Peer-review started: June 15, 2016
First decision: July 29, 2016
Revised: September 18, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: January 28, 2017
Processing time: 217 Days and 15.7 Hours
To investigate the role of contrast enhanced ultrasound (CEUS) in evaluating patients with renal function impairment (RFI) showing: (1) acute renal failure (ARF) of suspicious vascular origin; or (2) suspicious renal lesions.
We retrospectively evaluated patients addressed to CEUS over an eight years period to rule-out vascular causes of ARF (first group of 50 subjects) or assess previously found suspicious renal lesions (second group of 41 subjects with acute or chronic RFI). After preliminary grey-scale and color Doppler investigation, each kidney was investigated individually with CEUS, using 1.2-2.4 mL of a sulfur hexafluoride-filled microbubble contrast agent. Image analysis was performed in consensus by two readers who reviewed digital clips of CEUS. We calculated the detection rate of vascular abnormalities in the first group and performed descriptive statistics of imaging findings for the second group.
In the first group, CEUS detected renal infarction or cortical ischemia in 18/50 patients (36%; 95%CI: 23.3-50.9) and 1/50 patients (2%; 95%CI: 0.1-12), respectively. The detection rate of infarction was significantly higher (P = 0.0002; McNemar test) compared to color Doppler ultrasonography (10%). No vascular causes of ARF were identified in the remaining 31/50 patients (62%). In the second group, CEUS detected 41 lesions on 39 patients, allowing differentiation between solid lesions (21/41; 51.2%) vs complex cysts (20/41; 48.8%), and properly addressing 15/39 patients to intervention when feasible based on clinical conditions (surgery and cryoablation in 13 and 2 cases, respectively). Cysts were categorized Bosniak II, IIF, III and IV in 8, 5, 4 and 3 cases, respectively. In the remaining two patients, CEUS found 1 pseudolesion and 1 subcapsular hematoma.
CEUS showed high detection rate of renal perfusion abnormalities in patients with ARF, influencing the management of patients with acute or chronic RFI and renal masses throughout their proper characterization.
Core tip: Imaging in patients with renal function impairment (RFI) is challenging because of well-known limitations of conventional color Doppler ultrasound or risks related to the use of contrast media on computed tomography and magnetic resonance imaging. Contrast-enhanced ultrasound is a safer imaging tool in patients with RFI, showing 36% detection rate of renal infarction in patients with acute renal failure of suspicious vascular origin, and the capability of characterizing renal lesions in order to address patients to most proper treatment.
- Citation: Girometti R, Stocca T, Serena E, Granata A, Bertolotto M. Impact of contrast-enhanced ultrasound in patients with renal function impairment. World J Radiol 2017; 9(1): 10-16
- URL: https://www.wjgnet.com/1949-8470/full/v9/i1/10.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i1.10
Despite technical improvements, imaging of patients with renal function impairment (RFI) is challenging. Contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) provide panoramic representation of the kidneys, perirenal spaces, and vessels, leading to high diagnostic accuracy. However, iodinated contrast agents are potentially harmful in patients with RFI because of the risk of contrast-induced nephropathy (CIN)[1]. Although risk for nephrogenic systemic fibrosis (NSF) has been better defined over the last years, concerns still exist for the use of gadolinium chelates in patients with RFI, given uncertainty in pathogenic mechanisms and/or potential additional side effects related to gadolinium accumulation in the brain[1-4]. In practice, it is recommended to consider alternative imaging modalities in patients at risk with the use of iodinated or gadolinium contrast media[1].
Color-Doppler ultrasound (US) is the first imaging modality in patients with RFI. It is widely used to rule-out obstruction or investigate renal vessels and parenchymal abnormalities without the use of nephrotoxic agents[5]. In addition, US permits the detection of incidental, otherwise unknown renal lesions. However, there are well-known limitations of conventional Doppler modes in evaluating these patients, including difficult detection of perfusion abnormalities in globally hypoperfused kidneys, and unreliable characterization of renal masses other than simple cysts[5-8]. In particular, conventional Doppler modes do not allow differentiation between hypovascular tumors and complicated cysts[9,10], both of common occurrence in patients with RFI, nor can reliably assess the risk of malignancy of complex cystic masses.
Contrast-enhanced ultrasound (CEUS) has been advocated as the imaging modality of choice to evaluate patients with RFI, given the absence of nephrotoxicity and the ability of representing renal vascularization with excellent sensitivity and high spatial resolution[9,11]. According to the European federation of societies for ultrasound in medicine and biology (EFSUMB) guidelines, imaging with CEUS should be considered in every patient with RFI, when able to provide the clinically necessary information[1]. CEUS has the potential to compensate for limitations of conventional Doppler modes with a diagnostic performance comparable or superior to CT in the detection of perfusion abnormalities, lesion characterization (cystic vs solid), and categorization of cysts according to Bosniak criteria[10,12-14]. To our knowledge, however, evidence supporting the above indications results from reports on patients with normal renal function and experts opinion rather than specifically addressed studies, which currently lack.
The purpose of this study was to investigate the role of CEUS in a population of patients with RFI to assess the cause of renal function deterioration when perfusion abnormalities were clinically suspected or characterize renal lesions.
Referring institutional review board approved this study and waived for informed consent acquisition due to the retrospective design, in accordance with regulations of our country. By performing a computer search, we identified all patients with RFI who underwent renal CEUS over an 8-years period (January 2004-August 2012) to assess the cause of renal function deterioration, or to attempt characterization of renal masses. Patients of the first group were investigated to rule-out a vascular cause for renal function deterioration. They were patients with risk factors for renal infarction manifesting a rapid decline of the estimated glomerular filtration rate (eGFR). In this group, conventional Doppler modes were used to investigate the renal arteries and parenchymal vessels, while CEUS was subsequently performed to rule-out infarcted areas not identified with conventional modes. Patients of the second group had renal masses identified on previous conventional US or unenhanced CT. All of them showed eGFR < 60 mL/min per 1.73 m2 estimated from the serum creatinine values using the CKD-EPI equation[15]. Renal impairment was scored according to the grades of the National Kidney Foundation[16]. We assessed RFI according to the Kidney disease improving global outcomes (KDIGO) criteria for both acute renal failure (ARF)[17] and chronic renal failure (CRF)[18].
A total of 91 patients were enrolled (64 men, 27 women; age range 40-88 years; mean age 71.4 ± 11.02 years), showing renal impairment ranging from grade 3 to grade 5. Indications to CEUS were: (1) assessment of renal function deterioration in 50/91 patients; and (2) characterization of focal renal lesions in the remaining 41/91 patients.
CEUS was performed using different ultrasound equipment and contrast-specific modes (Table 1). After preliminary grey-scale and color Doppler investigation, CEUS examination was set with low acoustic power to achieve minimum microbubble destruction (mechanical index between 0.06 and 0.2, depending on the equipment used). Each kidney was evaluated separately after i.v. injection of a 1.2-2.4 mL dose of a sulfur hexafluoride-filled microbubble contrast agent (SonoVue, BR1, Bracco, Milan, Italy). A 20-gauge cannula was used for contrast injection, followed by a 10 mL normal saline flush. Digital cine-clips were acquired to allow for post-procedure re-evaluation.
| Ultrasound equipment | Contrast-specific mode | No. of patients |
| MyLab-70 (EsaOte) | CnTI™ (contrast tuned imaging) | 7 |
| ATL HDI5000 (Philips) | PIHI™ (pulse inversion harmonic imaging) | 15 |
| Sequoia 512 (Acuson Siemens) | CPS™ (contrast pulse sequencing) | 54 |
| iU22 (Philips) | PIHI-PM™ (pulse inversion harmonic imaging – power modulation) | 15 |
One radiologist with 18 years of experience in CEUS performed all the examinations. For the purpose of the study, he and a junior radiologist with five years of experience in this technique reviewed in consensus the images of conventional Doppler modes and the cine-clips of entire CEUS examinations, using a commercially available display workstation (OsiriX MD v.7.5, Pixmeo, Bernex, CH). Readers were blinded to histological diagnosis and/or follow-up results. No discrepancies were found between image interpretation at the time of examinations and during study review. Readers were asked to assess the presence of renal infarctions, characterize renal lesions as solid or cystic, and classify those with cystic appearance at CEUS according to the Bosniak criteria.
Readers assessed renal infarction on conventional Doppler modes in presence of parenchymal regions lacking color signal[19]. Concerning CEUS, we used the following diagnostic criteria: (1) infarction was diagnosed in presence of at least one well-defined, wedge-shaped non-enhancing area within an otherwise normal-appearing kidney[12]; (2) cortical ischemia was diagnosed in presence of enhancing interlobar and arcuate arteries with non-enhancing portions of the cortex[11]; and (3) a lesion was considered solid if more than half of the volume was represented by enhancing solid tissue, and cystic if composed predominantly of nonenhancing spaces[10,20]. Cystic lesions were graded according to the Bosniak criteria as previously described[13,14].
For the group of patients investigated to rule-out a vascular cause for renal function deterioration we calculated the detection rate of vascular abnormalities [(number of positive cases/total number of cases) × 100]. Analysis was performed on a per-patient basis by identifying at least one area of renal involvement. Significance of the difference between techniques in the detection rate of renal infarction was assessed with the McNemar test, using a reference alfa level of 0.01. Analysis was performed with a commercially available software (MedCalc v9.1, Mariakerke, Belgium).
For the group of patients with suspicious renal lesions we performed descriptive statistics of CEUS findings on a per-lesion basis.
Of fifty patients, 38 were males and 12 females (mean age: 71 ± 9 years, range 40-88 years). They presented with ARF either in previously well-functioning kidneys (31/50, 62%; 95%CI: 47.2-75.0), or complicating an already known CRF (19/50, 38%; 95%CI: 25.0-32.8). Causes of ARF were established based on clinical history and imaging follow-up in 44/50 cases (88%), renal biopsy in 3/50 cases (6.0%) and autopsy in the remaining 3/50 cases (6.0%).
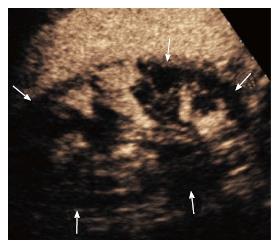
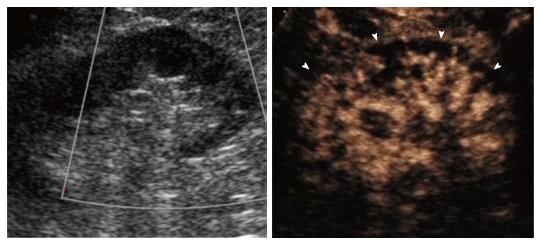
Renal infarction was found in 18/50 patients (36%; 95%CI: 23.3-50.9) using CEUS and 5/50 (10%; 95%CI: 3.7-22.6) using color Doppler US, corresponding to a significant difference in detection rate (P = 0.0002). In particular, CEUS found infarction in 13 additional subjects compared to color Doppler US (Figure 1). Moreover, CEUS identified acute cortical necrosis in one patient (2%; 95%CI: 0.1-12.0) presenting with non-specific hypoperfusion of the kidneys at color Doppler interrogation (Figure 2).
In the remaining 31/50 patients (62%; 95%CI: 47.2-75.0), there was no evidence of vascular abnormalities both on color Doppler US and CEUS. Final presumptive diagnosis was reached in 20/31 patients based on clinical and laboratory features, course of the disease and kidney biopsy in three subjects (two with interstitial nephritis and one with atheroembolic renal disease, respectively). Other three patients with atheroembolic renal disease had positive skin biopsy. In the remaining 12 patients, the cause of renal function deterioration remained undetermined. CEUS findings, pre-existing renal function and final diagnosis are reported in Table 2.
| Findings on CEUS | Side of CEUS findings | Pre-existing renal function | Cause of acute renal failure | No. of patients with biopsy |
| Renal infarction (n = 18) | Unilateral (n = 13) Bilateral (n = 5) | Chronic RFI (n = 10) No previous history of RFI (n = 8) | Suspicious embolization (n = 2) Placement of aortic endoprothesis (n = 5) Aortic dissection (n = 2) Ischemia (n = 3) Drug-induced nephrotoxicity (n = 1) Undetermined (n = 5) | None |
| Acute cortical necrosis pattern (n = 1) | Bilateral (n = 1) | No previous history of RFI (n = 1) | Post-surgical, hypovolemic acute tubular necrosis (n = 1) | None |
| No vascular abnormalities (n = 31) | None | Chronic RFI (n = 21) No previous history of RFI (n = 10) | Atheroembolic disease (n = 10) Acute pyelonephritis (n = 4) Interstitial nephritis (n = 2) Acute papillary necrosis (n = 1) Antiblastic drug-induced (n = 1) Dehydration (n = 1) Undetermined (n = 12) | 1 kidney biopsy, 3 skin biopsy 2 kidney biopsy |
Of 41 patients included in this group, 26 were male and 15 female (mean age: 70 ± 14 years, range 41-90 years). CEUS showed a total of 41 lesions in 39 patients.
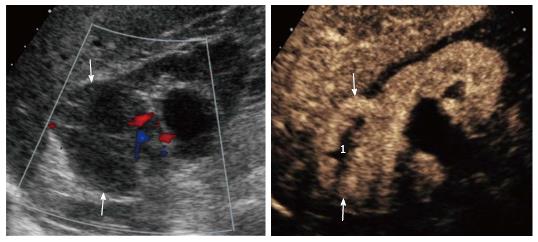
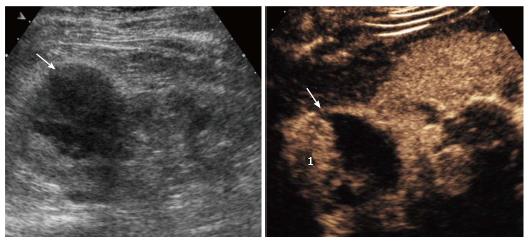
Twenty-one/41 lesions were solid in nature (51.2%; 95%CI: 35.4-66.8), whereas 20/41 lesions were assessed as complex cysts (48.8%; 95%CI: 33.2-64.6). Twelve out of 21 solid lesions were removed surgically, with final diagnosis of renal cancer, including 11 clear cell carcinomas (Figure 3) and 1 urothelial carcinoma. The remaining lesions included one oncocytoma diagnosed on autopsy, 7 indeterminate lesions addressed to imaging follow-up because of patients’ age and comorbidities contraindicating surgery, and one inoperable lesion addressed to angiographic embolization because of acute intratumoral hemorrhage.
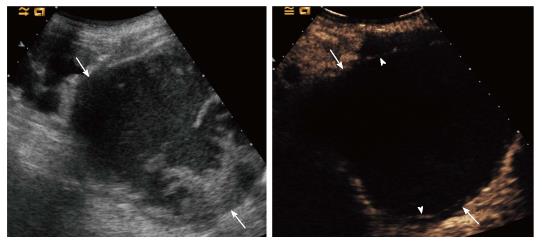
Cysts were classified according to Bosniak categories II, IIF, III and IV in 8, 5, 4 and 3 cases, respectively. All category IIF lesions, 1/4 category III and 1/3 category IV cysts remained stable over a 3-years imaging follow-up (Figure 4). Two category III cysts were a papillary and a clear cell renal cell carcinoma (RCC) on biopsy performed before percutaneous cryoablation. One category IV lesion was a clear cell RCC at nephrectomy (Figure 5). The remaining two patients (one with category III, one with category IV cysts) were not operated because of clinically relevant comorbidities. Lesions increased in size and complexity over time and were considered presumably malignant. The remaining two patients with suspicious renal tumor on conventional US had a pseudotumour and a subcapsular hematoma at CEUS, respectively.
Current EFSUMB guidelines recommend use of CEUS in patients with RFI[1]. Indeed, this technique can be performed during the same examination session of color Doppler US, thus acting as first-line and problem-solving imaging modality at the same time[9,11].
However, indication to CEUS in this scenario is based more on theoretical considerations and experts opinion than on results of validation studies. Indeed, the ability of CEUS to identify renal infarction and to characterize complex cystic masses, pseudolesions, and hypovascular lesions has been mostly demonstrated in patients with well-functioning kidneys[10,12,13,21]. To our knowledge, no specific studies focused on patients with renal failure. Moreover, there is lack of evidence on whether information obtained with CEUS in patients with RFI has a clinical impact for patient management.
Our results on a consecutive series of patients with renal failure investigated with CEUS show that this technique is effective in identifying renal infarction and characterizing renal masses. When a vascular cause for the deterioration of the renal function was suspected, CEUS either confirmed the diagnosis or, when negative, prompted further clinical workup and eventually identification of other causes of renal function deterioration. CEUS clearly outperformed US with color Doppler, with a significantly higher detection rate of renal infarction (36% vs 10%) (P = 0.0002). Moreover, CEUS was able to differentiate between renal infarctions and cortical ischemia, which showed no definite correspondence on color Doppler US. Therefore, CEUS proved to be effective as problem solving technique in these patients, with the advantage of avoiding radiation exposure or the use of nephrotoxic contrast agents. When renal lesions were identified in patients with renal failure, CEUS was able to discriminate between solid and cystic ones, as well as to categorize cysts according to the Bosniak criteria. Characterizing lesions with indeterminate appearance on conventional US modes as a presumably benign cysts prevented unnecessary operations in patients with renal failure (usually poor surgical candidates with high risk of complications), and further deterioration of the renal function.
Though cost-effectiveness analysis was beyond the purpose of this study, one can reasonably assume that evaluating the above patients with CEUS would led to prompt diagnosis and treatment while minimizing patients’ risk and costs compared to conventional diagnostic strategy combining US and CT/MRI (including related complications). One might argue that the use of CEUS is often limited to experienced centers, and no randomized controlled trials support the above statements. However, the experience acquired in reference centres and guidelines recommendations (e.g., EFSUMB ones) are now promoting an ever-increasing and widespread use of CEUS, as exemplified by extended indications to paediatric population[1]. We also believe that our study results might contribute as a reference for the planning of future studies designed to obtain high-level evidence in large populations with normal or impaired renal function. Potential effectiveness of CEUS diagnosis is further emphasized by the fact that this technique can be performed at patients’ bedside, which is of special advantage for critically ill ones.
This study has several limitations. The most important one is that the gold standard investigation has not been obtained for the majority of patients with renal function deterioration. Because of the concern for further deterioration of renal function, no additional imaging modalities were performed when the results of CEUS and clinical features were found sufficient for patient’s work-up. Only a limited number of patients with suspicious perfusion abnormalities had kidney biopsy (3/50 patients). As a consequence, causative diagnosis for renal function deterioration remained indeterminate in 5 patients with CEUS findings suggestive for renal infarction and 12 patients without CEUS evidence of vascular abnormalities. Gold standard investigation was not available also for 25/41 renal masses (8/21 solid lesions and 17/20 cysts), whereas 12 patients with presumably malignant renal lesions (7 solid indeterminate lesions, 1 inoperable solid lesion and 4 Bosniak III-IV cysts) were not operated because the surgical risk was considered excessive due to comorbidities. However, all operated or ablated lesions (n = 15) were found to have cancer at histological examination, thus emphasizing the effectiveness of CEUS in guiding most proper treatment.
Another major limitation of the present study is its retrospective design. This might have introduced a case-selection bias, because some cases may have not been recorded for inclusion. Additionally, a relatively small number of lesions have been evaluated, reflecting the fact that CEUS has been performed as a problem solving technique to assess very specific diagnostic questions.
Finally, in our series CEUS failed to detect perfusion abnormalities in patients with atheroembolic renal disease. We can only speculate on the anatomic basis for this finding: atheroembolic renal disease consists in patchy embolization of very small arteries (interlobular and afferent arterioles) by cholesterol crystals resulting in cortical ischemic areas which are likely too small to be detected with imaging methods.
In conclusion, our study shows that CEUS has a significant role as a problem-solving technique for detection of perfusion abnormalities and characterization of renal lesions in patients with renal failure. CEUS can be performed in emergency at the bedside. In our series, it was helpful in stratifying treatment decisions, as shown by the fact that all patients with suspicious renal cancer in whom surgery was not contraindicated were operated properly.
The authors thank Dr. Iliana Bednarova from the Institute of Radiology, University of Udine, for her help in revising English language.
Imaging in patients with acute or chronic renal function impairment (RFI) is challenging because of nephrotoxicity or the risk for nephrogenic systemic fibrosis (NSF) related to the use of computed tomography (CT) and magnetic resonance imaging contrast agents, respectively. Contrast-enhanced ultrasound (CEUS) is gaining widespread acceptance as the imaging modality of choice to evaluate patients with RFI, given the absence nephrotoxicity and the ability of representing renal vascularization with excellent sensitivity and high spatial resolution. However, the consensus on its use in this setting is related more to experts’ opinion and clinical practice than specifically addressed studies. Hence, evidence on this topic still lacks as a basis to support clinical practice and future trials.
CEUS has a pivotal role in assessing patients with RFI. However, despite the use of CEUS in this scenario, there is paucity of scientific evidence supporting it. The results of the study show that CEUS has a significant impact in managing patients with RFI and might contribute to strengthen the recommendation to use it as the imaging method of choice in this setting.
The authors provided an evidence-based background for supporting the use of CEUS in patients with RFI. CEUS is safer than contrast-enhanced computed tomography and/or magnetic resonance imaging in evaluating patients with RFI. This technique can be performed on patients’ bedside, thus allowing prompt diagnosis and management.
Their study shows that CEUS is a problem-solving technique in detecting perfusion abnormalities and characterizing renal lesions in patients with renal failure.
CEUS: An ultrasound technique using microbubble contrast agents.
This is a well-written paper.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Abou El-Ghar M, Markic D, Salvi PF, Scarpioni R, Watanabe T S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 674] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 2. | Thomsen HS. Nephrogenic systemic fibrosis: A serious late adverse reaction to gadodiamide. Eur Radiol. 2006;16:2619-2621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Thomsen HS, Morcos SK, Almén T, Bellin MF, Bertolotto M, Bongartz G, Clement O, Leander P, Heinz-Peer G, Reimer P. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2013;23:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 4. | Stojanov D, Aracki-Trenkic A, Benedeto-Stojanov D. Gadolinium deposition within the dentate nucleus and globus pallidus after repeated administrations of gadolinium-based contrast agents-current status. Neuroradiology. 2016;58:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Pozzi Mucelli R, Bertolotto M, Quaia E. Imaging techniques in acute renal failure. Contrib Nephrol. 2001;132:76-91. [PubMed] |
| 6. | Kim SH, Cho JY, Kim SY, Moon KC, Kwak C, Kim HH. Ultrasound Evaluation of Renal Masses: Gray-scale, Doppler, and More. Ultrasound Clinics. 2013;8:565-579. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Cantisani V, Bertolotto M, Weskott HP, Romanini L, Grazhdani H, Passamonti M, Drudi FM, Malpassini F, Isidori A, Meloni FM. Growing indications for CEUS: The kidney, testis, lymph nodes, thyroid, prostate, and small bowel. Eur J Radiol. 2015;84:1675-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Pozzi Mucelli R, Bertolotto M. Imaging techniques in acute renal failure. Kidney Int. 1998;66:S102-S105. |
| 9. | Bertolotto M, Derchi LE, Cicero C, Iannelli M. Renal Masses as Characterized by Ultrasound Contrast. Ultrasound Clin. 2013;8:581-592. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Bertolotto M, Cicero C, Perrone R, Degrassi F, Cacciato F, Cova MA. Renal Masses With Equivocal Enhancement at CT: Characterization With Contrast-Enhanced Ultrasound. AJR Am J Roentgenol. 2015;204:W557-W565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Granata A, Zanoli L, Insalaco M, Valentino M, Pavlica P, Di Nicolò PP, Scuderi M, Fiorini F, Fatuzzo P, Bertolotto M. Contrast-enhanced ultrasound (CEUS) in nephrology: Has the time come for its widespread use? Clin Exp Nephrol. 2015;19:606-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Bertolotto M, Martegani A, Aiani L, Zappetti R, Cernic S, Cova MA. Value of contrast-enhanced ultrasonography for detecting renal infarcts proven by contrast enhanced CT. A feasibility study. Eur Radiol. 2008;18:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Ascenti G, Mazziotti S, Zimbaro G, Settineri N, Magno C, Melloni D, Caruso R, Scribano E. Complex cystic renal masses: characterization with contrast-enhanced US. Radiology. 2007;243:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Quaia E, Bertolotto M, Cioffi V, Rossi A, Baratella E, Pizzolato R, Cov MA. Comparison of contrast-enhanced sonography with unenhanced sonography and contrast-enhanced CT in the diagnosis of malignancy in complex cystic renal masses. AJR Am J Roentgenol. 2008;191:1239-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [PubMed] |
| 16. | Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2145] [Cited by in RCA: 2517] [Article Influence: 125.9] [Reference Citation Analysis (0)] |
| 17. | KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements. 2012;2:8-12. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 18. | Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3156] [Cited by in RCA: 3210] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 19. | Hélénon O, el Rody F, Correas JM, Melki P, Chauveau D, Chrétien Y, Moreau JF. Color Doppler US of renovascular disease in native kidneys. Radiographics. 1995;15:833-854; discussion 854-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Tamai H, Takiguchi Y, Oka M, Shingaki N, Enomoto S, Shiraki T, Furuta M, Inoue I, Iguchi M, Yanaoka K. Contrast-enhanced ultrasonography in the diagnosis of solid renal tumors. J Ultrasound Med. 2005;24:1635-1640. [PubMed] |
| 21. | Mazziotti S, Zimbaro F, Pandolfo A, Racchiusa S, Settineri N, Ascenti G. Usefulness of contrast-enhanced ultrasonography in the diagnosis of renal pseudotumors. Abdom Imaging. 2010;35:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |