magn Reson Med. 2005;54:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Rydberg JN, Hammond CA, Grimm RC, Erickson BJ, Jack CR Jr, Huston J 3rd, Riederer SJ. Initial clinical experience in MR imaging of the brain with a fast fluid-attenuated inversion-recovery pulse sequence. Radiology. 1994;193:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 149] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Lavdas E, Tsougos I, Kogia S, Gratsias G, Svolos P, Roka V, Fezoulidis IV, Kapsalaki E. T2 FLAIR artifacts at 3-T brain magnetic resonance imaging. Clin Imaging. 2014;38:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000;217:331-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 699] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 35. | Holodny AI, Ollenschlager M. Diffusion imaging in brain tumors. Neuroimaging Clin N Am. 2002;12:107-124, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Ayanzen RH, Bird CR, Keller PJ, McCully FJ, Theobald MR, Heiserman JE. Cerebral MR venography: normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol. 2000;21:74-78. [PubMed] |
| 37. | Wu Z, Mittal S, Kish K, Yu Y, Hu J, Haacke EM. Identification of calcification with MRI using susceptibility-weighted imaging: a case study. J Magn Reson Imaging. 2009;29:177-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 38. | Lesser FD, Derbyshire SG, Lewis-Jones H. Can computed tomography and magnetic resonance imaging differentiate between malignant pathology and osteomyelitis in the central skull base? J Laryngol Otol. 2015;129:852-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Fischbein NJ, Kaplan MJ. Magnetic resonance imaging of the central skull base. Top Magn Reson Imaging. 1999;10:325-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Curtin HD, Chavali R. Imaging of the skull base. Radiol Clin North Am. 1998;36:801-817, v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Marques JP, Gruetter R, van der Zwaag W. In vivo structural imaging of the cerebellum, the contribution of ultra-high fields. Cerebellum. 2012;11:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Hawkes R, Blyth S, Chockkan V, Tano D, Ji Z, Mascher C. Structural and molecular compartmentation in the cerebellum. Can J Neurol Sci. 1993;20 Suppl 3:S29-S35. [PubMed] |
| 43. | Manto M. The cerebellum, cerebellar disorders, and cerebellar research--two centuries of discoveries. Cerebellum. 2008;7:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Marques JP, van der Zwaag W, Granziera C, Krueger G, Gruetter R. Cerebellar cortical layers: in vivo visualization with structural high-field-strength MR imaging. Radiology. 2010;254:942-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Tanenbaum LN. Clinical 3T MR imaging: mastering the challenges. Magn Reson Imaging Clin N Am. 2006;14:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Gramsch C, Nensa F, Kastrup O, Maderwald S, Deuschl C, Ringelstein A, Schelhorn J, Forsting M, Schlamann M. Diagnostic value of 3D fluid attenuated inversion recovery sequence in multiple sclerosis. Acta Radiol. 2015;56:622-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Kilsdonk ID, de Graaf WL, Soriano AL, Zwanenburg JJ, Visser F, Kuijer JP, Geurts JJ, Pouwels PJ, Polman CH, Castelijns JA. Multicontrast MR imaging at 7T in multiple sclerosis: highest lesion detection in cortical gray matter with 3D-FLAIR. AJNR Am J Neuroradiol. 2013;34:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Deoni SC, Catani M. Visualization of the deep cerebellar nuclei using quantitative T1 and rho magnetic resonance imaging at 3 Tesla. Neuroimage. 2007;37:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Maderwald S, Thürling M, Küper M, Theysohn N, Müller O, Beck A, Aurich V, Ladd ME, Timmann D. Direct visualization of cerebellar nuclei in patients with focal cerebellar lesions and its application for lesion-symptom mapping. Neuroimage. 2012;63:1421-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | D’Ambrosio A, Pagani E, Riccitelli GC, Colombo B, Rodegher M, Falini A, Comi G, Filippi M, Rocca MA. Cerebellar contribution to motor and cognitive performance in multiple sclerosis: An MRI sub-regional volumetric analysis. Mult Scler. 2017;23:1194-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Bogovic JA, Jedynak B, Rigg R, Du A, Landman BA, Prince JL, Ying SH. Approaching expert results using a hierarchical cerebellum parcellation protocol for multiple inexpert human raters. Neuroimage. 2013;64:616-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 750] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 53. | Stoodley CJ. Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front Syst Neurosci. 2014;8:92. [PubMed] [DOI] [Full Text] |
| 54. | Piccinin CC, Santos MC, Piovesana LG, Campos LS, Guimarães RP, Campos BM, Torres FR, França MC, Amato-Filho AC, Lopes-Cendes I. Infratentorial gray matter atrophy and excess in primary craniocervical dystonia. Parkinsonism Relat Disord. 2014;20:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Price M, Cardenas VA, Fein G. Automated MRI cerebellar size measurements using active appearance modeling. Neuroimage. 2014;103:511-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Weier K, Beck A, Magon S, Amann M, Naegelin Y, Penner IK, Thürling M, Aurich V, Derfuss T, Radue EW. Evaluation of a new approach for semi-automatic segmentation of the cerebellum in patients with multiple sclerosis. J Neurol. 2012;259:2673-2680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1859] [Cited by in RCA: 1792] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 58. | Wang JY, Ngo MM, Hessl D, Hagerman RJ, Rivera SM. Robust Machine Learning-Based Correction on Automatic Segmentation of the Cerebellum and Brainstem. PLoS One. 2016;11:e0156123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Knösche TR, Anwander A, Liptrot M, Dyrby TB. Validation of tractography: Comparison with manganese tracing. Hum Brain Mapp. 2015;36:4116-4134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 60. | Le Bihan D. Diffusion MRI: what water tells us about the brain. EMBO Mol Med. 2014;6:569-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 61. | Keser Z, Hasan KM, Mwangi BI, Kamali A, Ucisik-Keser FE, Riascos RF, Yozbatiran N, Francisco GE, Narayana PA. Diffusion tensor imaging of the human cerebellar pathways and their interplay with cerebral macrostructure. Front Neuroanat. 2015;9:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 62. | Mormina E, Briguglio M, Morabito R, Arrigo A, Marino S, Di Rosa G, Micalizzi A, Valente EM, Salpietro V, Vinci SL. A rare case of cerebellar agenesis: a probabilistic Constrained Spherical Deconvolution tractographic study. Brain Imaging Behav. 2016;10:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Mormina E, Arrigo A, Calamuneri A, Granata F, Quartarone A, Ghilardi MF, Inglese M, Di Rocco A, Milardi D, Anastasi GP. Diffusion tensor imaging parameters’ changes of cerebellar hemispheres in Parkinson’s disease. Neuroradiology. 2015;57:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Mormina E, Longo M, Arrigo A, Alafaci C, Tomasello F, Calamuneri A, Marino S, Gaeta M, Vinci SL, Granata F. MRI Tractography of Corticospinal Tract and Arcuate Fasciculus in High-Grade Gliomas Performed by Constrained Spherical Deconvolution: Qualitative and Quantitative Analysis. AJNR Am J Neuroradiol. 2015;36:1853-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 65. | Neto Henriques R, Correia MM, Nunes RG, Ferreira HA. Exploring the 3D geometry of the diffusion kurtosis tensor--impact on the development of robust tractography procedures and novel biomarkers. Neuroimage. 2015;111:85-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Jenabi M, Peck KK, Young RJ, Brennan N, Holodny AI. Identification of the Corticobulbar Tracts of the Tongue and Face Using Deterministic and Probabilistic DTI Fiber Tracking in Patients with Brain Tumor. AJNR Am J Neuroradiol. 2015;36:2036-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2830] [Cited by in RCA: 2608] [Article Influence: 144.9] [Reference Citation Analysis (0)] |
| 68. | Gore JC. Principles and practice of functional MRI of the human brain. J Clin Invest. 2003;112:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6985] [Cited by in RCA: 7201] [Article Influence: 240.0] [Reference Citation Analysis (0)] |
| 70. | Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848-13853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3157] [Cited by in RCA: 3273] [Article Influence: 172.3] [Reference Citation Analysis (0)] |
| 71. | Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040-13045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4468] [Cited by in RCA: 3909] [Article Influence: 244.3] [Reference Citation Analysis (0)] |
| 72. | Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586-8594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 913] [Cited by in RCA: 872] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 73. | Yacoub E, Shmuel A, Pfeuffer J, Van De Moortele PF, Adriany G, Andersen P, Vaughan JT, Merkle H, Ugurbil K, Hu X. Imaging brain function in humans at 7 Tesla. Magn Reson Med. 2001;45:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 344] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 74. | Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240:318-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 300] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 75. | Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, Bolan PJ, Brindle KM, Cudalbu C, Dinçer A. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270:658-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 473] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 76. | Li BS, Wang H, Gonen O. Metabolite ratios to assumed stable creatine level may confound the quantification of proton brain MR spectroscopy. Magn Reson Imaging. 2003;21:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Mascalchi M, Brugnoli R, Guerrini L, Belli G, Nistri M, Politi LS, Gavazzi C, Lolli F, Argenti G, Villari N. Single-voxel long TE 1H-MR spectroscopy of the normal brainstem and cerebellum. J Magn Reson Imaging. 2002;16:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Weber-Fahr W, Ende G, Braus DF, Bachert P, Soher BJ, Henn FA, Büchel C. A fully automated method for tissue segmentation and CSF-correction of proton MRSI metabolites corroborates abnormal hippocampal NAA in schizophrenia. Neuroimage. 2002;16:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Currie S, Hadjivassiliou M, Wilkinson ID, Griffiths PD, Hoggard N. Magnetic resonance spectroscopy of the normal cerebellum: what degree of variability can be expected? Cerebellum. 2013;12:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Sajja BR, Wolinsky JS, Narayana PA. Proton magnetic resonance spectroscopy in multiple sclerosis. Neuroimaging Clin N Am. 2009;19:45-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 81. | Jahng GH, Li KL, Ostergaard L, Calamante F. Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean J Radiol. 2014;15:554-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 82. | Talbot PR, Lloyd JJ, Snowden JS, Neary D, Testa HJ. Choice of reference region in the quantification of single-photon emission tomography in primary degenerative dementia. Eur J Nucl Med. 1994;21:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 83. | Lacalle-Aurioles M, Alemán-Gómez Y, Guzmán-De-Villoria JA, Cruz-Orduña I, Olazarán J, Mateos-Pérez JM, Martino ME, Desco M. Is the cerebellum the optimal reference region for intensity normalization of perfusion MR studies in early Alzheimer’s disease? PLoS One. 2013;8:e81548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Hartkamp NS, Petersen ET, De Vis JB, Bokkers RP, Hendrikse J. Mapping of cerebral perfusion territories using territorial arterial spin labeling: techniques and clinical application. NMR Biomed. 2013;26:901-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M. A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology. 2015;57:1181-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 86. | van Gelderen P, de Zwart JA, Duyn JH. Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magn Reson Med. 2008;59:788-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 87. | Compston A, Lassmann H, McDonald I. Chapter 1 - The story of multiple sclerosis. McAlpine’s Multiple Sclerosis (Fourth Edition). Edinburgh: Churchill Livingstone 2006; 3-68. [DOI] [Full Text] |
| 88. | Tintore M, Otero-Romero S, Río J, Arrambide G, Pujal B, Tur C, Galán I, Comabella M, Nos C, Arévalo MJ. Contribution of the symptomatic lesion in establishing MS diagnosis and prognosis. Neurology. 2016;87:1368-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Fazekas F, Offenbacher H, Fuchs S, Schmidt R, Niederkorn K, Horner S, Lechner H. Criteria for an increased specificity of MRI interpretation in elderly subjects with suspected multiple sclerosis. Neurology. 1988;38:1822-1825. [PubMed] |
| 90. | Ormerod IE, Miller DH, McDonald WI, du Boulay EP, Rudge P, Kendall BE, Moseley IF, Johnson G, Tofts PS, Halliday AM. The role of NMR imaging in the assessment of multiple sclerosis and isolated neurological lesions. A quantitative study. Brain. 1987;110:1579-1616. [PubMed] |
| 91. | Favaretto A, Lazzarotto A, Poggiali D, Rolma G, Causin F, Rinaldi F, Perini P, Gallo P. MRI-detectable cortical lesions in the cerebellum and their clinical relevance in multiple sclerosis. Mult Scler. 2016;22:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Calabrese M, Reynolds R, Magliozzi R, Castellaro M, Morra A, Scalfari A, Farina G, Romualdi C, Gajofatto A, Pitteri M. Regional Distribution and Evolution of Gray Matter Damage in Different Populations of Multiple Sclerosis Patients. PLoS One. 2015;10:e0135428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Bendfeldt K, Kuster P, Traud S, Egger H, Winklhofer S, Mueller-Lenke N, Naegelin Y, Gass A, Kappos L, Matthews PM. Association of regional gray matter volume loss and progression of white matter lesions in multiple sclerosis - A longitudinal voxel-based morphometry study. Neuroimage. 2009;45:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 94. | Edwards SG, Gong QY, Liu C, Zvartau ME, Jaspan T, Roberts N, Blumhardt LD. Infratentorial atrophy on magnetic resonance imaging and disability in multiple sclerosis. Brain. 1999;122:291-301. [PubMed] |
| 95. | Ramasamy DP, Benedict RH, Cox JL, Fritz D, Abdelrahman N, Hussein S, Minagar A, Dwyer MG, Zivadinov R. Extent of cerebellum, subcortical and cortical atrophy in patients with MS: a case-control study. J Neurol Sci. 2009;282:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 96. | Anderson VM, Fisniku LK, Altmann DR, Thompson AJ, Miller DH. MRI measures show significant cerebellar gray matter volume loss in multiple sclerosis and are associated with cerebellar dysfunction. Mult Scler. 2009;15:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Anderson VM, Wheeler-Kingshott CA, Abdel-Aziz K, Miller DH, Toosy A, Thompson AJ, Ciccarelli O. A comprehensive assessment of cerebellar damage in multiple sclerosis using diffusion tractography and volumetric analysis. Mult Scler. 2011;17:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 98. | Preziosa P, Rocca MA, Mesaros S, Pagani E, Drulovic J, Stosic-Opincal T, Dackovic J, Copetti M, Caputo D, Filippi M. Relationship between damage to the cerebellar peduncles and clinical disability in multiple sclerosis. Radiology. 2014;271:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 99. | Deppe M, Tabelow K, Krämer J, Tenberge JG, Schiffler P, Bittner S, Schwindt W, Zipp F, Wiendl H, Meuth SG. Evidence for early, non-lesional cerebellar damage in patients with multiple sclerosis: DTI measures correlate with disability, atrophy, and disease duration. Mult Scler. 2016;22:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | Tomassini V, d’Ambrosio A, Petsas N, Wise RG, Sbardella E, Allen M, Tona F, Fanelli F, Foster C, Carnì M. The effect of inflammation and its reduction on brain plasticity in multiple sclerosis: MRI evidence. Hum Brain Mapp. 2016;37:2431-2445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 101. | Rocca MA, Pagani E, Absinta M, Valsasina P, Falini A, Scotti G, Comi G, Filippi M. Altered functional and structural connectivities in patients with MS: a 3-T study. Neurology. 2007;69:2136-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 102. | Pardini M, Bonzano L, Roccatagliata L, Mancardi GL, Bove M. The fatigue-motor performance paradox in multiple sclerosis. Sci Rep. 2013;3:2001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | Weier K, Penner IK, Magon S, Amann M, Naegelin Y, Andelova M, Derfuss T, Stippich C, Radue EW, Kappos L. Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PLoS One. 2014;9:e86916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 104. | Damasceno A, Damasceno BP, Cendes F. The clinical impact of cerebellar grey matter pathology in multiple sclerosis. PLoS One. 2014;9:e96193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Yeoman LJ, Howarth L, Britten A, Cotterill A, Adam EJ. Gantry angulation in brain CT: dosage implications, effect on posterior fossa artifacts, and current international practice. Radiology. 1992;184:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Sbardella E, Upadhyay N, Tona F, Prosperini L, De Giglio L, Petsas N, Pozzilli C, Pantano P. Dentate nucleus connectivity in adult patients with multiple sclerosis: functional changes at rest and correlation with clinical features. Mult Scler. 2017;23:546-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 107. | Loitfelder M, Filippi M, Rocca M, Valsasina P, Ropele S, Jehna M, Fuchs S, Schmidt R, Neuper C, Fazekas F. Abnormalities of resting state functional connectivity are related to sustained attention deficits in MS. PLoS One. 2012;7:e42862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 108. | Roccatagliata L, Vuolo L, Bonzano L, Pichiecchio A, Mancardi GL. Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with the secondary progressive subtype. Radiology. 2009;251:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 109. | Albert M, Barrantes-Freer A, Lohrberg M, Antel JP, Prineas JW, Palkovits M, Wolff JR, Brück W, Stadelmann C. Synaptic pathology in the cerebellar dentate nucleus in chronic multiple sclerosis. Brain Pathol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 110. | Tedeschi E, Palma G, Canna A, Cocozza S, Russo C, Borrelli P, Lanzillo R, Angelini V, Postiglione E, Morra VB. In vivo dentate nucleus MRI relaxometry correlates with previous administration of Gadolinium-based contrast agents. Eur Radiol. 2016;26:4577-4584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 111. | Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA. 2010;107:8452-8456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 575] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 112. | Sweet JA, Walter BL, Gunalan K, Chaturvedi A, McIntyre CC, Miller JP. Fiber tractography of the axonal pathways linking the basal ganglia and cerebellum in Parkinson disease: implications for targeting in deep brain stimulation. J Neurosurg. 2014;120:988-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 113. | Borghammer P, Østergaard K, Cumming P, Gjedde A, Rodell A, Hall N, Chakravarty MM. A deformation-based morphometry study of patients with early-stage Parkinson’s disease. Eur J Neurol. 2010;17:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 114. | Benninger DH, Thees S, Kollias SS, Bassetti CL, Waldvogel D. Morphological differences in Parkinson’s disease with and without rest tremor. J Neurol. 2009;256:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 115. | O’Callaghan C, Hornberger M, Balsters JH, Halliday GM, Lewis SJ, Shine JM. Cerebellar atrophy in Parkinson’s disease and its implication for network connectivity. Brain. 2016;139:845-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 116. | Zhang K, Yu C, Zhang Y, Wu X, Zhu C, Chan P, Li K. Voxel-based analysis of diffusion tensor indices in the brain in patients with Parkinson’s disease. Eur J Radiol. 2011;77:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 117. | Nicoletti G, Caligiuri ME, Cherubini A, Morelli M, Novellino F, Arabia G, Salsone M, Quattrone A. A Fully Automated, Atlas-Based Approach for Superior Cerebellar Peduncle Evaluation in Progressive Supranuclear Palsy Phenotypes. AJNR Am J Neuroradiol. 2017;38:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 118. | Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, Chan P. Regional homogeneity changes in patients with Parkinson’s disease. Hum Brain Mapp. 2009;30:1502-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 347] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 119. | Dirkx MF, den Ouden H, Aarts E, Timmer M, Bloem BR, Toni I, Helmich RC. The Cerebral Network of Parkinson’s Tremor: An Effective Connectivity fMRI Study. J Neurosci. 2016;36:5362-5372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 120. | Planetta PJ, Kurani AS, Shukla P, Prodoehl J, Corcos DM, Comella CL, McFarland NR, Okun MS, Vaillancourt DE. Distinct functional and macrostructural brain changes in Parkinson’s disease and multiple system atrophy. Hum Brain Mapp. 2015;36:1165-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 121. | Nicoletti G, Rizzo G, Barbagallo G, Tonon C, Condino F, Manners D, Messina D, Testa C, Arabia G, Gambardella A. Diffusivity of cerebellar hemispheres enables discrimination of cerebellar or parkinsonian multiple system atrophy from progressive supranuclear palsy-Richardson syndrome and Parkinson disease. Radiology. 2013;267:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 122. | Lin DJ, Hermann KL, Schmahmann JD. The Diagnosis and Natural History of Multiple System Atrophy, Cerebellar Type. Cerebellum. 2016;15:663-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 123. | Batla A, Sánchez MC, Erro R, Ganos C, Stamelou M, Balint B, Brugger F, Antelmi E, Bhatia KP. The role of cerebellum in patients with late onset cervical/segmental dystonia?--evidence from the clinic. Parkinsonism Relat Disord. 2015;21:1317-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 124. | Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, Meunier S, Terrier A, Lehéricy S. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology. 2007;69:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 125. | Preibisch C, Berg D, Hofmann E, Solymosi L, Naumann M. Cerebral activation patterns in patients with writer’s cramp: a functional magnetic resonance imaging study. J Neurol. 2001;248:10-17. [PubMed] |
| 126. | Baker RS, Andersen AH, Morecraft RJ, Smith CD. A functional magnetic resonance imaging study in patients with benign essential blepharospasm. J Neuroophthalmol. 2003;23:11-15. [PubMed] |
| 127. | Klockgether T. Update on degenerative ataxias. Curr Opin Neurol. 2011;24:339-345. [PubMed] [DOI] [Full Text] |
| 128. | Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology. 2014;42:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 454] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 129. | Bürk K, Abele M, Fetter M, Dichgans J, Skalej M, Laccone F, Didierjean O, Brice A, Klockgether T. Autosomal dominant cerebellar ataxia type I clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain. 1996;119:1497-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 163] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 130. | Schulz JB, Borkert J, Wolf S, Schmitz-Hübsch T, Rakowicz M, Mariotti C, Schöls L, Timmann D, van de Warrenburg B, Dürr A. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. Neuroimage. 2010;49:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 131. | Klockgether T, Petersen D, Grodd W, Dichgans J. Early onset cerebellar ataxia with retained tendon reflexes. Clinical, electrophysiological and MRI observations in comparison with Friedreich’s ataxia. Brain. 1991;114:1559-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 132. | Mascalchi M. The cerebellum looks normal in Friedreich ataxia. AJNR Am J Neuroradiol. 2013;34:E22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 133. | Selvadurai LP, Harding IH, Corben LA, Stagnitti MR, Storey E, Egan GF, Delatycki MB, Georgiou-Karistianis N. Cerebral and cerebellar grey matter atrophy in Friedreich ataxia: the IMAGE-FRDA study. J Neurol. 2016;263:2215-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 134. | Al-Maawali A, Blaser S, Yoon G. Diagnostic approach to childhood-onset cerebellar atrophy: a 10-year retrospective study of 300 patients. J Child Neurol. 2012;27:1121-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 135. | Lin DD, Barker PB, Lederman HM, Crawford TO. Cerebral abnormalities in adults with ataxia-telangiectasia. AJNR Am J Neuroradiol. 2014;35:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 136. | Sardanelli F, Parodi RC, Ottonello C, Renzetti P, Saitta S, Lignana E, Mancardi GL. Cranial MRI in ataxia-telangiectasia. Neuroradiology. 1995;37:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 137. | Tavani F, Zimmerman RA, Berry GT, Sullivan K, Gatti R, Bingham P. Ataxia-telangiectasia: the pattern of cerebellar atrophy on MRI. Neuroradiology. 2003;45:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 138. | Guimarães RP, D’Abreu A, Yasuda CL, França MC Jr, Silva BH, Cappabianco FA, Bergo FP, Lopes-Cendes IT, Cendes F. A multimodal evaluation of microstructural white matter damage in spinocerebellar ataxia type 3. Mov Disord. 2013;28:1125-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 139. | Kang JS, Klein JC, Baudrexel S, Deichmann R, Nolte D, Hilker R. White matter damage is related to ataxia severity in SCA3. J Neurol. 2014;261:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 140. | Hernandez-Castillo CR, Vaca-Palomares I, Galvez V, Campos-Romo A, Diaz R, Fernandez-Ruiz J. Cognitive Deficits Correlate with White Matter Deterioration in Spinocerebellar Ataxia Type 2. J Int Neuropsychol Soc. 2016;22:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 141. | Mascalchi M, Toschi N, Giannelli M, Ginestroni A, Della Nave R, Nicolai E, Bianchi A, Tessa C, Salvatore E, Aiello M. Progression of microstructural damage in spinocerebellar ataxia type 2: a longitudinal DTI study. AJNR Am J Neuroradiol. 2015;36:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 142. | Clemm von Hohenberg C, Schocke MF, Wigand MC, Nachbauer W, Guttmann CR, Kubicki M, Shenton ME, Boesch S, Egger K. Radial diffusivity in the cerebellar peduncles correlates with clinical severity in Friedreich ataxia. Neurol Sci. 2013;34:1459-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 143. | Della Nave R, Ginestroni A, Tessa C, Salvatore E, Bartolomei I, Salvi F, Dotti MT, De Michele G, Piacentini S, Mascalchi M. Brain white matter tracts degeneration in Friedreich ataxia. An in vivo MRI study using tract-based spatial statistics and voxel-based morphometry. Neuroimage. 2008;40:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 144. | Rizzo G, Tonon C, Valentino ML, Manners D, Fortuna F, Gellera C, Pini A, Ghezzo A, Baruzzi A, Testa C. Brain diffusion-weighted imaging in Friedreich’s ataxia. Mov Disord. 2011;26:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 145. | Vieira Karuta SC, Raskin S, de Carvalho Neto A, Gasparetto EL, Doring T, Teive HA. Diffusion tensor imaging and tract-based spatial statistics analysis in Friedreich’s ataxia patients. Parkinsonism Relat Disord. 2015;21:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 146. | Sahama I, Sinclair K, Fiori S, Doecke J, Pannek K, Reid L, Lavin M, Rose S. Motor pathway degeneration in young ataxia telangiectasia patients: A diffusion tractography study. Neuroimage Clin. 2015;9:206-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 147. | Stefanescu MR, Dohnalek M, Maderwald S, Thürling M, Minnerop M, Beck A, Schlamann M, Diedrichsen J, Ladd ME, Timmann D. Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich’s ataxia. Brain. 2015;138:1182-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 148. | Duarte JV, Faustino R, Lobo M, Cunha G, Nunes C, Ferreira C, Januário C, Castelo-Branco M. Parametric fMRI of paced motor responses uncovers novel whole-brain imaging biomarkers in spinocerebellar ataxia type 3. Hum Brain Mapp. 2016;37:3656-3668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 149. | Wu T, Wang C, Wang J, Hallett M, Zang Y, Chan P. Preclinical and clinical neural network changes in SCA2 parkinsonism. Parkinsonism Relat Disord. 2013;19:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 150. | Cocozza S, Saccà F, Cervo A, Marsili A, Russo CV, Giorgio SM, De Michele G, Filla A, Brunetti A, Quarantelli M. Modifications of resting state networks in spinocerebellar ataxia type 2. Mov Disord. 2015;30:1382-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 151. | Hernandez-Castillo CR, Galvez V, Mercadillo RE, Díaz R, Yescas P, Martinez L, Ochoa A, Velazquez-Perez L, Fernandez-Ruiz J. Functional connectivity changes related to cognitive and motor performance in spinocerebellar ataxia type 2. Mov Disord. 2015;30:1391-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 152. | Harding IH, Corben LA, Storey E, Egan GF, Stagnitti MR, Poudel GR, Delatycki MB, Georgiou-Karistianis N. Fronto-cerebellar dysfunction and dysconnectivity underlying cognition in friedreich ataxia: The IMAGE-FRDA study. Hum Brain Mapp. 2016;37:338-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 153. | Thomann PA, Schläfer C, Seidl U, Santos VD, Essig M, Schröder J. The cerebellum in mild cognitive impairment and Alzheimer’s disease - a structural MRI study. J Psychiatr Res. 2008;42:1198-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 154. | Colloby SJ, O’Brien JT, Taylor JP. Patterns of cerebellar volume loss in dementia with Lewy bodies and Alzheimer׳s disease: A VBM-DARTEL study. Psychiatry Res. 2014;223:187-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 155. | Łojkowska W, Witkowski G, Bednarska-Makaruk M, Wehr H, Sienkiewicz-Jarosz H, Graban A, Bochyńska A, Wiśniewska A, Gugała M, Sławińska K. Correlations between cerebellar and brain volumes, cognitive impairments, ApoE levels, and APOE genotypes in patients with AD and MCI. Curr Alzheimer Res. 2013;10:964-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 156. | Möller C, Vrenken H, Jiskoot L, Versteeg A, Barkhof F, Scheltens P, van der Flier WM. Different patterns of gray matter atrophy in early- and late-onset Alzheimer’s disease. Neurobiol Aging. 2013;34:2014-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 157. | Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT. Age, Alzheimer disease, and brain structure. Neurology. 2009;73:1899-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |