Published online Jul 28, 2016. doi: 10.4329/wjr.v8.i7.707
Peer-review started: January 26, 2016
First decision: March 25, 2016
Revised: April 5, 2016
Accepted: April 14, 2016
Article in press: April 18, 2016
Published online: July 28, 2016
Processing time: 181 Days and 2.6 Hours
AIM: To compare breath-hold cartesian volumetric interpolated breath-hold examination (cVIBE) and free-breathing radial VIBE (rVIBE) and determine whether rVIBE could replace cVIBE in routine liver magnetic resonance imaging (MRI).
METHODS: In this prospective study, 15 consecutive patients scheduled for routine MRI of the abdomen underwent pre- and post-contrast breath-hold cVIBE imaging (19 s acquisition time) and free-breathing rVIBE imaging (111 s acquisition time) on a 1.5T Siemens scanner. Three radiologists with 2, 4, and 8 years post-fellowship experience in abdominal imaging evaluated all images. The radiologists were blinded to the sequence types, which were presented in a random order for each patient. For each sequence, the radiologists scored the cVIBE and rVIBE images for liver edge sharpness, hepatic vessel clarity, presence of artifacts, lesion conspicuity, fat saturation, and overall image quality using a five-point scale.
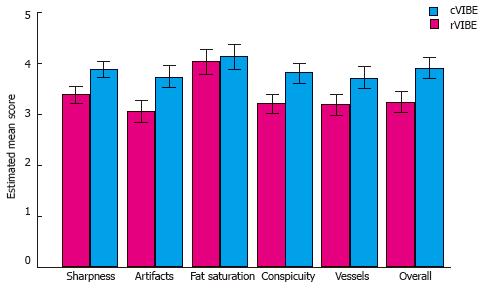
RESULTS: Compared to rVIBE, cVIBE yielded significantly (P < 0.001) higher scores for liver edge sharpness (mean score, 3.87 vs 3.37), hepatic-vessel clarity (3.71 vs 3.18), artifacts (3.74 vs 3.06), lesion conspicuity (3.81 vs 3.2), and overall image quality (3.91 vs 3.24). cVIBE and rVIBE did not significantly differ in quality of fat saturation (4.12 vs 4.03, P = 0.17). The inter-observer variability with respect to differences between rVIBE and cVIBE scores was close to zero compared to random error and inter-patient variation. Quality of rVIBE images was rated as acceptable for all parameters.
CONCLUSION: rVIBE cannot replace cVIBE in routine liver MRI. At 1.5T, free-breathing rVIBE yields acceptable, although slightly inferior image quality compared to breath-hold cVIBE.
Core tip: This is a prospective study comparing the image quality of the current standard of care T1 weighted magnetic resonance imaging (MRI) technique for liver MRI, the breath-hold cartesian volumetric interpolated breath-hold examination (cVIBE), with a new free-breathing imaging technique, radial VIBE (rVIBE), at 1.5T. Image quality of rVIBE is acceptable but slightly inferior compared to cVIBE.
- Citation: Yedururi S, Kang HC, Wei W, Wagner-Bartak NA, Marcal LP, Stafford RJ, Willis BJ, Szklaruk J. Free-breathing radial volumetric interpolated breath-hold examination vs breath-hold cartesian volumetric interpolated breath-hold examination magnetic resonance imaging of the liver at 1.5T. World J Radiol 2016; 8(7): 707-715
- URL: https://www.wjgnet.com/1949-8470/full/v8/i7/707.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i7.707
Conventional pre-contrast and dynamic post-contrast three-dimensional T1-weighted fat-suppressed gradient-echo volumetric interpolated breath-hold examination (VIBE) using cartesian sampling (cVIBE) plays a well-established role in magnetic resonance imaging (MRI) of the abdomen. However, the utility of this sequence depends on the ability of patients to hold their breath. Respiratory motion artifacts can cause significant blurring and severely limit the evaluation of abdominal viscera in patients unable to hold their breath. This inability is frequently encountered in patients with altered mental status, pediatric patients who cannot follow breath-hold commands, and patients undergoing examination under sedation or anesthesia, which compromises their ability to follow commands.
The liver is the largest organ in the abdomen and due to its location immediately below the diaphragm, can move up to 5.5 cm during free breathing[1]. Liver lesions have been reported to move in all three planes during quiet respiration[2]. As a result, assessment of the liver is noticeably hampered by respiratory motion artifacts in patients who are unable to hold their breath.
Several approaches have been proposed to address the problem of respiratory motion-related artifact. One of these is radial VIBE (rVIBE), which can be performed during free breathing, in contrast to cVIBE, which requires the patient to hold their breath for approximately 20 s. Radial VIBE is a three-dimensional T1-weighted imaging technique that uses rectilinear sampling in the z direction and radial sampling in the xy plane. Because it can be performed during free breathing, rVIBE has been described as a valuable alternative T1-weighted gradient-echo sequence for MRI of the chest, abdomen, and pelvis in pediatric and adult patients who cannot hold their breath[3-13]. The rVIBE technique has been reported to be particularly helpful for imaging of the liver[4,6,9], which is often the organ most affected by respiratory motion artifact.
Previous studies comparing rVIBE and cVIBE have reported slightly conflicting results. Azevedo et al[3] reported that free-breathing radial 3D gradient echo (GRE) had slightly inferior, although acceptable, image quality compared to breath-hold 3D GRE VIBE. This study included cooperative and non-cooperative patients; however, the imaging protocol for non-cooperative patients did not include the breath-hold 3D GRE sequence. Chandarana et al[8] reported higher image quality measures for radial GRE compared to cartesian VIBE. Notably, cartesian VIBE was acquired during a breath-hold in cooperative patients, and during free-breathing in non-cooperative patients. Thus, the cartesian VIBE sequence would be expected to result in widely differing image quality between cooperative and non-cooperative patients.
The purpose of this prospective study is to test if the new free-breathing rVIBE technique could replace the current standard of care T1-weighted MR imaging technique (i.e., breath-hold cVIBE) in routine liver MRI at 1.5T by comparing free-breathing radial VIBE and breath-hold cartesian VIBE sequences in a single group of patients. We focused on image quality in the liver, which is the largest mobile organ in the abdomen, and thus highly sensitive to motion artifacts. Our hypothesis was that free-breathing rVIBE would result in similar or acceptable image quality for liver MRI compared to breath-hold cVIBE.
Our prospective study was approved by the Institutional Review Board as a Quality Improvement project, and informed consent was obtained from all patients before the MRI examination. All patients scheduled for MRI of the abdomen on a single 1.5T scanner between April 2014 and August 2014 were offered participation in this study and the maximum of 15 patients allowed for QI projects agreed to participate. The indications for the MRI examinations included characterization of incidentally-detected liver, adrenal, or renal lesions on prior computed tomography (n = 4); follow-up of liver metastases (n = 4), pancreatic cystic lesions (n = 3), or extrahepatic malignancy (n = 3); and liver transplant for primary intrahepatic cholangiocarcinoma (n = 1). Of note, the patient’s ability to hold their breath and the type of contrast agent used were not part of the inclusion criteria.
Prior to the study, we tested rVIBE sequences in three healthy volunteers, while varying parameters such as the number of radial views (range 300 to 1200), matrix size and flip angle. Increasing the number of spokes led to a longer acquisition time, and decreasing the number of spokes increased radiating streak artifacts. Since we wished to use a rVIBE sequence that closely matched the existing cVIBE sequence in image sequence parameters and could be completed within 2 min, we decided on 600 spokes as a reasonable trade-off between image acquisition time and image quality. A prototype fat-saturated three-dimensional rVIBE was acquired with free breathing before and after contrast administration. The acquisition time was 111 s for 88 views with 3-mm spacing (interpolated from 6 mm) over a 420 mm × 420 mm field of view reconstructed to a 256 × 256 matrix from 600 radial views with 256 samples in the readout direction using a 400 Hz/pixel bandwidth. The repetition time/echo time/flip angle for the acquisition was 4.1 ms/1.9 ms/12º. It was necessary to wrap two coils around the patient for the rVIBE sequence in order to enhance signal reception and improve image quality.
The images from rVIBE acquisition were compared with the images from a current standard of care fat-saturated three-dimensional breath-hold cVIBE acquisition. The breath-hold cVIBE acquisition time was 19.4 s for 104 views with 2.5-mm spacing (interpolated from 5 mm) over a 420-mm field of view using a 288 × 145 acquisition matrix (interpolated by a factor of 2 for viewing) and a parallel imaging acceleration factor of 2. An asymmetric field of view (up to 70%) in the phase-encoding direction (anterior-posterior) was also employed to decrease breath-hold times. The receiver bandwidth for this acquisition was 405 Hz/pixel with TR/TE/FA of 4.1 ms/1.9 ms/12º.
All patients were scanned on the same 1.5T clinical MRI scanner (MAGNETOM Aera; Siemens AG, Munich, Germany). The system operated D13 software and was equipped with the XQ gradient package (amplitude, 45 mT/m; slew rate, 200 T/m per second). Patients were positioned supine with their feet first and their arms at their sides. A 24-channel spine matrix coil was used for posterior signal reception, and one or two (two preferred) 18-channel body matrix coils were used for anterior and lateral signal reception. Routine clinical MRI of the abdomen at our institution includes coronal T2-weighted single-shot turbo spin-echo, T1-weighted gradient-echo in-phase and out-of-phase, T2-weighted turbo spin-echo with fat saturation, diffusion-weighted echo planar imaging, and pre-contrast, dynamic and 5-min post-contrast cVIBE sequences. We added two additional sequences for this study; the pre-contrast rVIBE and the post-contrast rVIBE. The pre-contrast rVIBE sequence was acquired immediately following the pre-contrast cVIBE sequence and the post contrast rVIBE sequence was acquired immediately following the post contrast cVIBE sequence. The post-contrast images were acquired after the intravenous administration of a gadolinium contrast agent [either gadobutrol (n = 12) injected at 2 mL/s or gadoxetate disodium (n = 3) injected at 1 mL/s] at a dose of 0.1 mL/kg up to a maximum dose of 10 mL immediately followed by a 30-mL normal saline flush.
Three abdominal radiologists with 2, 4, and 8 years of experience (HCK, NAW, LPM) independently evaluated the images. The pre-contrast rVIBE, post-contrast rVIBE, pre-contrast cVIBE, and post-contrast cVIBE sequences were presented in a random order for each patient and the radiologists were blinded to image parameters. Only these 4 sequences were available for review. Readers did not have access to clinical history, prior studies, or other abdominal MRI sequences obtained on the same day. Using a five-point scale (1: Non-diagnostic; 2: Poor and unacceptable; 3: Fair and acceptable; 4: Good and diagnostic; and 5: Very good) for each parameter, readers scored the liver edge sharpness, hepatic vessel clarity, presence of artifacts, lesion conspicuity (when present), fat saturation, and overall image quality. The criteria for assigning scores for each parameter are detailed in Table 1. The number of lesions varied between patients. Some patients had no lesions. The liver lesions varied from simple cysts to malignant tumors. In patients with no liver lesions, the radiologists scored their estimated confidence level for detecting and delineating a liver lesion on the basis of the overall image quality. The radiologists subjectively evaluated the conspicuity of the lesions in the entire liver parenchyma on each series, especially the interface of the lesion with the adjacent liver parenchyma. Due to variability in the number of lesions, the sensitivity of each sequence for lesion detection was not part of this subjective evaluation.
| Image qualityparameter | Scoring criteria |
| Liver edge sharpness | 1 Non diagnostic; severe blurring |
| 2 Poor and unacceptable; moderate to severe blurring resulting in considerable loss of anatomic detail | |
| 3 Fair and acceptable; mild blurring resulting in acceptable images with minimal loss of anatomic detail | |
| 4 Good and diagnostic; barely perceptible blurring | |
| 5 Very good and sharp | |
| Artifacts | 1 Non-diagnostic; severe artifacts |
| 2 Poor and unacceptable; moderate to severe artifacts resulting in significant loss of information | |
| 3 Fair and acceptable; mild artifacts and very minimal loss of information | |
| 4 Good and diagnostic; no perceptible artifacts in the area of interest and minimal artifacts at the margins | |
| 5 Very good; no artifacts | |
| Hepatic vessel clarity | 1 Uninterpretable |
| 2 Severely blurred | |
| 3 Moderately blurred | |
| 4 Mildly blurred | |
| 5 Sharp | |
| Lesion conspicuity | 1 Most lesions not seen |
| 2 Most lesions barely seen but margins and internal heterogeneity not well delineated | |
| 3 All lesions seen with at least 50% of the lesions showing good delineation of margins and internal heterogeneity | |
| 4 All lesions seen with at least 75% of lesions showing good delineation of the margins and internal heterogeneity | |
| 5 All lesions seen with good delineation of margins and internal heterogeneity | |
| Fat saturation | 1 Unacceptable |
| 2 Poor | |
| 3 Fair | |
| 4 Good | |
| 5 Very good | |
| Overall image quality | 1 Non-diagnostic |
| 2 Poor and unacceptable | |
| 3 Fair and acceptable | |
| 4 Good and diagnostic | |
| 5 Very good |
Statistical review of the study was performed by a biostatistician (WW). Image quality scores were summarized using mean, standard deviation, and range for each image quality parameter, sequence, contrast and reader. A score of 3 or above was considered acceptable. A multivariate linear mixed model was used to estimate and compare scores between sequences, adjusting for contrast and reader for each image quality parameter. The mixed model took into account the correlations between scores from the same image. Tukey-Kramer adjustment was used for pairwise comparisons between sequences to control the overall type I error rate at 5%. All tests were two-sided, and P values of 0.05 or less were considered statistically significant. Statistical analyses were carried out using SAS version 9 (SAS Institute, Cary, NC).
Eight men and seven women with median age of 57 years (range 40-78 years) participated in the study. Mean scores for each image quality parameter for pre- and post-contrast rVIBE and cVIBE are summarized in Table 2. The mean scores of each parameter for the pre-contrast rVIBE images were inferior to those of the pre-contrast cVIBE images. Similarly, the scores of the post-contrast rVIBE images were inferior to those of the post-contrast cVIBE images. Pre- and post-contrast rVIBE images had acceptable image quality scores (at least 3) for all parameters measured.
| Image qualityparameter | Pre-contrast | Post-contrast | ||
| rVIBE | cVIBE | rVIBE | cVIBE | |
| Liver edge sharpness | 3.37 | 3.76 | 3.38 | 3.39 |
| Hepatic vessel clarity | 3.26 | 3.61 | 3.09 | 3.81 |
| Artifacts | 3.07 | 3.61 | 3.04 | 3.87 |
| Lesion conspicuity | 3.16 | 3.61 | 3.24 | 4.00 |
| Fat saturation | 3.96 | 4.02 | 4.10 | 4.22 |
| Overall image quality | 3.26 | 3.77 | 3.21 | 4.04 |
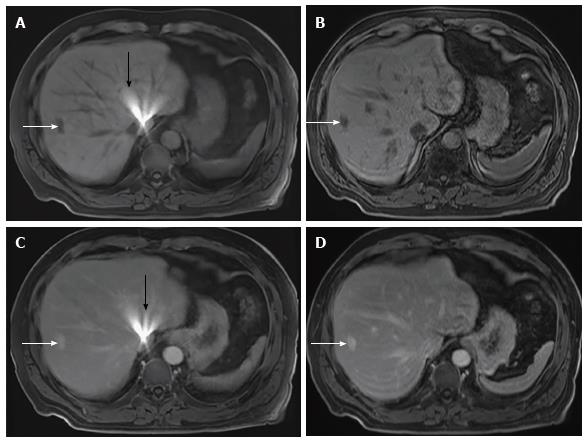
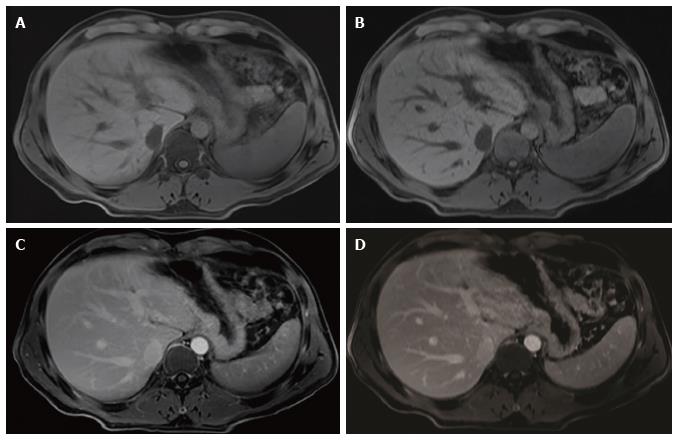
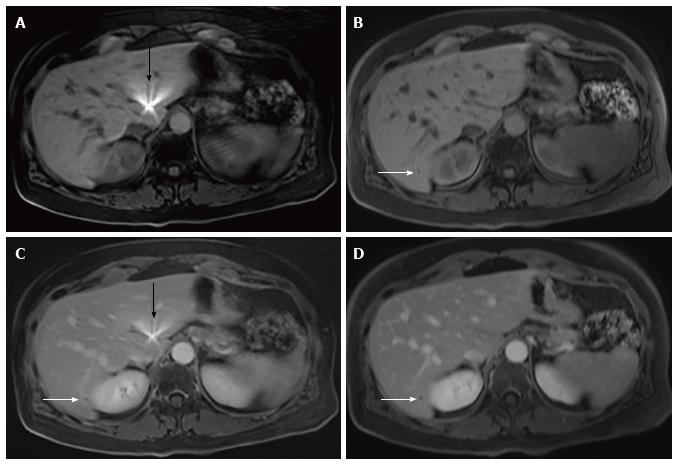
The mean differences between rVIBE and cVIBE scores were the same for the pre- and post-contrast data, so the pre- and post-contrast scores were combined for statistical modeling, yielding combined rVIBE and combined cVIBE scores. The mean combined scores for each image quality parameter, with 95%CIs and P values, are shown in Table 3 and Figure 1. After adjusting for contrast and reader effect, cVIBE images had significantly higher scores, compared to rVIBE images, for all parameters (P values of < 0.0001) except fat saturation (P = 0.17). Images from representative patients are shown in Figure 2, Figure 3, Figure 4 and Figure 5.
| Image qualityparameter | rVIBE | cVIBE | P value | ||
| Mean | 95%CI | Mean | 95%CI | ||
| Liver edge sharpness | 3.37 | 3.21-3.53 | 3.87 | 3.71-4.03 | < 0.001 |
| Hepatic vessel clarity | 3.18 | 2.95-3.40 | 3.71 | 3.49-3.93 | < 0.001 |
| Artifacts | 3.06 | 2.85-3.27 | 3.74 | 3.53-3.95 | < 0.001 |
| Lesion conspicuity | 3.20 | 3.01-3.39 | 3.81 | 3.62-4.00 | < 0.001 |
| Fat saturation | 4.03 | 3.78-4.27 | 4.12 | 3.88-4.37 | 0.17 |
| Overall image quality | 3.24 | 3.02-3.45 | 3.91 | 3.69-4.12 | < 0.001 |
We estimated the difference between the cVIBE and rVIBE scores since it measured the perceived difference in image quality by the individual readers rather than the absolute scores. For example, the hepatic vessel clarity scores on one patient for post-contrast cVIBE and rVIBE given by the three readers were 3 and 2.5; 4 and 3; and 4 and 3.5, respectively. In this example, the difference between the cVIBE and rVIBE scores for each reader was 0.5, 1, and 0.5, respectively. This difference was used to estimate inter-observer variability. The inter-observer variability with respect to differences between rVIBE and cVIBE scores was close to zero for all the image quality parameters compared to random error and inter-patient variation, i.e., the reproducibility between readers was good with respect to the differences between cVIBE and rVIBE image scores. The estimated inter-observer variance for liver edge sharpness was 0; for artifacts, 0.06; for hepatic vessel clarity, 0.023; for lesion conspicuity, 0.01; for fat saturation, 0.07; for overall image quality, 0.04.
In our prospective study, we subjectively compared the current standard-of-care T1-weighted 3D gradient-echo imaging technique at our institution, the breath-hold cVIBE, with a new technique that has the potential to be the new standard of care, the free-breathing rVIBE, on a 1.5T MRI scanner. During the MRI examinations for this study, only a single contrast injection was performed for each patient and the window to acquire two separate sequences during the arterial and portal venous phases was not technically feasible. Hence, we acquired and compared pre-contrast and delayed post-contrast cVIBE and rVIBE images. Note is made of differences in the voxel size between rVIBE and cVIBE due to inherent differences in the sampling of radial and cartesian acquisitions. We have shown that free-breathing rVIBE yielded image quality that was slightly inferior to breath-hold cVIBE, although still acceptable. Therefore, free-breathing rVIBE may be used as an alternative to cVIBE in patients with limited breath-hold capacity.
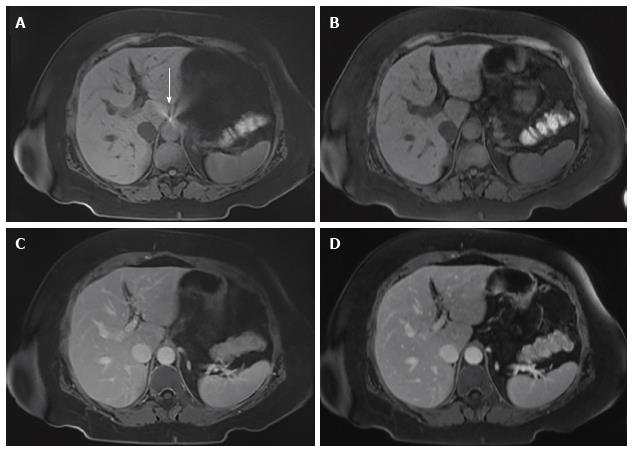
During the preliminary evaluation of rVIBE, we selected an rVIBE sequence that closely matched the existing cVIBE in image parameters. In spite of this, there were noticeable differences between rVIBE and cVIBE sequences, which often allowed the blinded readers to distinguish between them. The rVIBE images have a distinguishable grainy (i.e., noisy or pixelated) appearance and an unusually smooth configuration at the edges/boundaries compared with the images acquired with cVIBE. One patient with an atrial septal defect closure device (Figure 2) showed a bright artifact near the liver dome region which was more pronounced with rVIBE than with cVIBE, likely due to signal averaging from multiple slices acquired during free breathing.
cVIBE is known to cause phase wrap and ghosting artifacts due to parallel imaging acquisition. In our series, we observed minimal ghosting artifacts at the periphery of the image. None of the patients in our study had significant phase wrap artifacts. cVIBE is also known to suffer from artifacts due to breathing motion. None of the images obtained with cVIBE were significantly degraded by respiratory motion artifacts. Had there been considerable degradation of image quality of our cVIBE images due to these two types of artifacts, the rVIBE images would likely have scored higher than the cVIBE images.
Previous studies comparing rVIBE with cVIBE have also reported that rVIBE sequences result in acceptable image quality[3,8]. Chandarana et al[8] compared rVIBE with cVIBE in abdominopelvic MRI in 73 children. Forty-six of those patients were sedated and underwent free-breathing cVIBE and rVIBE, and the other 27 were imaged with an attempted breath hold for cVIBE and free breathing for rVIBE. Chandarana et al[6] reported significantly higher scores for rVIBE compared to cVIBE with respect to overall image quality, hepatic edge sharpness, hepatic vessel clarity, and respiratory motion robustness. This is likely due to the large number of sedated patients whose cVIBE could have suffered from free-breathing artifacts. Azevedo et al[3] compared overall image quality, extent of artifacts, and lesion detectability and conspicuity on abdominal MRI in 55 patients. Of these, the 39 cooperative patients had both three-dimensional free-breathing rVIBE and three-dimensional breath-hold gradient-recalled echo VIBE sequences, and the 16 non-cooperative patients only had the free-breathing rVIBE sequence. Their results were similar to ours, with lower but acceptable scores for rVIBE compared with three-dimensional gradient-recalled echo VIBE.
The limitations of our study included the small number of patients (n = 15). We were limited by the internal guidelines for QI projects. In addition, the breath-hold capacity of patients was not taken into account for patient selection. None of our patients required anesthesia or sedation. Those patients requiring sedation or anesthesia would have potentially benefited the most from the rVIBE sequence. Another limitation was the inability to completely blind the radiologists to the sequence types since the readers were able to learn to identify the rVIBE sequences in many cases, due to a distinguishable appearance of the rVIBE images.
In conclusion, free-breathing rVIBE imaging yields an acceptable image quality in the evaluation of the liver. rVIBE can be used as an alternative to breath-hold cVIBE for liver imaging in patients who cannot cooperate with breath-hold instructions. rVIBE cannot replace cVIBE in routine liver MRI at 1.5T. Based on our results we have modified our imaging protocol and have incorporated rVIBE as a post-contrast imaging sequence for MRI of the abdomen in patients who cannot cooperate with breath hold instructions.
T1 weighted fat suppressed gradient-echo volumetric interpolated breath-hold examination (VIBE) using cartesian sampling (cVIBE) plays a well-established role in magnetic resonance imaging (MRI) of the abdomen. However, respiratory motion artifacts can result in significant blurring and compromise the image quality of VIBE in patients who are unable to hold breath. Several new techniques including free-breathing radial VIBE (rVIBE) were developed to address the problem of respiratory motion artefact. The purpose of the study is to test if the new free-breathing radial VIBE could replace the current gold standard breath hold cartesian VIBE in routine liver MR imaging at 1.5T.
This study shows that at 1.5T MR, the image quality of free-breathing rVIBE is acceptable but slightly inferior to the current standard of care breath-hold VIBE. Therefore, free-breathing rVIBE can be used as an alternative to cVIBE in patients with limited breath-hold capacity.
Although free-breathing rVIBE can be used as an alternative to cVIBE in patients who cannot cooperate with breath-hold instructions, it cannot replace the cVIBE in routine liver MRI at 1.5T.
The authors have modified our imaging protocol and have incorporated rVIBE as a post-contrast imaging sequence for the MRI of the abdomen in patients who cannot cooperate with breath hold instructions.
VIBE stands for volumetric interpolated breath-hold examination and is a T1 weighted gradient echo sequence. cVIBE uses Cartesian sampling and requires up to 20 s breath-hold by the patient to result in optimal image quality. rVIBE is a free-breathing technique.
This is an interesting study that compares breath-hold cVIBE and free-breathing rVIBE to determine whether rVIBE could replace cVIBE in routine liver MRI on a 1.5T MR system. The results are helpful for our clinical practice.
Specialty Type: Radiology, Nuclear Medicine and Medical Imaging
Country of Origin: United States
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Li YZ, Shen J S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Suramo I, Päivänsalo M, Myllylä V. Cranio-caudal movements of the liver, pancreas and kidneys in respiration. Acta Radiol Diagn (Stockh). 1984;25:129-131. [PubMed] |
| 2. | Shimizu S, Shirato H, Xo B, Kagei K, Nishioka T, Hashimoto S, Tsuchiya K, Aoyama H, Miyasaka K. Three-dimensional movement of a liver tumor detected by high-speed magnetic resonance imaging. Radiother Oncol. 1999;50:367-370. [PubMed] |
| 3. | Azevedo RM, de Campos RO, Ramalho M, Herédia V, Dale BM, Semelka RC. Free-breathing 3D T1-weighted gradient-echo sequence with radial data sampling in abdominal MRI: preliminary observations. AJR Am J Roentgenol. 2011;197:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Chandarana H, Block TK, Rosenkrantz AB, Lim RP, Kim D, Mossa DJ, Babb JS, Kiefer B, Lee VS. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Invest Radiol. 2011;46:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 245] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 5. | Bamrungchart S, Tantaway EM, Midia EC, Hernandes MA, Srirattanapong S, Dale BM, Semelka RC. Free breathing three-dimensional gradient echo-sequence with radial data sampling (radial 3D-GRE) examination of the pancreas: Comparison with standard 3D-GRE volumetric interpolated breathhold examination (VIBE). J Magn Reson Imaging. 2013;38:1572-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Chandarana H, Feng L, Block TK, Rosenkrantz AB, Lim RP, Babb JS, Sodickson DK, Otazo R. Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling. Invest Radiol. 2013;48:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Kim KW, Lee JM, Jeon YS, Kang SE, Baek JH, Han JK, Choi BI, Bang YJ, Kiefer B, Block KT. Free-breathing dynamic contrast-enhanced MRI of the abdomen and chest using a radial gradient echo sequence with K-space weighted image contrast (KWIC). Eur Radiol. 2013;23:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Chandarana H, Block KT, Winfeld MJ, Lala SV, Mazori D, Giuffrida E, Babb JS, Milla SS. Free-breathing contrast-enhanced T1-weighted gradient-echo imaging with radial k-space sampling for paediatric abdominopelvic MRI. Eur Radiol. 2014;24:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Fujinaga Y, Ohya A, Tokoro H, Yamada A, Ueda K, Ueda H, Kitou Y, Adachi Y, Shiobara A, Tamaru N. Radial volumetric imaging breath-hold examination (VIBE) with k-space weighted image contrast (KWIC) for dynamic gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI of the liver: advantages over Cartesian VIBE in the arterial phase. Eur Radiol. 2014;24:1290-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Wright KL, Chen Y, Saybasili H, Griswold MA, Seiberlich N, Gulani V. Quantitative high-resolution renal perfusion imaging using 3-dimensional through-time radial generalized autocalibrating partially parallel acquisition. Invest Radiol. 2014;49:666-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Song HK, Dougherty L. Dynamic MRI with projection reconstruction and KWIC processing for simultaneous high spatial and temporal resolution. Magn Reson Med. 2004;52:815-824. [PubMed] |
| 12. | Amano Y, Takahama K, Kumita S. Noncontrast-enhanced three-dimensional magnetic resonance aortography of the thorax at 3.0 T using respiratory-compensated T1-weighted k-space segmented gradient-echo imaging with radial data sampling: preliminary study. Invest Radiol. 2009;44:548-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |