Published online Jul 28, 2016. doi: 10.4329/wjr.v8.i7.700
Peer-review started: February 16, 2016
First decision: March 23, 2016
Revised: March 31, 2016
Accepted: May 10, 2016
Article in press: May 11, 2016
Published online: July 28, 2016
Processing time: 160 Days and 8.9 Hours
AIM: To evaluate the pudendal nerve segments that could be identified on magnetic resonance neurography (MRN) before and after surgical marking of different nerve segments.
METHODS: The hypothesis for this study was that pudendal nerve and its branches would be more easily seen after the surgical nerve marking. Institutional board approval was obtained. One male and one female cadaver pelvis were obtained from the anatomy board and were scanned using 3 Tesla MRI scanner using MR neurography sequences. All possible pudendal nerve branches were identified. The cadavers were then sent to the autopsy lab and were surgically dissected by a peripheral nerve surgeon and an anatomist to identify the pudendal nerve branches. Radiological markers were placed along the course of the pudendal nerve and its branches. The cadavers were then closed and rescanned using the same MRN protocol as the pre-marking scan. The remaining pudendal nerve branches were attempted to be identified using the radiological markers. All scans were read by an experienced musculoskeletal radiologist.
RESULTS: The pre-marking MR Neurography scans clearly showed the pudendal nerve at its exit from the lumbosacral plexus in the sciatic notch, at the level of the ischial spine and in the Alcock’s Canal in both cadavers. Additionally, the right hemorrhoidal branch could be identified in the male pelvis cadaver. The perineal and distal genital branches could not be identified. On post-marking scans, the markers were used as identifiable structures. The location of the perineal branch, the hemorroidal branch and the dorsal nerve to penis (in male cadaver)/clitoris (in female cadaver) could be seen. However, the visualization of these branches was suboptimal. The contralateral corresponding nerves were poorly seen despite marking on the surgical side. The nerve was best seen on axial T1W and T2W SPAIR images. The proximal segment could be seen well on 3D DW PSIF sequence. T2W SPACE was not very useful in visualization of this small nerve or its branches.
CONCLUSION: Proximal pudendal nerve is easily seen on MR neurography, however it is not possible to identify distal branches of the pudendal nerve even after surgical marking.
Core tip: The radiologist should comment on lesions detected on magnetic resonance neurography along the course of the pudendal branch nerves or their approximate location, that could be helpful in image guided pain injections directed at the distal branches. However, they should not over-diagnose pudendal branch neuropathy or avoid mistaking the nearby vessels as distal pudendal branches.
- Citation: Chhabra A, McKenna CA, Wadhwa V, Thawait GK, Carrino JA, Lees GP, Dellon AL. 3T magnetic resonance neurography of pudendal nerve with cadaveric dissection correlation. World J Radiol 2016; 8(7): 700-706
- URL: https://www.wjgnet.com/1949-8470/full/v8/i7/700.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i7.700
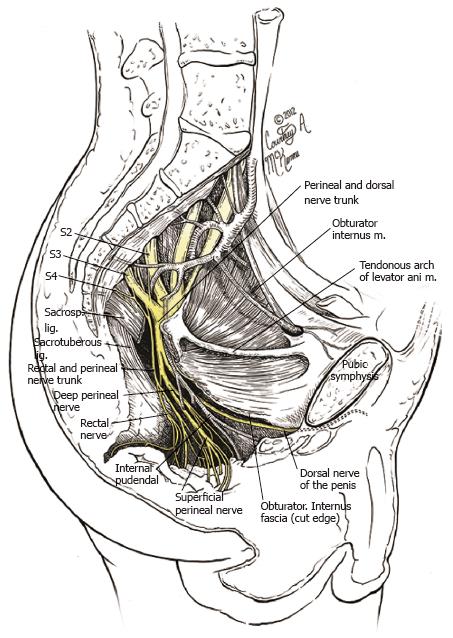
Pudendal nerve is a mixed motor and sensory nerve arising from lumbosacral plexus that courses along the pelvic floor (Figure 1). It is constituted by contributions from ventral rami of S2, S3 and S4 nerve roots. The main trunk courses between the coccygeus and piriformis muscles and exits at the lower aspect of the greater sciatic foramen, giving the inferior hemorroidal branch (inferior rectal branch). It re-enters the pelvis via the lesser sciatic foramen, travelling along with internal pudendal vessels underneath the obturator fascia in the pudendal canal, where it gives the superficial perineal branches and further deep gives rise to the dorsal nerve of penis/clitoris and posterior scrotal/labial nerves[1,2].
Pudendal neuropathy causing pudendal neuralgia is gaining much attention as an important etiology of chronic pelvic pain syndrome[1-5]. Pudendal neuropathy can be caused by nerve entrapment or injury, resulting from repetitive motion such as in bike racing, trauma such as pelvic fracture, stretch/traction or perineal tears during delivery, or pelvic tumors compressing the nerve in its path. The symptoms of pudendal neuralgia include erectile/orgasmic dysfunction, vulvodynia, and fecal or stress urinary incontinence. Due to poorly understood anatomy and complex branching patterns of the pudendal nerve, the condition is often misdiagnosed and maltreated, thereby increasing the morbidity in the patients[6-9]. High-resolution magnetic resonance neurography (MRN) is an imaging technique to identify and characterize peripheral neuropathies[10,11]. Pudendal neuropathy has been studied by Filler et al[12] and different sites of entrapment have been described, namely at the sciatic notch, ischial spine, Alcock’s canal, and selected distal site involvement of terminal branches, including hemorroidal, perineal and dorsal nerve of penis/clitoris. However, it is not clear whether all or which particular segments of the pudendal nerve can be confidently identified on MRN. The objective of this study was to evaluate which particular segments of the pudendal nerve and its branches could be possibly identified on 3 Tesla MRN sequences before and after surgical cadaveric dissection and marking of different segments of the pudendal nerve. The hypothesis was that smaller branches of pudendal nerve would be easily seen after the surgical nerve marking.
One male and one female fresh cadaver specimens were obtained from the regional Anatomy Board. The specimens were transected at the level of the superior iliac crest and the proximal thigh. The bladder, rectum and pelvic organs were kept intact and the intestines were removed. There were no signs of any previous surgery. The specimens were stored in the morgue for twenty-four hours following which they were scanned individually on the Magnetom Trio MR Scanner (Siemens, Erlangen, Germany). To replicate anatomical imaging picture similar to the one routinely obtained on 3 Tesla MRN, the technique described in previous articles[11,13] was used. The pre-marking scan sequences included, axial T1W (TR-575 ms, TE-11 ms, Sl-2 mm, base resolution-704), axial T2W SPAIR (spectral adiabatic inversion recovery; TR-5600 ms, TE-53 ms, Sl-2 mm, base resolution-704), coronal T1W (TR-915 ms, TE-14 ms, Sl-3 mm, base resolution-768), 3D isotropic T2W SPACE (sampling perfection with application optimized contrasts using varying flip angle evolutions; TR-1300 ms, TE-84 ms, Sl-1 mm, base resolution-512) and 3D isotropic diffusion weighted (DW) PSIF (reversed steady state free precession; TR-10 ms, TE-2.8 ms, Sl-0.9 mm, base resolution-128), totaling an acquisition time of 45 min per pelvis block. After the MRN scans were performed, the pelvis blocks were sent to the autopsy lab where they were dissected by specialist peripheral nerve surgeon and an anatomist. The surgical approach was directed to identify the different pudendal nerve branches.
The female pelvis was dissected first. Two rectal branches were identified. The perineal branch was then located after cutting the sacrospinous ligament and removing the obturator internus fascia. The dorsal nerve to clitoris was identified by tracing the crus of clitoris along its edge. After the dissection, four markers (1.5 cm, circular) were placed at specific points along the pudendal nerve branches for potential identification in post dissection MR Scans. The first marker was placed directly on the sacrospinous ligament along the first rectal branch. The second marker was placed on the perineal branch followed by the third over the dorsal nerve to clitoris in Alcock’s canal, and the fourth at the distal end of the inferior pubic ramus canal where the dorsal nerve to clitoris exited.
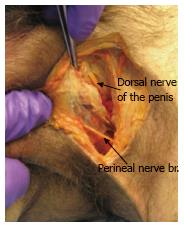
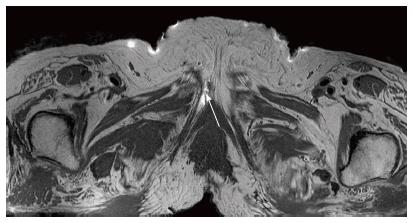
The male pelvis block was then dissected using the same surgical approach. The perineal branch was identified first and was found to be lower than located in the female dissection. The dorsal nerve of penis was located by following the inferior pubic ramus (Figure 2). The sacrotuberous ligament was cut and the rectal branch was identified entering Alcock’s canal proximally and travelling straight across the ischiorectal fossa towards the anus. The obturator internus fascia was cut and a large perineal branch was identified directly under the fascia. Four markers were placed - the first under the dorsal nerve to penis, second on the perineal branch (anterior side), the third over the sacrospinous ligament (along the rectal branch) and the fourth marker was placed along the perineal branch (posterior side).
Both specimens were sutured to close all incisions and prepared for the post-dissection scanning. The post-marking MRN protocol with pulse sequence parameters similar to the pre-dissection (pre-marking) scan protocol was used. The specimens were then returned to the Anatomy Board. The MRN studies was read by an experienced musculoskeletal fellowship trained radiologist and in discussion with the surgeon who placed the markers.
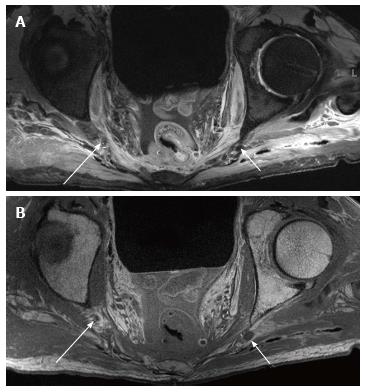
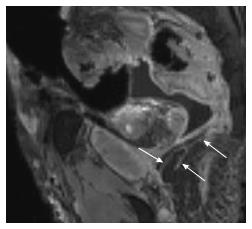
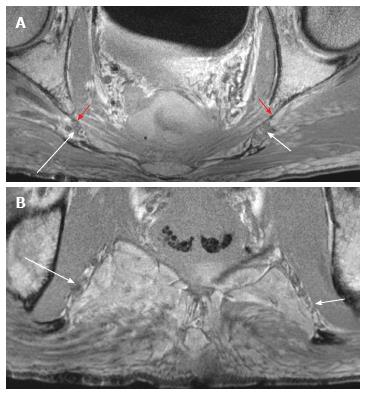
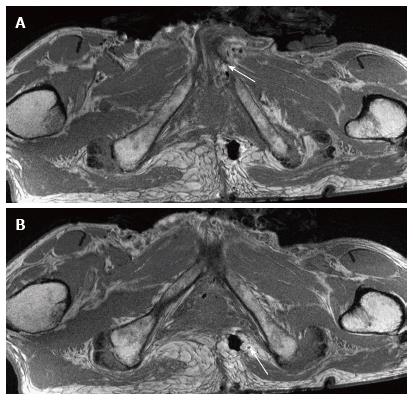
Pre-marking MRN scans clearly showed the pudendal nerve as it exited the lumbosacral plexus in the sciatic notch, at the level of the ischial spine and in the Alcock’s canal, in both male and female pelvic scans (Figures 3-5). In the male scan, additionally, right hemorroidal branch could also be seen with some difficulty. Other smaller branches could not be identified. The nerve was best seen on axial T1W and T2W SPAIR images and fascicular appearance could be delineated. On oblique sagittal reconstructed 3D DW PSIF, the proximal segment of the nerve could be identified along its long axis. The nerve could also be seen on coronal T1W image at its entrance into the Alcock’s canal, with limited visualization (Figure 5C). T2W SPACE was not very useful in visualization of this small nerve or its branches.
After the nerve and its branches were marked, the location of perineal, hemorroidal branches and dorsal nerve to penis/clitoris could be identified (Figures 6 and 7). Although the nerve braches themselves were suboptimally seen, the markers were used as identifiable structures and thus it was possible to find the location of the nerves on MRN using the relation of the markers to the nerve segments. The contralateral corresponding nerves were poorly seen despite marking on the surgical side.
The pudendal nerve arises from the lumbo-sacral plexus constituted by ventral rami of the second, third, and fourth sacral nerve roots (S2, 3, 4). The nerve passes between piriformis and coccygeus muscles and exits the pelvis through the lower part of greater sciatic foramen, crosses the ischial spine between the sacrospinous and sacrotuberous ligaments. It then re-enters the pelvis through the lesser sciatic foramen and courses distally through the pudendal canal (Alcock’s canal) along with the internal pudendal vessels. This canal lies along the lateral wall of ischiorectal fossa and its outer covering is formed by the obturator fascia. At the level of the perineum, it normally gives terminal branches, namely the perineal nerve(s) and the dorsal nerve of the penis or clitoris[14,15]. This is the classical description of the pudendal nerve described in most anatomical texts. However, it has recently been shown that the anatomy of the pudendal nerve is extremely variable, and there could be two pudendal nerves trunks transversing the sacrotuberous ligament in many cases, and that there are two exit(s) from Alcock’s canal, one for the dorsal branch and one for the perineal branch of the pudendal nerve[16].
Pudendal neuralgia is a painful mononeuropathy and is a common cause of chronic pelvic pain without any obvious etiological agent. The pain is localized to the vulva, vagina, clitoris, perineum, and rectum in females and to the glans penis, scrotum excluding testicles, perineum, and rectum in males[1,5,6]. The possibility of this being related to pudendal nerve compression has recently been questioned, as nerve compressive syndromes in other sites such as carpal tunnel syndrome or peroneal neuropathy frequently occur without distal pain and neuralgia[3].
In any case, imaging is frequently used to exclude a mass lesion or focal fibrosis along the course of the nerve[12]. Recently, ultrasound (US) has been shown to identify some segments of the pudendal nerve[14]. However, US is an operator dependent technique and small areas of scarring in the pelvis along the course of the pudendal nerve may not be identified using US. MRN is a more objective technique and can show lesions, such as focal scarring or tumors along the course of the nerve. The aim of this study was to detect the pudendal nerve segments that could be identified on MRN with a good degree of confidence. The major proximal segments of the pudendal nerve could be identified in both male and female pelvis and on both pre- and post-marking scans of the pelvis. The fascicular appearance on axial images and selective identification on 3D DW PSIF images was possible due to poor fat and vascular signal suppression on the latter technique. 3D SPACE technique although, useful for large branch nerve delineation[17-19], was not particularly helpful in visualizing pudendal nerve or its branches. The smaller pudendal branches were not visible on any sequence on the pre-marking scans and suboptimally seen on the post-marking scans. Therefore, although reader should comment on lesions detected on MRN along the course of the pudendal branch nerves or their approximate location could be helpful in image guided pain injections directed at the distal branches, the reader should not over diagnose pudendal branch neuropathy or avoid mistaking the nearby vessels as distal pudendal branches. It should be noted that one can also find the nerve by electrical stimulation for treatment of pudendal neuralgia[20].
Our limitations include small sample size and lack of intravenous contrast administration. However, due to cost involved with the nature of the study and collaborators required, studying two cadaveric blocks of opposite sex was justified. Additionally, since most MRN examinations are traditionally non-contrast, we did not opacify the vessels. Finally, fascicular appearance was easily identified in the proximal pudendal nerve leading to its easy delineation.
To conclude, the radiologist reading the pudendal nerve MRN scans should be cognizant that proximal pudendal nerve is easily seen on the high resolution MRN scans, however it is not possible to identify distal branches of the pudendal nerve.
Magnetic resonance neurography (MRN) is increasingly being used for evaluation of peripheral neuropathies, such as pudendal neuralgia. However, it is not clear if all segments of pudendal nerve can be confidently identified on MRN.
MRN is an imaging technique optimized for evaluation of peripheral nerves and their associated pathologies. The purpose of this study was to identify segments of the pudendal nerve and its branches that could be possibly identified on MRN sequences before and after surgical cadaveric dissection and marking of different segments of the nerve.
The proximal pudendal nerve segment is easily seen on MRN; however it is not possible to confidently identify the distal branches of the pudendal nerve even after surgical marking.
This study serves as a reminder to the radiologist to comment on the lesions along the course of the pudendal nerve, but not to over-diagnose pudendal neuropathies, especially in the distal segments, which cannot be confidently seen.
MRN is a modification of MRI for the direct imaging of peripheral nerves. The pudendal nerve is a mixed motor and sensory nerve arising from the S1, S2 and S3 nerve roots.
In this study, the authors investigated the role of MRN in identification of segments of the pudendal nerve before and after cadaveric dissection and marking of the segments. It was found that the proximal segments of the nerve can be easily seen on MRN sequences, but the distal segments cannot be seen even after surgical marking.
Manuscript Source: Invited manuscript
Specialty Type: Radiology, Nuclear Medicine and Medical Imaging
Country of Origin: United States
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen F, Naja ZM, Nouh MR S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Hibner M, Desai N, Robertson LJ, Nour M. Pudendal neuralgia. J Minim Invasive Gynecol. 2010;17:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Koninckx PR, Corona R, de Cicco C, Verguts J, Ussia A. Pudendal neuralgia. J Minim Invasive Gynecol. 2010;17:666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Stav K, Dwyer PL, Roberts L. Pudendal neuralgia. Fact or fiction? Obstet Gynecol Surv. 2009;64:190-199. [PubMed] |
| 4. | Vancaillie T, Eggermont J, Armstrong G, Jarvis S, Liu J, Beg N. Response to pudendal nerve block in women with pudendal neuralgia. Pain Med. 2012;13:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Antolak SJ, Hough DM, Pawlina W, Spinner RJ. Anatomical basis of chronic pelvic pain syndrome: the ischial spine and pudendal nerve entrapment. Med Hypotheses. 2002;59:349-353. [PubMed] |
| 6. | Calabrò RS, Gervasi G, Marino S, Mondo PN, Bramanti P. Misdiagnosed chronic pelvic pain: pudendal neuralgia responding to a novel use of palmitoylethanolamide. Pain Med. 2010;11:781-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Kennedy J. Neurologic injuries in cycling and bike riding. Phys Med Rehabil Clin N Am. 2009;20:241-28, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Chaliha C. Postpartum pelvic floor trauma. Curr Opin Obstet Gynecol. 2009;21:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Fisher HW, Lotze PM. Nerve injury locations during retropubic sling procedures. Int Urogynecol J. 2011;22:439-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Pham M, Bäumer T, Bendszus M. Peripheral nerves and plexus: imaging by MR-neurography and high-resolution ultrasound. Curr Opin Neurol. 2014;27:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Wadhwa V, Thakkar RS, Maragakis N, Höke A, Sumner CJ, Lloyd TE, Carrino JA, Belzberg AJ, Chhabra A. Sciatic nerve tumor and tumor-like lesions - uncommon pathologies. Skeletal Radiol. 2012;41:763-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Filler AG. Diagnosis and treatment of pudendal nerve entrapment syndrome subtypes: imaging, injections, and minimal access surgery. Neurosurg Focus. 2009;26:E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Lee PP, Chalian M, Bizzell C, Williams EH, Rosson GD, Belzberg AJ, Eng J, Carrino JA, Chhabra A. Magnetic resonance neurography of common peroneal (fibular) neuropathy. J Comput Assist Tomogr. 2012;36:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Tagliafico A, Perez MM, Martinoli C. High-Resolution ultrasound of the pudendal nerve: normal anatomy. Muscle Nerve. 2013;47:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Mahakkanukrauh P, Surin P, Vaidhayakarn P. Anatomical study of the pudendal nerve adjacent to the sacrospinous ligament. Clin Anat. 2005;18:200-205. [PubMed] |
| 16. | Furtmüller GJ, McKenna CA, Ebmer J, Dellon AL. Pudendal nerve 3-dimensional illustration gives insight into surgical approaches. Ann Plast Surg. 2014;73:670-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Vargas MI, Viallon M, Nguyen D, Beaulieu JY, Delavelle J, Becker M. New approaches in imaging of the brachial plexus. Eur J Radiol. 2010;74:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Chhabra A, Thawait GK, Soldatos T, Thakkar RS, Del Grande F, Chalian M, Carrino JA. High-resolution 3T MR neurography of the brachial plexus and its branches, with emphasis on 3D imaging. AJNR Am J Neuroradiol. 2013;34:486-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Ahlawat S, Wadhwa V, Belzberg AJ, Batra K, Chhabra A. Spectrum of suprascapular nerve lesions: normal and abnormal neuromuscular imaging appearances on 3-T MR neurography. AJR Am J Roentgenol. 2015;204:589-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Naja MZ, Al-Tannir MA, Maaliki H, El-Rajab M, Ziade MF, Zeidan A. Nerve-stimulator-guided repeated pudendal nerve block for treatment of pudendal neuralgia. Eur J Anaesthesiol. 2006;23:442-444. [PubMed] |