Published online Aug 28, 2016. doi: 10.4329/wjr.v8.i8.757
Peer-review started: December 18, 2015
First decision: February 29, 2016
Revised: May 14, 2016
Accepted: June 14, 2016
Article in press: June 16, 2016
Published online: August 28, 2016
Processing time: 254 Days and 18.8 Hours
Erdheim-Chester disease (ECD) is an uncommon, non-familial, non-Langerhans cell histiocytosis, which involves skeletal system and soft tissue usually in middle aged and elderly patients. The characteristic radiologic features include bilateral, symmetric cortical osteosclerosis of the diaphyseal and metaphyseal parts of the long bones, or bilateral symmetrically abnormal intense 99mTechnetium labelling of the metaphyseal-diaphyseal region of the long bones, and computed tomography scan findings of “coated aorta” or “hairy kidneys”. ECD in childhood with osteolytic lesion is extremely rare. We describe an unusual case with an expansile lytic bone lesion at presentation in a case of acute lymphoblastic leukemia.
Core tip: Erdheim-Chester disease (ECD) is characterised by bilateral, symmetric cortical osteosclerosis of the diaphyseal and metaphyseal parts of the long bones. Occurrence of osteolytic lesions and presentation in childhood are extremely rare. We describe an unusual case of ECD with an expansile lytic bone lesion in a case of acute lymphoblastic leukemia.
- Citation: Vallonthaiel AG, Mridha AR, Gamanagatti S, Jana M, Sharma MC, Khan SA, Bakhshi S. Unusual presentation of Erdheim-Chester disease in a child with acute lymphoblastic leukemia. World J Radiol 2016; 8(8): 757-763
- URL: https://www.wjgnet.com/1949-8470/full/v8/i8/757.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i8.757
Erdheim-Chester disease (ECD) results from excessive proliferation of CD68-positive and CD1a-negative foamy histiocytes and “lipid-laden” macrophages in different organs and tissues. It was first described in 1930 as “lipid granulomatosis” by Erdheim and Chester[1]. More than 550 cases have been described[2] and most are in middle aged and elderly patients. Bone is most commonly affected. The pathognomonic radiologic features are bilateral, symmetric cortical osteosclerosis of the diaphyseal and metaphyseal regions of the long bones, symmetric bilateral abnormally intense 99mTechnetium (Tc) labelling of the distal ends of the long bones, and, “coated aorta” and “hairy kidneys” which are seen on computed tomography (CT) scan due to soft-tissue sheathing of aorta and infiltration of the perirenal fat by the histiocytic infiltrate respectively[3,4]. Occurrence in childhood and presence of osteolytic lesions are uncommon[5,6]. Only a single case with multiple osteolytic lesions in a child with acute lymphoblastic lymphoma (ALL) has been reported[5]. But an expansile osteolytic lesion as the presenting clinical feature is unique to our case and has not been reported in the literature.
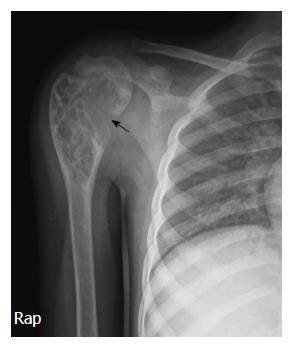
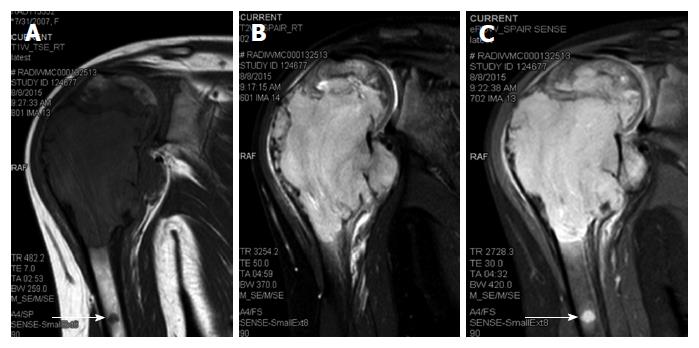
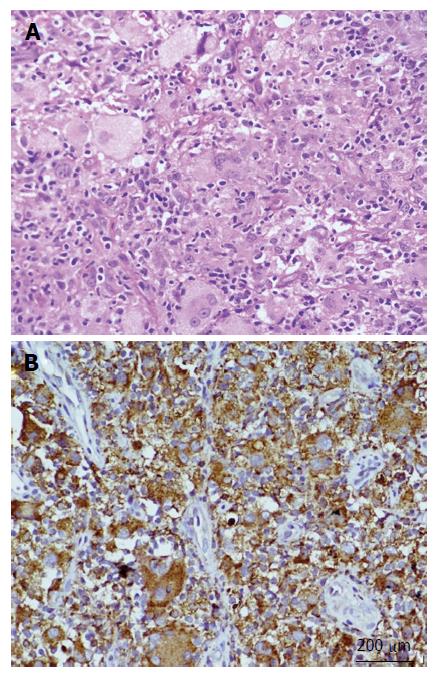
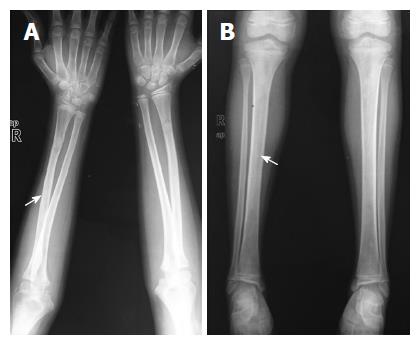
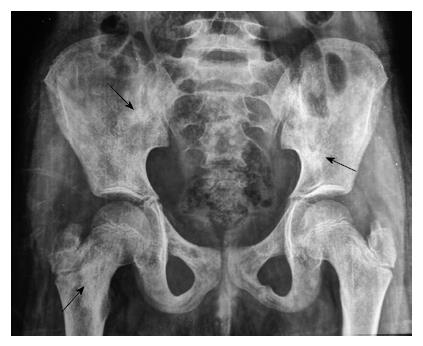
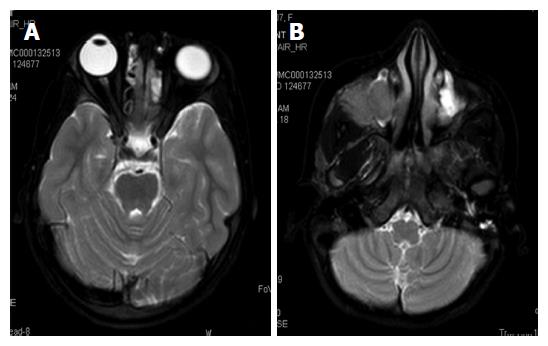
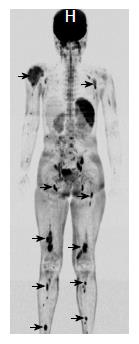
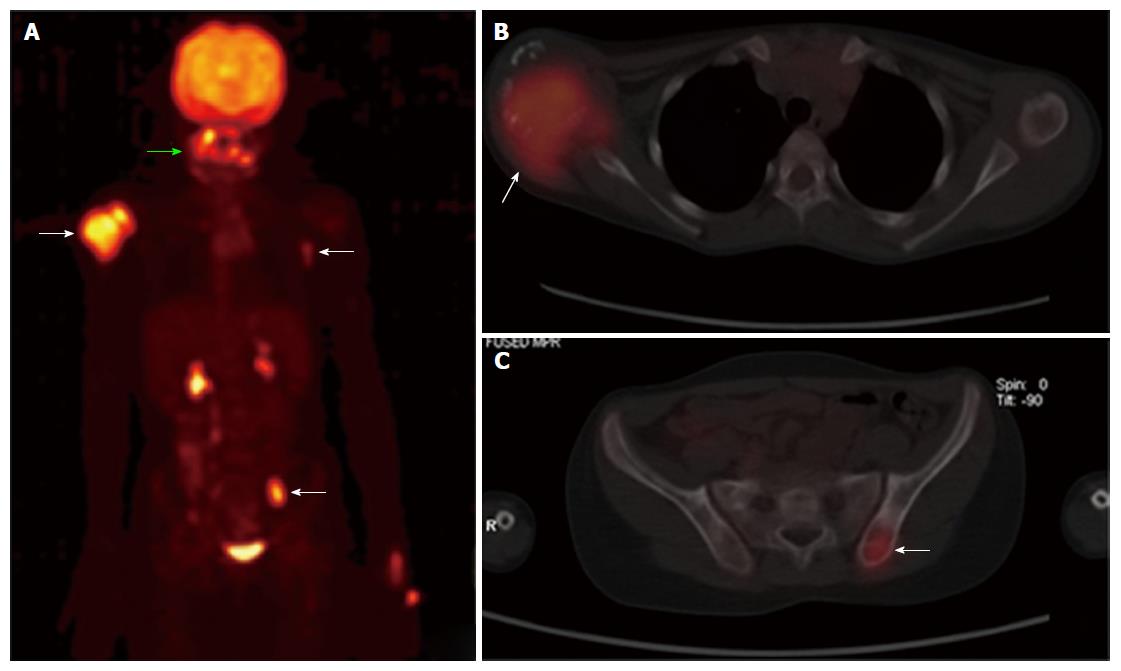
A 6-year-old female was diagnosed with B-cell ALL and treated as per International Network for Cancer Treatment and Research programs protocol. Complete clinical and haematological remission was achieved after induction therapy. Two years later, during maintenance therapy, she complained of pain in the right arm. Physical examination revealed a tender swelling in the right upper arm; while other systems were unremarkable. An X-ray of right arm revealed a multiseptated, expansile, lytic lesion with narrow zone of transition in the metaphysis of upper end of the right humerus with cortical discontinuity on medial aspect (arrow). No specific matrix mineralization or periosteal reaction was noted (Figure 1). Magnetic resonance imaging (MRI) of the right shoulder showed a solid expansile intramedullary mass replacing the normal marrow fat. It was hypointense on T1-weighted images, and hyperintense on T2-weighted images. There was a cortical break in medial upper humeral diaphysis with extension into soft tissue. A skip lesion in the humeral shaft with similar signal characteristics was also noted (white arrows) (Figure 2). A biopsy from the lesion showed fragmented bony trabeculae with widening of intertrabecular spaces by an infiltrate comprised of foamy histiocytic cells, lipid laden histiocytes, a few multinucleated giant cells, lymphocytes and fibroblastic cells (Figure 3A). No emperipolesis, mitosis or necrosis was seen. Histiocytic cells and giant cells were immunopositive with CD68 (1:50) (Novocastra, United Kingdom) (Figure 3B) and negative for CD1a (1:100) (Thermo Scientific, United States), Langerin (1:200) (Novocastra, United Kingdom), S100 (1:800) (Dako, United States), and CD23 (1:100) (Spring Bioscience, United States). Microscopic features were those of ECD. Subsequent skeletal survey revealed symmetric bilateral osteosclerosis of the metaphysis and diaphysis of both the long bones of the forearms and legs (arrows) (Figure 4) as well as patchy osteosclerosis involving pelvic bones and proximal femoral shafts (arrows) (Figure 5). Whole body MRI was performed in a 1.5 T MRI scanner (Acheiva 1.5 T, Philips, The Netherlands) and coronal whole body soft tissue inversion recovery sequence was acquired, followed by detailed evaluation of the foci of abnormality, using axial and coronal T2-weighted and T2-weighted turbo spin echo fat-suppressed sequences. Coronal whole body diffusion weighted imaging with background suppression imaging were performed using b-value of 0 and 400. The whole body MRI showed presence of bilateral ethmoid and maxillary sinusitis along with replacement of normal marrow by focal lesions involving maxilla (Figure 6), mandible, right humerus (involving epi-meta and proximal diaphysis), left scapula, head of left humerus, dorsal vertebra, sacrum, bilateral femoral and tibial diaphyses (Figure 7). Positron emission tomography-computed tomography (PET-CT) acquisition was done 45 to 60 min after injection of 10 mCi 18fludeoxyglucose (18FDG) by intravenous route from level of orbits to mid-thigh. PET-CT revealed increased tracer uptake in bilateral maxilla, head of the right humerus, and bilateral pubic and iliac bones (Figure 8) along with ground glass appearance in bilateral lungs. MR and PET-CT scans of the brain were normal. Besides mild pain and swelling in the right shoulder, the patient is otherwise doing well. She has been given conservative treatment with anti-inflammatory drug and kept under close follow up.
ECD is a primary histiocytic disorder with unknown aetiology and usually occurs during fifth to seventh decades of life with a slight male predominance[7]. It can involve multiple organ systems or any tissue of the body; however, bone is most frequently (96%) affected with a predilection for the femur, tibia and fibula[8]. Involvement of the humerus and axial skeleton is uncommon[9]. Soft tissue lesions are generally seen in the heart with endocardial, myocardial and pericardial involvement, coronaries, large vessels, lung parenchyma, pleura, retroperitoneum with or without ureteral obstruction and hydronephrosis, adrenal glands, retro-orbital region, maxillary sinus, central nervous system including intra- and extra-axial compartments, skin, etc.[2]. At least one soft tissue involvement is present in more than 50% of patients[8,10]. The clinical presentation depends upon the organ of involvement and the common manifestations are bone pain (in 50% of cases)[3] diabetes insipidus, proptosis, renal, cardiovascular, central nervous system and retroperitoneal involvement[7,10]. The classic triad of ECD includes bone pain, diabetes insipidus and painless bilateral exophthalmos. The commonest cutaneous manifestation is xanthelasma. Some authors have categorized ECD as central nervous system, cardiac, vascular, endocrine, retroperitoneal, pulmonary dominant and multisystem type depending on the organ/tissue involvement[2]. ECD is rare in children and only seven cases have been reported[3]. Though clinical features are like that of adult patients; no cardiovascular involvement has been reported in children[5,6]. Localized pain and swelling in the right shoulder were the clinical presentation in our case.
Bone is most frequently (96% of cases) affected in ECD and the typical radiographic features include bilateral symmetric osteosclerosis of the metadiaphyseal region of long bones. Lytic lesions are infrequent and occur in < 10% of cases[5,7]. An expansive lytic lesion involving the epi-, meta- and proximal diaphysis of humerus, which was the presenting clinical feature in our case, has not been described earlier. Even though the exact pathogenesis needs to be elucidated, the lytic lesions may be due to localised increase in osteoclastic activity or reduced host bone response to the lesion. However, subsequent skeletal survey revealed typical features of ECD in the bilateral long bones of upper and lower extremities as well as osteosclerotic lesions in the pelvic bones and the femur. Epiphyseal and subperiosteal lesions are rare[9] and better visualized by MRI than plain radiograph or CT[3,8]. The classical CT scan findings of ECD are “coated aorta” and “hairy kidneys” which are seen in 23% and 68% cases respectively[3]. In the present case besides right humerus lesions, MRI also revealed focal lesions in the maxilla, mandible, left scapula, head of the left humerus, dorsal vertebrae, sacrum, bilateral femoral and tibial diaphysis, and right maxilla.
99mTc bone scintigraphy demonstrates bilateral, symmetric, abnormal labelling in the metaphyseal-diaphyseal parts of long bones especially in the lower limbs[8]. FDG PET-CT scan can assess extent of involvement including extraskeletal components, activity and progression of disease, and help monitoring of therapy[11] (Table 1). In addition to above bone and soft tissue lesions, the PET-CT scan in our case also showed mild bilateral lung parenchymal involvement which was also described by Wittenberg et al[12]. Lung lesions mainly affect the interlobular septae and are not associated with a significant prognostic factor for ECD[3].
| Age | Middle aged and elderly patients predominantly affected |
| Site | Any tissue or organ can be affected and clinical manifestations depend upon the organ of involvement. Bone is most frequently affected (> 90%), however, at least one soft tissue component is seen in more than 50% of patients |
| Pathophysiology | Shows polyclonal proliferation of histiocytes associated with abnormal Th1 immune response. The recent studies however have suggested a clonal origin by demonstrating BRAFV600E mutations in more than 50% cases |
| Diagnostic criteria (Radiologic) | Bilateral, symmetric cortical osteosclerosis of the diaphyseal and metaphyseal regions of the long bones |
| Symmetric bilateral abnormally intense Tc labelling of the distal ends of the long bones | |
| (Histopathologic) | Characteristic “coated aorta” or “hairy kidneys” on CT scan; Xanthogranulomatosis or polymorphic granuloma with foamy/lipid laden histiocytes with immunoreactivity to CD68, but negative for CD1a and Langerin |
| Treatment | IFN-α and pegylated IFN-α are preferred for the treatment |
| Anakinra (recombinant IL1R antagonist) and infliximab (anti-TNF-α antibody) may be used for second-line treatment | |
| Vemurafenib (an inhibitor of BRAF) especially for the patients with severe and refractory BRAFV600E histiocytoses | |
| Follow-up (with PET-CT) | Useful for assessing extent of involvement both skeletal and extraskeletal components, activity and progression of disease, and monitoring of therapy |
Radiologic differential diagnoses of ECD are renal osteodystrophy, Pagets disease, myelofibrosis, osteoblastic metastases, chronic osteomyelitis, metabolic bone disorders[8,13] and in children with lytic lesion differentials include Langerhans cell histiocytosis (LCH), infection and metastatic neuroblastoma[5]. Imaging findings are typical and pathognomonic for ECD in most of the cases. However, histopathologic examination is required to confirm the diagnosis and exclude the other possibilities and coexisting diseases such as LCH and Rosai-Dorfman disease (RDD).
Microscopic features of ECD include foamy histiocytic infiltrate with lipid laden macrophages/histiocytes, fibroblastic proliferation, lymphocytic infiltrate, granuloma formation with or without Touton giant cells and fibrosis[8]. LCH and RDD can rarely coexist either alone or in combination with ECD. Histiocytes of ECD are immunoreactive to CD68, CD163 and Factor XIIIa; while, negative for CD1a and langerin, which are positive in LCH. In our case, histiocytes were negative for S100, CD1a and langerin. Absence of emperipolesis in our case also ruled out the possibility of RDD (Table 2). The association of ALL and ECD, similar to our case, has been reported only in a single child; but she had osteolytic lesions in multiple long bones and skull bones without any osteosclerosis[5].
| Radiologic differential diagnoses | |
| With osteosclerotic lesions | Renal osteodystrophy, Pagets disease, myelofibrosis, osteoblastic metastases, chronic osteomyelitis, metabolic bone disorders |
| With osteolytic lesions in children | Langerhans cell histiocytosis, infection, metastatic neuroblastoma |
| Histologic | |
| Langerhans cell histiocytosis | Positive for S100, CD1a, and Langerin |
| Rosai-Dorfman disease | Histiocytes show emperipolesis; positive for S100 and CD68; negative for CD1a, and Langerin |
| Juvenile xanthogranuloma | Lack characteristic radiologic features of ECD; positive for factor XIIIa, CD68, CD163, fascin, and CD14, negative for CD1a, S100, and Langerin |
| Solitary/multicentric reticulohistiocytoma | Lack characteristic radiologic features of ECD; positive for factor XIIIa, CD68, CD163, fascin, and CD14; negative for CD1a, S100, and Langerin |
ECD was considered as non-clonal condition associated with abnormal Th1 immune response producing several proinflammatory cytokines like interferon-α, interleukin (IL)-1/IL1-RA, IL-6, IL-12 and monocyte chemotactic protein-1[2,8,14]. The recent studies, however, have demonstrated v-raf murine sarcoma viral oncogene homolog B1V600E (BRAFV600E) mutations in more than 50% cases, suggesting the clonal origin[2,3]. The origin of ECD is thought to be from CD34(+) myeloid stem cells, which also give rise to various haematolymphoid malignancies[8]. The association between ECD with other histiocytic disorders like Langerhans cell histiocytosis, Rosai-Dorfman disease and rare cases of haematologic malignancies like Hodgkin lymphoma[15] and ALL could be due to the origin from common precursor cells.
Current recommendations for evaluation of ECD are imaging studies including skeletal survey, CT scan of chest, abdomen, and pelvis, fluorine-18-fluorodeoxyglucose- positron emission tomography (FDG-PET) of the entire body including brain and distal extremities, MRI of the brain and the heart with gadolinium, histopathological examination and v-raf murine sarcoma viral oncogene homolog B1 (BRAF) mutation analysis[2]. Immunohistochemistry using the BRAFV600E mutant specific antibody (anti-BRAF V600E) has high sensitivity and specificity[16]. However, it was negative in our case.
Corticosteroids, cytotoxic chemotherapies, radiotherapy and surgery were the treatment modalities prior to the discovery of interferon (IFN)-α[2]. Currently, IFN-α and pegylated IFN-α are preferred for the treatment and are associated with improved survival[17]. Treatment should be continued indefinitely if tolerated. An attempt at treatment cessation may be done for individual cases with minimal disease burden[2]. Anakinra (recombinant IL1R antagonist) and infliximab [anti-tumor necrosis factor (TNF)-α antibody] reduce the inflammatory mediators and may be used for second-line treatment. A pilot study using vemurafenib, an inhibitor of BRAF harbouring the V600E mutation, has shown dramatic clinical improvement in a few patients[18]. Treatment with LCH-protocol therapies in children with ECD has proved to be unsuccessful. FDG-PET scan is recommended for monitoring and assessing treatment response, every 3 to 6 mo for all patients following the initiation of treatment, and the frequency can be reduced once the disease has stabilized[2,19]. No specific therapy has been given to our patient and she has been kept under close follow-up.
Prognosis of patients with ECD has been reported to be dismal[14] and is generally worse with cardiovascular and central nervous system involvement. Overall mean survival is 32 mo and majority of patients die within 3 years from renal, cardiovascular, pulmonary or central neurological complications[7]. Arnaud et al[17] have reported 1-year and 5-year survival rates to be 96% and 68% respectively. ECD is a rare disease and may occur in association with other neoplastic and non-neoplastic diseases with atypical clinical presentation. High degree of suspicion and proper evaluation are required for the diagnosis of the disease, proper management and prediction of prognosis.
A 6-year-old female who had achieved remission after treatment for B-cell acute lymphoblastic lymphoma (ALL), presented with pain in the right upper arm.
Physical examination revealed a tender swelling in the right upper arm, suspicious of primary bone neoplasm.
Langerhans cell histiocytosis.
All labs were within normal limits including bone marrow aspirate for blasts.
An X-ray of right arm revealed a multiseptated, expansile, lytic lesion with narrow zone of transition in the metaphysis of upper end of the right humerus with cortical discontinuity without specific matrix mineralization or periosteal reaction while magnetic resonance images of the right shoulder showed a solid expansile intramedullary mass replacing the normal marrow fat, suggestive of Langerhan cell histiocytosis (LCH).
Polymorphic population with foamy and lipid laden histiocytes, which are immunopositive with CD68, but negative for CD1a and Langerin, compatible with Erdheim-Chester disease (ECD).
Patient is kept on symptomatic treatment and a close follow-up.
ECD presenting in childhood, especially after ALL and with osteolytic lesions is extremely rare. Only one case with multiple pure osteolytic lesions after ALL has been reported in the literature.
ECD is a non-LCH characterised by bilateral symmetrical osteosclerosis of diaphyseal and metaphyseal region of long bones.
ECD may be mistaken for LCH which is the most common differential diagnosis of osteolytic lesions in childhood. High degree of suspicion and proper evaluation are required for an accurate diagnosis.
It is an excellent, well-written and documented work.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: India
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garcia-Gomez FJ, Matsushita K, Monti L S- Editor: Kong JX L- Editor: A E- Editor: Zhang FF
| 1. | Chester W. Uber lipoidgranulomatose. Virchows Arch A Pathol Anat Histol. 1930;279:561-602. [RCA] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 296] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Diamond EL, Dagna L, Hyman DM, Cavalli G, Janku F, Estrada-Veras J, Ferrarini M, Abdel-Wahab O, Heaney ML, Scheel PJ. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 422] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 3. | Haroche J, Arnaud L, Amoura Z. Erdheim-Chester disease. Curr Opin Rheumatol. 2012;24:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Serratrice J, Granel B, De Roux C, Pellissier JF, Swiader L, Bartoli JM, Disdier P, Weiller PJ. “Coated aorta”: a new sign of Erdheim-Chester disease. J Rheumatol. 2000;27:1550-1553. [PubMed] |
| 5. | Krishna VV, James TE, Chang KT, Yen SS. Erdheim-Chester disease with rare radiological features in a 14-year old girl with pre-B Acute Lymphocytic Leukemia and Diabetes Mellitus. J Radiol Case Rep. 2014;8:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Song SY, Lee SW, Ryu KH, Sung SH. Erdheim-Chester disease with multisystem involvement in a 4-year-old. Pediatr Radiol. 2012;42:632-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Veyssier-Belot C, Cacoub P, Caparros-Lefebvre D, Wechsler J, Brun B, Remy M, Wallaert B, Petit H, Grimaldi A, Wechsler B. Erdheim-Chester disease. Clinical and radiologic characteristics of 59 cases. Medicine (Baltimore). 1996;75:157-169. [PubMed] |
| 8. | Mazor RD, Manevich-Mazor M, Shoenfeld Y. Erdheim-Chester Disease: a comprehensive review of the literature. Orphanet J Rare Dis. 2013;8:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | Dion E, Graef C, Miquel A, Haroche J, Wechsler B, Amoura Z, Zeitoun D, Grenier PA, Piette JC, Laredo JD. Bone involvement in Erdheim-Chester disease: imaging findings including periostitis and partial epiphyseal involvement. Radiology. 2006;238:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Antunes C, Graça B, Donato P. Thoracic, abdominal and musculoskeletal involvement in Erdheim-Chester disease: CT, MR and PET imaging findings. Insights Imaging. 2014;5:473-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Arnaud L, Malek Z, Archambaud F, Kas A, Toledano D, Drier A, Zeitoun D, Cluzel P, Grenier PA, Chiras J. 18F-fluorodeoxyglucose-positron emission tomography scanning is more useful in followup than in the initial assessment of patients with Erdheim-Chester disease. Arthritis Rheum. 2009;60:3128-3138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Wittenberg KH, Swensen SJ, Myers JL. Pulmonary involvement with Erdheim-Chester disease: radiographic and CT findings. AJR Am J Roentgenol. 2000;174:1327-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Ihde LL, Forrester DM, Gottsegen CJ, Masih S, Patel DB, Vachon LA, White EA, Matcuk GR. Sclerosing bone dysplasias: review and differentiation from other causes of osteosclerosis. Radiographics. 2011;31:1865-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Al-Quran S, Reith J, Bradley J, Rimsza L. Erdheim-Chester disease: case report, PCR-based analysis of clonality, and review of literature. Mod Pathol. 2002;15:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Bulycheva EN, Baykov VV, Zaraĭskiĭ MI, Salogub GN. Rare form of erdheim-chester disease presenting with isolated central skeletal lesions treated with a combination of alfa-interferon and zoledronic Acid. Case Rep Hematol. 2015;2015:876752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Long GV, Wilmott JS, Capper D, Preusser M, Zhang YE, Thompson JF, Kefford RF, von Deimling A, Scolyer RA. Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am J Surg Pathol. 2013;37:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 17. | Arnaud L, Pierre I, Beigelman-Aubry C, Capron F, Brun AL, Rigolet A, Girerd X, Weber N, Piette JC, Grenier PA. Pulmonary involvement in Erdheim-Chester disease: a single-center study of thirty-four patients and a review of the literature. Arthritis Rheum. 2010;62:3504-3512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Haroche J, Cohen-Aubart F, Emile JF, Arnaud L, Maksud P, Charlotte F, Cluzel P, Drier A, Hervier B, Benameur N. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 387] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 19. | García-Gómez FJ, Acevedo-Báñez I, Martínez-Castillo R, Tirado-Hospital JL, Cuenca-Cuenca JI, Pachón-Garrudo VM, Álvarez-Pérez RM, García-Jiménez R, Rivas-Infante E, García-Morillo JS. The role of 18FDG, 18FDOPA PET/CT and 99mTc bone scintigraphy imaging in Erdheim-Chester disease. Eur J Radiol. 2015;84:1586-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |