Peer-review started: June 24, 2016
First decision: August 16, 2016
Revised: September 6, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: January 28, 2017
Processing time: 212 Days and 8.1 Hours
Functional magnetic resonance imaging (fMRI) is employed in many behavior analysis studies, with blood oxygen level dependent- (BOLD-) contrast imaging being the main method used to generate images. The use of BOLD-contrast imaging in fMRI has been refined over the years, for example, the inclusion of a spin echo pulse and increased magnetic strength were shown to produce better recorded images. Taking careful precautions to control variables during measurement, comparisons between different specimen groups can be illustrated by fMRI imaging using both quantitative and qualitative methods. Differences have been observed in comparisons of active and resting, developing and aging, and defective and damaged brains in various studies. However, cognitive studies using fMRI still face a number of challenges in interpretation that can only be overcome by imaging large numbers of samples. Furthermore, fMRI studies of brain cancer, lesions and other brain pathologies of both humans and animals are still to be explored.
Core tip: We summarize the use of blood oxygen level dependent-contrast imaging in functional magnetic resonance imaging (fMRI) by introducing and comparing the various experimental and analysis methods used, as well as describing the results obtained, and the challenges that might occur in order to derive a hypothesis for further studies and exploration. In addition, an overview of fMRI following sensory stimulation in different specimen groups in both humans and animals is provided.
- Citation: Chow MSM, Wu SL, Webb SE, Gluskin K, Yew DT. Functional magnetic resonance imaging and the brain: A brief review. World J Radiol 2017; 9(1): 5-9
- URL: https://www.wjgnet.com/1949-8470/full/v9/i1/5.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i1.5
There have recently been a significant number of behavior response analysis studies that have made use of magnetic resonance imaging (MRI), and in particular functional magnetic resonance imaging (fMRI). The majority of these studies make use of blood oxygen level-dependent- (BOLD-) contrast imaging, which involves mapping particular regions of a functioning brain, from the changes in blood oxygen.
BOLD-contrast imaging fMRI has a good enough spatial resolution for the localization of activated brain areas and their delineation from neighbouring regions to be visualised. The voxel representing the area of activation is usually defined as covering a few million neurons[1]. In addition, the BOLD response lags 1 to 2 s behind the stimulus in order for the vascular system to respond, and in general it peaks at 5 s after the stimulus. A continuation of the same stimulus would downregulate the BOLD response[1-3]. A refractory period of just a few seconds is frequently inadequate for BOLD imaging after activation to fade (see below), depending on whether the mode of activation is motor, sensory or emotional.
To eliminate noise in the recording, the stimulus must be repeated several times. This process often takes a few minutes to complete, and the results can then be compared across different individuals or animals[1,4]. With regards to the latter, rodents, pigs and monkeys have all been employed in behavior response analysis studies with BOLD-contrast imaging fMRI[5-9]. This technique and thus the quality of images generated, has been improved by using both a spin echo pulse and increasing the magnetic strength[10].
One region of the brain that is popular for fMRI mapping due to it being relatively easy to generate a stimulus, is the sensory part of the brain, including the lateral geniculate bodies and the cortex[11]. Over the last ten years, our group has employed a number of different inputs of both sensory and motor activations for fMRI mapping, with some success. These include chewing, the opposition of the thumb (in humans) and passively flexing the elbow (in animals). These different types of activation trigger both the motor and the sensory systems, such as proprioception and movement[12,13]. However, only a limited number of clinical studies on head injuries, Alzheimer’s disease and drug use have been documented using this method so far[4].
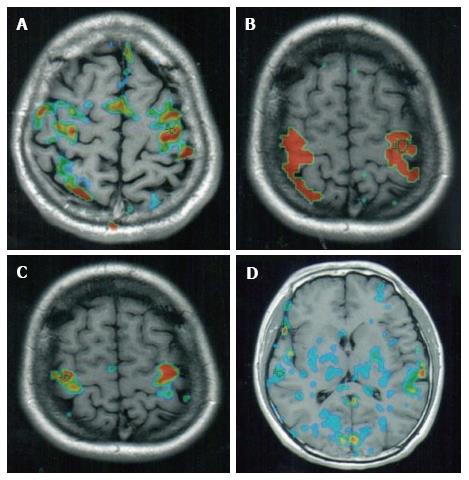
In the course of performing fMRI and comparing different specimen groups, it is often necessary to illustrate comparisons with quantitation. This can be achieved via a number of methods. For example, the different levels of oxygen usage are frequently illustrated with a pseudocolor scale in the images acquired. In the commonly used four-color scale, red indicates the highest level of oxygen uptake, yellow is an intermediate level of uptake, green indicates the normal level of oxygen, and blue indicates oxygen levels that are lower than normal (i.e., down-regulation; Figure 1). Utilizing this type of color scale, the researcher can easily compare the volumes of the variously-colored regions either in a specific part of the brain or globally in the whole brain. Indeed, some well-funded research laboratories can afford to purchase software specifically designed to calculate the morphometry for this purpose. Another method used for quantitating fMRI images, which is perhaps more applicable to the smaller, less affluent groups involves manually counting squares on a simple grid. This is placed on successive slices of the fMRI image, after which volume data can be calculated. Though tedious, this method can yield results that are as accurate as those generated from expensive software packages.
In addition to quantitative analysis, qualitative evaluation is also important, for instance to determine the specific regions of the brain that are activated during a particular type of movement. Some of the areas that are activated might be nonspecific, in which case the investigator has to carefully consider each site of activation to determine if any logical deduction might be obtained. In this respect, at least six individuals, when available, should be used for every experiment in each group in order to obtain the n-numbers required to compare the data statistically. Indeed, in some of our previous studies, we aimed to recruit at least a dozen individuals per group. While this was not difficult to achieve with studies using animals, it was sometimes difficult to solicit this number of human volunteers or patients. In addition, we found that fMRI studies are particularly hard to evaluate when the experiments involve cognitive changes that might be affected by emotions. The results of psychometrical tests were variable in different individuals, especially in those with neurosis.
fMRI recordings require the subject (whether human or animal), to perform a particular action in order to trigger a dynamic uptake of oxygen into the brain. This is because fMRI recordings are based on the subsequent increase in oxygen demand from the brain tissue upon the execution of a certain stimulus, whether it is motor, sensory or emotional. One memorable case involved human subjects being asked to repeat a set words in the correct sequence, which elicited a notable increase of blood oxygen levels in the inferior frontal area (BA44) of the brain[14]. In this study, an uptake of oxygen was normally elicited after repeated and continuous stimulus, and the acquisition of the BOLD-contrast image was usually completed within several seconds after the stimulation ended (in this case after 6 to 18 s). The acquisition of subsequent BOLD-contrast images took > 1 s per slice, e.g., 1.6 s[14]. The images acquired were then superimposed on the neuroanatomical image of the corresponding slices, which facilitated the localization of the sites of recording[14].
In previous studies, we engaged human volunteers in active movements such as the opposition of the finger and thumb, flexion of the arm or mastication (e.g., by chewing gum)[12,15]. In animals, such as pigs, rodents and monkeys, however, it is often difficult to capture fMRI images while they are freely and actively moving. Thus, passive movements tend to be performed instead. These include flexion and extension of the limbs and visual stimulation, as well as treatment with drugs.
In order to determine the effect of a specific behaviour on the amount of oxygen uptake in particular regions of the brain using fMRI, images acquired during movement and at rest must be compared. Indeed, valid data can be acquired by subtracting the results recorded at rest from those recorded during movement. Thus, subjects must be imaged as they perform a certain type of movement and then again when they are at rest. It would also be interesting to compare data from fMRI images acquired at different durations of rest after movement, as this might provide useful information regarding changes in the brain that occur during recovery after movement.
A typical example of a standard fMRI recording is depicted in Figure 1. Figure 1A indicates a recording that was acquired when the test subject was at rest. When we conducted this experiment, we were interested to find that in a number of individuals, some sporadic activity occurred in the cortex even at rest. In the recording of the individual shown in Figure 1, varying levels of activity were registered in the motor, sensory and visual areas of the brain, as well as in the midline of the cortex. Upon stimulation (achieved by thumb and finger opposition), clear and intensive reactions became apparent in the BOLD-contrast images, but these were limited to the motor and sensory areas alone (Figure 1B). Figure 1C depicts the corresponding fMRI image from the same individual following two minutes of stimulation and then one-minute of rest. It is still possible to recognise some residual increased brain activity in the post stimulation resting state. These figures demonstrate the importance of the timing used for the evaluation and comparison of data, as well as the use of the same individual for collecting corresponding sections in each series.
Another comparison method involves depicting of all the active sites of BOLD-contrast fMRI images in the whole brain both at rest and during stimulation. Both of these different methods of comparison are useful in their own way, with the comparison of corresponding slices providing a quantitation of comparison on specific sites, whereas the whole brain images provide a global picture of all the active sites.
In addition to recording the uptake of blood oxygen levels into the brain, the downregulation of blood oxygen has also recently been imaged by fMRI, in order to evaluate subjects with brain damage or brain defects. Figure 1D is an fMRI slice of the brain of long-term heroin addict. The large numbers of blue spots indicate regions of the brain where the blood oxygen levels are lower than normal (as described above, green indicates normal levels of oxygen). In the drug addict’s brain, the blood oxygen was low (i.e., downregulated) in the grey matter, and also in the fibers, especially those in the corpus callosum (Figure 1D). These data confirm those acquired from ordinary MRI of addicts on abusive drugs, which also demonstrated degenerative sites[16], especially degenerative fibers or grey matter in the cortex, or in groups of nuclei in the cerebellum.
fMRI recordings are useful for evaluating the changes in the overall activity of specific brain regions, not only in adults, but also in developing and aging animals. For example, in one study, fMRI was conducted in the neonatal pig. At this stage of development the brain is relatively immature, and it was shown that stimulation of either sensory or motor activities in the body elicited a wide global and non-discrete response in the brain[7]. Several months later, stimulation of the same activities only produced induced BOLD responses in discrete and related areas of the brain. This example clearly shows that nonselective responses were elicited in the immature brain, whereas in the maturing or fully mature brain, the particular neuronal groups that fired were specific to the related functioning areas. The same type of global nonspecific firing observed in the fMRI of the immature brain can also be observed in some brain diseases such as schizophrenia and bipolar disorders. Follow-up cytochemical and histological studies conducted with the pig concluded that during the development and maturation of the brain, superfluous pathways were pruned significantly, and the number of inhibitory contacts that refine the specificity of each pathway of the brain increased. This study and others, demonstrated the usefulness of utilizing BOLD and fMRI for understanding psychiatric and neurological diseases, and they facilitated the collection of pathological specimens along the way at the same time. The use of fMRI imaging in conjunction with cytochemistry and/or classical pathohistological techniques has the potential to become a very powerful tool to help with the analysis of neurological diseases and mental disorders.
In addition to imaging the developing brain, fMRI can be applied to the aging brain. In a study employing humans of different ages (i.e., young, middle-aged and old), BOLD-contrast imaging of fMRI was recorded in each group as they performed the same motor activities[12]. The results obtained were interesting and significant. More brain area activations were recorded in individuals in the “old” group (i.e., around 70 years of age), when compared with individuals from the young and middle-aged groups as they conducted the same motor activity. It was concluded that the older brain is less efficient, and so larger areas are required to achieve a certain job. To substantiate this proposition, it was necessary to obtain a similar result in animals. As mentioned previously, specific active movements are a challenge to initiate in animals but passive movements are easier to control although the results obtained are more difficult to interpret. Therefore, in one a series of experiments we conducted, we used sensory stimulation in order to evaluate the aging hypothesis by applying a weight to the tail of rodents of different ages[17]. The results obtained with the rodents complimented those of the human studies in that the sensory stimulus triggered increased blood oxygen levels in larger areas of the brains of the older animals than in the young groups. It is therefore tempting to conclude that larger areas of the brain are recruited in the aging groups than in the young groups, to conduct the same functional activity. Perhaps this is due to brains being less efficient overall on aging. On the other hand, it is also possible that on aging it is normal for additional areas of the brain to be used to engage in activities. This would therefore illustrate the plasticity of the brain during aging rather than simply a reduction in the efficiency over time.
Cognitive studies using fMRI are extremely important, but the results can be a challenge to interpret. The main difficulties lie in the psychometric nature of the individual to be recorded. For instance, some individuals are very anxious and when they are asked unrelated questions, this elicits responses in areas of the brain that are not normally engaged. Interpretation of data therefore depends on being able to collect a large enough number of samples to be able to exclude false positive results. fMRI imaging has largely been conducted with humans (including normal individuals, patients, addicts, and aged individuals), as well as in animal models of addiction and aging[18,19]. In the case of patients with various diseases, the way forward for fMRI and its potential are largely still under-explored. However, it might be very insightful to find out how areas of the brain areas react in patients with brain cancer, for example. It would also be interesting to explore any changes that might occur in focal pathological areas and in normal areas surrounding the pathology; as well as what changes take place in the brain as the disease progresses. These are just a few of the studies that might be conducted using BOLD-contrast fMRI.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gao BL, Pan HC, Takahashi H S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Huettel SA, Song AW, McCarthy G. Functional Magnetic resonance imaging. Sunderland, Mass: Sinauer Associates 2009; 1-501. |
| 2. | Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp. 1997;5:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Kahn I, Desai M, Knoblich U, Bernstein J, Henninger M, Graybiel AM, Boyden ES, Buckner RL, Moore CI. Characterization of the functional MRI response temporal linearity via optical control of neocortical pyramidal neurons. J Neurosci. 2011;31:15086-15091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Rombouts SA, Bakhof L, Shelten P. Clinical applications of functional brain MRI. 1st edition. Oxford: Oxford University Press 2008; 1-352. |
| 5. | Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68-78. [PubMed] |
| 6. | Desai M, Kahn I, Knoblich U, Bernstein J, Atallah H, Yang A, Kopell N, Buckner RL, Graybiel AM, Moore CI. Mapping brain networks in awake mice using combined optical neural control and fMRI. J Neurophysiol. 2011;105:1393-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 7. | Fang M, Lorke DE, Li J, Gong X, Yew JC, Yew DT. Postnatal changes in functional activities of the pig’s brain: a combined functional magnetic resonance imaging and immunohistochemical study. Neurosignals. 2005;14:222-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Yu H, Li Q, Wang D, Shi L, Lu G, Sun L, Wang L, Zhu W, Mak YT, Wong N. Mapping the central effects of chronic ketamine administration in an adolescent primate model by functional magnetic resonance imaging (fMRI). Neurotoxicology. 2012;33:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Sun L, Li Q, Li Q, Zhang Y, Liu D, Jiang H, Pan F, Yew DT. Chronic ketamine exposure induces permanent impairment of brain functions in adolescent cynomolgus monkeys. Addict Biol. 2014;19:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Carr VA, Rissman J, Wagner AD. Imaging the human medial temporal lobe with high-resolution fMRI. Neuron. 2010;65:298-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Kim SG, Lee SP, Goodyear B, Silva AC, Moonen C, Bandettini PA. Medical Radiology: Diagnostic imaging, Functional MRI: Spatial resolution of BOLD and other fMRI techniques. Berlin: Springer 2000; 453-463. |
| 12. | Fang M, Li J, Lu G, Gong X, Yew DT. A fMRI study of age-related differential cortical patterns during cued motor movement. Brain Topogr. 2005;17:127-137. [PubMed] |
| 13. | Fang M, Li J, Rudd JA, Wai SM, Yew JC, Yew DT. fMRI mapping of cortical centers following visual stimulation in postnatal pigs of different ages. Life Sci. 2006;78:1197-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Gore JC. Principles and practice of functional MRI of the human brain. J Clin Invest. 2003;112:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Jiang YL, Tian W, Lu G, Rudd JA, Lai KF, Yeung LY, Wai MS, Li YY, Huang ML, Yew DT. Patterns of cortical activation following motor tasks and psychological-inducing movie cues in heroin users: an fMRI study. Int J Psychiatry Med. 2014;47:25-40. [PubMed] |
| 16. | Wang C, Zheng D, Xu J, Lam W, Yew DT. Brain damages in ketamine addicts as revealed by magnetic resonance imaging. Front Neuroanat. 2013;7:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Zhang L, Li Q, Wolff LT, Antonio GE, Yeung DK, Zhang A, Wu Y, Yew DT. Changes of brain activity in the aged SAMP mouse. Biogerontology. 2007;8:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Chan WM, Xu J, Fan M, Jiang Y, Tsui TY, Wai MS, Lam WP, Yew DT. Downregulation in the human and mice cerebella after ketamine versus ketamine plus ethanol treatment. Microsc Res Tech. 2012;75:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Wai MS, Rudd JA, Chan WY, Antonio GE, Yew DT. The effect of Ginkgo biloba on the cerebellum of aging SAMP mouse--a TUNEL, bcl-2, and fMRI study. Microsc Res Tech. 2007;70:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |