Published online Jul 28, 2016. doi: 10.4329/wjr.v8.i7.683
Peer-review started: January 22, 2016
First decision: March 1, 2016
Revised: March 14, 2016
Accepted: May 10, 2016
Article in press: May 11, 2016
Published online: July 28, 2016
Processing time: 185 Days and 23.5 Hours
AIM: To find out if magnetic resonance (MR)-signal characteristics of hepatocellular carcinomas (HCC) correlate with perfusion parameters assessed by volume perfusion computed tomography (VPCT).
METHODS: From October 2009 to January 2014, 26 (mean age, 69.3 years) patients with 36 HCC lesions who underwent both VPCT and MR liver imaging were analysed. We compared signal intensity in the T1w- and T2w-images and wash-in/wash-out kinetics on post-contrast MR images with mean values of blood flow (BF, mL/100 mL per minute), blood volume (BV, mL/100 mL), k-trans (mL/100 mL per minute), arterial liver perfusion (mL/100 mL per minute), portal venous perfusion and hepatic perfusion index (HPI, %) obtained by VPCT. Signal intensity on magnetic resonance imaging (MRI) was classified hyper/iso/hypointense compared with surrounding liver parenchyma.
RESULTS: Signal intensity on native T1w- and T2w-images was hyper/iso/hypo in 4/16/16 and 21/14/1 lesions, respectively. Wash-in and wash-out contrast kinetics were found on MRI in 33 of 36 lesions (91.7%) and 25 of 36 lesions (69.4%), respectively. The latter was observed significantly more often in higher graded lesions (P < 0.005). HPI was 94.7% ± 6.5%. There was no significant relationship between lesion’s MR-signal intensity, MR signal combinations, size and any of the VPCT-perfusion parameters. However HPI was constantly high in all HCC lesions.
CONCLUSION: VPCT parameters add limited value to MR-lesion characterization. However in HCC lesions with atypical MR signal characteristics HPI can add a parameter to ensure HCC diagnosis.
Core tip: The study shows no correlation between hepatocellular carcinomas (HCC) perfusion parameters and any of the possible magnetic resonance (MR)-signal characteristics of HCC as displayed at MR imaging as well as tumor size. However hepatic perfusion index measured with help of volume perfusion computed tomography (VPCT) was very high in all HCC lesions and the arterial liver perfusion decreased with increasing tumor dedifferentiation. For this purpose, we advocate the additional use of perfusion-based liver tumor identification by VPCT for accurate HCC detection and characterisation in case of unclear MR signal characteristics.
- Citation: Grözinger G, Bitzer M, Syha R, Ketelsen D, Nikolaou K, Lauer U, Horger M. Correlation of magnetic resonance signal characteristics and perfusion parameters assessed by volume perfusion computed tomography in hepatocellular carcinoma: Impact on lesion characterization. World J Radiol 2016; 8(7): 683-692
- URL: https://www.wjgnet.com/1949-8470/full/v8/i7/683.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i7.683
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide and accounts for 90% of primary malignant neoplasm of the liver[1]. Early detection with subsequent adequate stage-dependant therapy with surgery, liver transplantation, embolization therapy or sorafenib therapy significantly improves survival[2]. Imaging plays an incremental role in the diagnosis of HCC with most cases being meanwhile diagnosed non-invasively based on typical perfusion characteristics like the presence of wash-in and wash-out contrast kinetics or interval growth[3]. For the characterization of lesions larger than 1 cm in diameter the use of two different perfusion-based imaging modalities such as multi-phase CT, contrast enhanced ultrasound or dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) are advocated[3-6]. These imaging modalities have been under investigation for many years resulting in a plenty of reports dealing with the value of MRI signal intensity characteristics based on T1w and T2w sequences or diffusion-weighted imaging and in the last years also with the role of enhancement patterns of hepatocellular contrast agents[7-9].
MRI is in particular preferred for liver tumor diagnosis due to its better tissue contrast. HCCs are known to show manifold combinations of tumor signal intensities with different MR sequences which is generally believed to improve lesion classification. However, during the multistep process of hepatocarcinogenesis different precursor stages of advanced HCC are encountered, every of them displaying a variety of signal characteristics which are challenging and knowingly not specific enough for making a definite diagnosis[10]. Hence, the considerable overlap in signal intensity between regenerative nodules, adenomatous hyperplasia, low- and high-grade dysplastic nodules and early HCC has focused the attention on their different sources of blood supply and the potential benefit of perfusion-based characterisation by separate calculation of the arterial (mainly hepatic arterial) and portal venous contribution to the tumor vascularization. There is knowingly, a gradual increase in the number of unpaired arteries from cirrhotic nodules to dysplastic nodules and early HCC which is reflected by a continuous shift towards increased arterialisation of hepatic nodules[11,12]. In the past, the only technique capable to accomplish this task was the combination of CT hepatic arteriography (CTHA) and CT arterial portography (CTAP). Unfortunately, this imaging technique is invasive, costly and afflicted with a high false-positive rate[13]. As a relatively novel alternative, volume perfusion CT (VPCT) offers the possibility of real-time perfusion measurements coupled with software programs that facilitate separate calculation of arterial and portal-venous liver perfusion [(arterial liver perfusion (ALP) and portal venous perfusion (PVP)]. For VPCT, tissue density is measured after intravenous contrast agent application. For this purpose, the total liver volume is scanned at different points in time. The density in two different region of interests (ROIs), in an afferent artery and the desired tissue, is compared in the subsequent mathematical analysis.
In a previous work, correlation between e.g., T2w-signal intensity and tumor vascularization was found in patients with HCC using CTHA and CTAP[14]. Encouraged by novel software programs which are able to quantify perfusion parameters more accurately, we set out to seek for imaging fingerprints able to more accurately and non-invasively characterize HCC.
So, the aim of our study was to retrospectively determine if functional perfusion parameters by means of VPCT differ in HCC lesions with typical and atypical MR pattern including enhancement patterns (presence of wash-in and wash-out in dynamic studies) and if there is a link to histology. Is there a chance to facilitate lesion characterisation with the additional use of VPCT?
This retrospective evaluation was part of a prospective monitoring study in patients with HCC undergoing TACE which was approved by the local Institutional review board. This data has not yet been published. Additionally, retrospective data analysis was also approved by the local Institutional Review Board and all patients gave their written consent.
Between October 2009 and January 2014, 26 consecutive patients (18 male; 8 female; mean age, 69.3 years; range, 48-86 years,) with 36 histologically as HCC proven hepatic lesions in patients with liver cirrhosis who underwent both VPCT and MR liver imaging were retrospectively analysed for correlation of imaging findings.
Inclusion criteria for this study were liver cirrhosis with biopsy confirmed HCC and timely closely (less than one month) performed dual VPCT and MR imaging[3]. Exclusion criteria were impaired renal function, known allergic diathesis to iodized contrast agents, contraindications to MRI like claustrophobia, implanted pacemakers or refusal to participate in this study.
All patients had confirmed liver cirrhosis. Underlying cause of cirrhosis was: hepatitis C virus infection (HCV) (n = 8); hepatitis B virus infection (HBV) (n = 2); alcohol abuse (n = 6); combination of viral hepatitis + alcohol abuse (n = 4); nonalcoholic steatohepatitis (NASH) (n = 5) and hemochromatosis (n = 1).
VPCT examinations were all performed on a 128-row CT scanner (Somatom Definition AS+, Siemens Healthcare, Forchheim, Germany). The CT protocol consisted of a non-enhanced abdominal low-dose CT which was obtained to localize the liver porta. Subsequently, a scan range of up to 9.7 cm z-axis coverage was planned over the involved liver, followed by a volume perfusion CT of the tumor (VPCT) using adaptive spiral scanning technique. Perfusion parameters were: 80 kV, 100/120 mAs (for patients </> 70 kg, respectively), collimation 64 mm × 0.6 mm with z-flying focal spot and 26 CT repeated scans of the entire liver tumor within a total scan time of 40 s. The effective dose for liver perfusion measurements was 7 mSv. During perfusion scanning, the patients were asked to resume shallow breathing for the entire duration of the study. Fifty millilitre ultravist 370 (Bayer Vital Leverkusen, Germany) was injected at a flow rate of 5 mL/s in an antecubital vein followed by a saline flush of 50 mL NaCl at 5 mL/s, and a start delay of 7 s. From the VPCT raw data, axial images with a slice thickness of 3 mm for perfusion analysis were reconstructed without overlap, using a smooth tissue convolution kernel (B10f). All images were transferred to an external workstation (Multi-Modality Workplace, Siemens) for analysis.
Conventional MR imaging were performed on two 1.5T MR units (Siemens Magnetom Avanto and Siemens Magnetom Aera, both Siemens Healthcare, Erlangen, Germany) with a protocol that included the following sequences as a minimum: Axial non-contrast GRE T1-weighted, axial and coronal respiratory-gated T2-weighted (TSE), as well as dynamic post-contrast axial fat-saturated T1-weighted images. The T1-weighted sequences were performed before and after i.v. administration of gadolinium-based (non-hepatocellular) contrast medium (0.1 mL/kg body weight Gadovist, Beyer-Schering Pharma, Leverkusen, Germany). The post-gadolinium T1-weighted sequence was performed with parameters that were identical to non-enhanced T1-weighted sequences using fat-saturation and up to 5 consecutive series with 20-30 s scanning time pro series. Slice thickness varied among the sequences being 5-6 mm with 10% gap. Matrix size varied between 256 mm × 128 mm and 320 mm × 150 mm. The field of view was adapted to the patient size (250-350 mm).
Data evaluation was performed by 2 experienced radiologists with 20 and 5 year experience (Marius horrger and Gerd Grözinger) in consensus. All data sets were transferred to a dedicated workstation (Syngo MMWP, VE 36A, Siemens Healthcare, Forchheim, Germany) and CT-perfusion maps were calculated using a commercial software (Syngo Volume Perfusion CT Body (Siemens Healthcare, Forchheim, Germany).
CT perfusion analysis software is based on a combination of the maximum slope model and Patlak model using time density curves (TDC) to determine perfusion parameters[7]. Calculation of ALP and PVP corresponding to the dual blood supply of the liver by hepatic artery and portal vein is done by using the time of peak splenic enhancement as a separation point of arterial and portal-venous phase by drawing ROI in portal vein and spleen respectively. Therefore, a dedicated calculation algorithm (liver) is being used. Arterial TDC for ALP is calculated dividing maximum arterial slope by maximum aortic enhancement. Portal-venous TDC for PVP is calculated dividing maximum portal-venous slope by maximum portal-vein enhancement. Hepatic Perfusion index (HPI) expressed in % represents ALP divided by the sum of ALP and PVP. Blood flow (BF), blood volume (BV) and k-trans (defined as the sum of the flow within the microvasculature and capillary permeability) were calculated using a tumor calculation algorithm which applies only for one-vessel supply of the tumor. For the HCC lesions quantitative perfusion parameters BF, BV, ALP, PVP and HPI using both calculation methods were obtained. The size of the ROIs used for measurements of mean/max values of perfusion parameters had an average diameter of 2.4 cm.
Automated motion correction and noise reduction of the datasets were applied by using an integrated motion correction algorithm with non-rigid deformable registration for anatomic alignment. ROI were placed in the abdominal aorta, portal vein, and spleen for separation of arterial and portal-venous phases. For evaluation of functional VPCT parameters a freehand ROI was drawn around the circumference of the tumor and the largest diameter of each lesion was measured. BF, ALP and PVP is indicated as ml per 100 mL of tissue per minute, BV is indicated as mL per 100 mL of tissue and HPI is indicated in percent.
The same two radiologists retrospectively analysed all MR images of the corresponding liver tumors. MR signal characteristics of the tumors were analysed on both native T1-weighted and T2-weighted images and rated as hyperintense, isointense or hypointense relative to surrounding liver parenchyma. Additionally, the presence of contrast wash-in on arterial phase images and/or wash-out on late phase images was registered on dynamic images. Additionally the size of all hepatic tumors was measured and the growth pattern of liver tumors was rated infiltrative vs encapsulated.
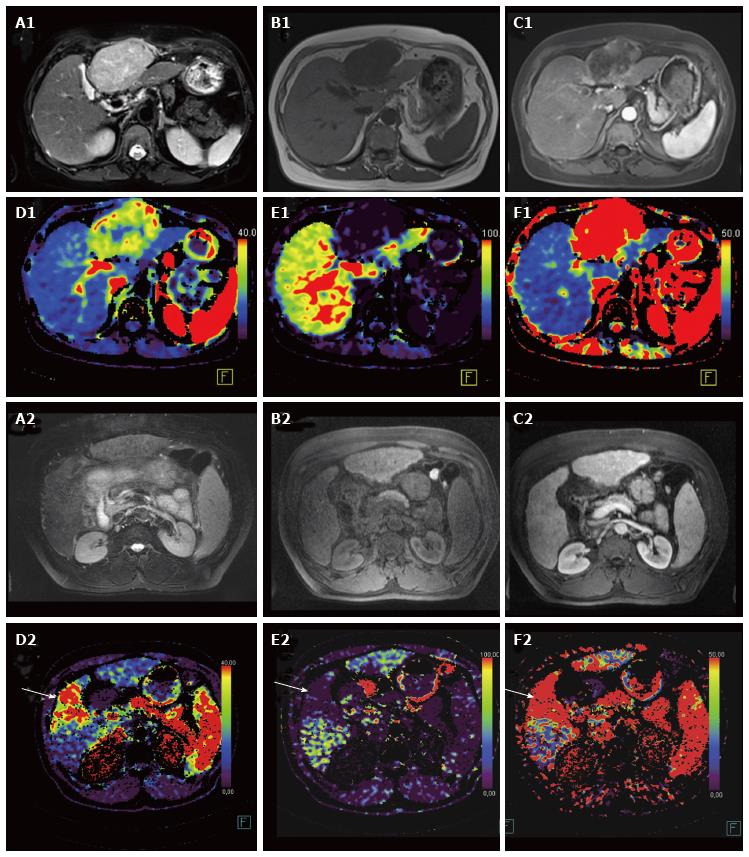
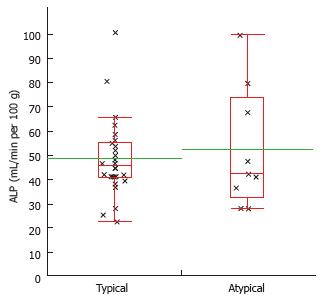
HCC-lesions fulfilling all of the following criteria: Hyperintense signal in T2 weighted sequences; T1 hypointense signal; wash-in and wash-out kinetic at the MR-study were classified as “typical” lesions. Otherwise the lesions were classified as “atypical” (Figure 1).
JMP software was used for statistical analysis (JMP Version 10.0, SAS Institute, Cary NC, United States). Summary statistics of the mean and max measurements of the perfusion CT parameters BF, BV and k-trans as well as of their standard deviations are reported as means, medians and ranges. Nominal data (parameters of MR-signal characteristics) was compared using a χ2 test. For continuous data, normal distribution was analyzed with the Shapiro-Wilk test. In case of a normal distribution, an analysis of variance (ANOVA) was used for multiple parameters or a t test in case of a two sided comparison in order to assert if the measurements obtained when comparing VPCT parameters. If data was not normally distributed a Kruskal-Wallis-test was applied. All tests were performed at the 0.05 level of significance.
Twenty-six patients with a total of 36 lesions were analysed with both modalities MRI and VPCT. These lesions all newly occurred in patients with known liver cirrhosis. Information about the tumor tissue sampling site in patients that underwent biopsy was available. The median lesion size was 3.8 cm, ranging from 1.5 to 7.7 cm. Histology was available in all 36 lesions with following tumor grading: G1 = 15; G2 = 18; G3 = 3. The tumor growth pattern was classified infiltrative (n = 5) and encapsulated (n = 31).
Results of perfusion measurements calculated by the maximum slope (BF) + Patlak (BV and k-trans) method are shown in Table 1. Additionally, measurements of the degree of arterial and portal venous supply (mL/100 g tissue) and HPI (%) of all lesions calculated using the liver calculation algorithm yielded following results: ALP (mean/SD, 49.8 ± 18.5 mL/100 mL per minute), PVP (mean/SD, 2.1 ± 2.6 mL/100 mL per minute), and HPI (94.7 ± 6.5%), respectively (Table 1).
| BF (mL/100 g tissue/min) | BV (mL/100 mL) | k-trans (mL/100 g tissue/min) | ALP (mL/100 g tissue/min) | PVP (mL/100 g tissue/min) | HPI (%) | |
| All lesions (n = 36) | 62.0 ± 23.6 | 15.3 ± 9.2 | 35.8 ± 18.4 | 49.8 ± 18.5 | 2.3 ± 2.5 | 94.4 ± 6.3 |
Results of perfusion measurements are shown in Table 2. All lesions show a high degree of arterialization (lowest a HPI 65.0%) independent of histological grading. At the same time, the total amount of blood supply over the hepatic artery (ALP) decreased with increasing tumor dedifferentiation (G2 and G3), however results were not statistically significant.
| Histologic grading | BF (mL/100 g tissue/min) | BV (mL/100 mL) | k-trans (mL/100 g tissue/min) | ALP (mL/100 g tissue/min) | PVP (mL/100 g tissue/min) | HPI (%) |
| G1 (n = 15) | 66.5 ± 30.5 | 15.8 ± 7.8 | 45.8 ± 18.4 | 56.4 ± 23.7 | 1.9 ± 2.1 | 95.2 ± 6.0 |
| G2 (n = 18) | 57.5 ± 18.9 | 13.7 ± 4.1 | 30.9 ± 13.7 | 46.3 ± 12.7 | 2.5 ± 2.9 | 93.9 ± 7.1 |
| G3 (n = 3) | 67.9 ± 2.8 | 22.1 ± 28.3 | 32.2 ± 8.5 | 41.2 ± 16.6 | 2.3 ± 0.3 | 93.9 ± 5.1 |
| P value | 10.52 | 10.34 | 10.21 | 10.24 | 10.78 | 10.71 |
Signal intensity on T1-weighted and T2-weighted images was hyper-/iso-/hypointense in 5/16/15 and 21/14/1 lesions, respectively (Table 3). Hyperintense signal on T1 was more frequent in better differentiated HCC; however this was statistically not significant.
| ALP | PVP | HPI | G1 (n = 15) | G2 (n = 18) | G3 (n = 3) | |
| T2-Signal characteristics | ||||||
| Hyperintense (n = 21) | 47.3 ± 18.7 | 3.0 ± 2.7 | 92.5 ± 7.1 | 8 | 13 | 0 |
| Isointense (n = 14) | 51.2 ± 17.3 | 1.1 ± 1.7 | 97.3 ± 4.4 | 6 | 5 | 3 |
| Hypointense (n = 1) | 80.7 | 0 | 100 | 1 | 0 | 0 |
| P value for student t test P value hyperintens vs isointense | 1P = 0.20 P = 0.54 | 1P = 0.06 P = 0.02 | 1P = 0.07 P = 0.03 | 28.5 P = 0.08 | ||
| T1-Signal characteristics | ||||||
| Hyperintense (n = 5) | 59.7 ± 16.5 | 3.6 ± 2.6 | 92.8 ± 3.2 | 4 | 1 | 0 |
| Isointense (n = 16) | 46.5.0 ± 9.9 | 1.4 ± 2.2 | 96.2 ± 1.6 | 5 | 8 | 3 |
| Hypointense (n = 15) | 50.1 ± 25.0 | 2.5 ± 2.8 | 93.5 ± 1.7 | 6 | 9 | 0 |
| P value for | 1P = 0.44 | 1P = 0.25 | 1P = 0.44 | 28.1 2P = 0.09 | ||
| Wash-in | ||||||
| Wash-in (n = 33) | 50.7 ± 18.9 | 2.3 ± 2.6 | 94.3 ± 6.6 | 15 | 15 | 3 |
| No wash-in (n = 3) | 40.6 ± 12.0 | 0.6 ± 0.6 | 98.4 ± 2.1 | 0 | 3 | 0 |
| P value for | 3P = 0.37 | 3P = 0.25 | 3P = 0.046 | 24.4 P = 0.11 | ||
| Wash-out | ||||||
| Wash-out (n = 25) | 49.7 ± 18.2 | 2.1 ± 2.3 | 94.8 ± 5.8 | 6 | 16 | 3 |
| No wash-out (n = 11) | 50.2 ± 20.2 | 2.1 ± 3.2 | 94.4 ± 8.2 | 9 | 2 | 0 |
| P value for | 3P = 0.94 | 3P = 0.98 | 3P = 0.89 | 211.6 P = 0.005 | ||
| Growth-pattern | ||||||
| Infiltrative (n = 5) | 53.4 ± 21.0 | 1.0 ± 1.1 | 94.0 ± 6.8 | 2 | 3 | 0 |
| Encapsulated (n = 31) | 49.0 ± 18.6 | 2.4 ± 2.7 | 97.4 ± 3.7 | 12 | 16 | 3 |
| P value for | 3P = 0.67 | 3P = 0.07 | 3P = 0.13 | 21 P = 0.60 | ||
Wash-in and wash-out contrast kinetics were found at MRI in 33 of 36 lesions (91.7%) and 25 of 36 lesions (69.4%), respectively. A wash-out kinetic was observed significantly more often in higher graded lesions [G2, 16/18 (89%) and G3, 3/3 (100%)] than in G1 tumors [6/15; (40%)], (χ2 =11.6, P = 0.005). 26 lesions exhibited a “typical” MR-pattern whereas 10 lesions presented an “atypical” pattern.
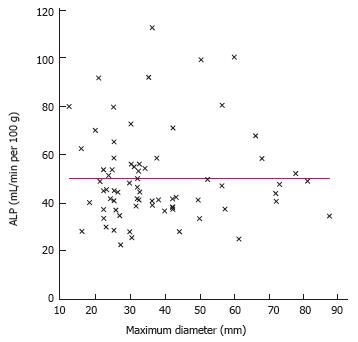
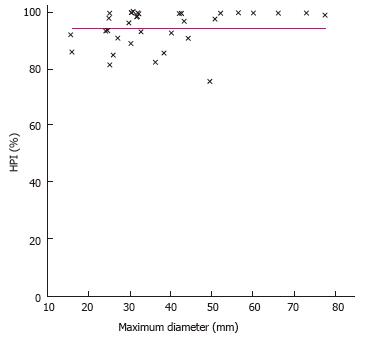
Tumor size was not found to be related to a distinct signal pattern in MR. Furthermore, the degree of arterialization (HPI) or the absolute amount of arterial perfusion was also not related to the maximum diameter of the tumor (Figures 2 and 3).
Analysis of different signal patterns in T1- and T2-weighted sequences as well as the presence of a wash-in and wash-out kinetic did not result in significantly altered VPCT parameters (scattered plots, Figures 4 and 5, Table 3).
No significant correlation was found between the magnitudes of perfusion parameters and the tumor growth pattern (encapsulated vs infiltrative).
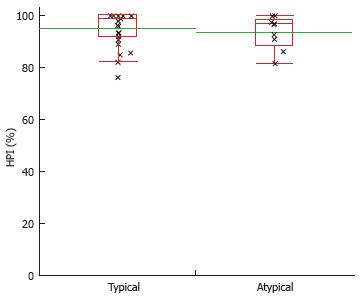
There was no significant difference between perfusion parameters measured by VPCT and MR imaging characteristics in the subgroups of “typical”vs“atypical” HCCs.
Despite different combinations of MR signal patterns, all HCC-lesions typically showed a strong arterial blood supply and a high HPI in VPCT imaging (Figures 1, 3 and 4, Table 4).
| MR-pattern | BF (mL/100 g tissue/min) | BV (mL/100 mL) | k-trans (mL/min per minute) | ALP (mL/100 g tissue/min) | PVP (mL/100 g tissue) | HPI (%) |
| Typical lesions (n = 26) | 63.3 ± 22.3 | 15.4 ± 9.8 | 36.4 ± 12.7 | 48.9 ± 16.3 | 2.0 ± 2.7 | 95.0 ± 6.6 |
| Atypical lesions (n = 10) | 58.1 ± 28.1 | 15.2 ± 7.4 | 34.4 ± 20.0 | 52.5 ± 24.6 | 2.5 ± 2.3 | 93.7 ± 6.3 |
| t test | P = 0.62 | P = 0.94 | P = 0.84 | P = 0.68 | P = 0.58 | P = 0.61 |
Our results show that the high degree of arterialization of HCC as expressed by the HPI assessed with help of VPCT in patients with liver cirrhosis and histologically proven HCC is independent of the highly variable MRI imaging characteristics. Accordingly in our series of biopsy prove HCC lesions HPI proved to be a confident imaging marker for arterialisation of HCC lesions and thus provides an additional marker for the diagnosis of HCC in all different gradings. By this VPCT enhances confidence in the HCC diagnosis especially in the case of an unusual native MR signal constellation or unusual contrast enhancement pattern in multiphase MRI. However even VPCT parameters failed to enable a grading of HCC based on imaging and functional fingerprints. In this series neither the lesions size, nor the growth pattern proved to have an impact on perfusion parameters or MR signal characteristics. In our opinion, these results encourage the use of perfusion-based lesion characterization as an additional tool for HCC diagnosis in liver cirrhotic patients by using, e.g., VPCT quantification especially in case of inconclusive signal combinations in MRI.
Characterization of liver lesions in patients with liver cirrhosis is essential for early diagnosis of malignant transformation. Knowingly, during the multistep process of hepatocarcinogenesis, tumor signal intensity on MRI typically changes from initially isointense to liver parenchyma in all sequences towards hyperintense on T2w and hypointense on T1w in advanced HCC[15]. In between, different signal intensity combinations are possible, which render this approach less reliable. Accordingly, Hecht et al[16] found that T2w imaging was insensitive for identifying HCC or dysplastic nodules by correlation with histology. Moreover, several variant types of HCC have been pathologically described, each of them exhibiting radically different textures with great impact on MR-signal[17]. However, histological data confirmed that throughout the subtypes. there is a tendency towards increased arterial supply with increasing nodule dedifferentiation paralleled by a drop in portal venous blood contribution[18].
Therefore, accurate assessment of the degree of tumor arterialization is expected to be a very specific fingerprint for identification of dedifferentiation in addition to variable conventional MR signal intensity combinations.
Reports correlating CT-arterioportal angiography results (CTHA and CTAP) with MR signal characteristics demonstrated a preponderance (70%) of signal hypointensity on T2w for liver nodules that were not arterialized and of hyperintensity on T2w (85%) in arterialized lesions. However, reliable quantification of the dual blood supply of liver nodules was not possible with this technique. Hence, the use of novel confident perfusion measurement techniques likes VPCT are expected to elucidate the nature and concomitantly the prognostic role of hepatic nodules in patients with liver cirrhosis.
First of all, the results of our study show that quantitative evaluation of perfusion parameters (e.g., BF, BV, k-trans, ALP, PVP and HPI) by VPCT is feasible.
At this point our results yielded a robust correlation between degree of arterialisation (HPI) and HCC histology. They also show that tumor vascularization is variable which reflects tumor-specific histological characteristics, but that all HCCs are highly vascularized presenting an almost exclusive arterial blood supply as shown by HPI. Concomitantly, MR signal characteristics proved heterogeneous with a variety of signal intensity constellations throughout the standard sequences. Importantly, calculated perfusion parameters did not significantly correlate with any tumor signal intensity combination (e.g., T1w-hypointense and T2w-hyperintense) expected in overt HCC, thus challenging earlier reports. However, we registered a slight trend towards increased signal intensity on T1w in better differentiated tumors. However, histological analysis in these cases could not confirm the presence of fat. Our results also contradict earlier statements addressing the issue of estimation of the histological grade of malignancy by measuring the intranodular blood supply using non-invasive imaging tools like CTHA + CTAP[14] as in our study even low grade HCCs in general showed an almost exclusively arterial blood supply as indicated by HPI. Notably, there was a trend towards increased ALP in well-differentiated HCC in our series which is in line with the results of van den Bos et al[7], but this finding did not reach statistical significance. This difference might be caused by a less homogenous attenuation of undifferentiated HCC due for instance to necrosis and intratumoral AV-shunts. This hypothesis is supported by a decreased ALP in higher grading (56.4 ± 23.7 mL/100 g tissue/min vs 41.2 ± 16.6 mL/100 g tissue/min) which is in line with earlier reports on this issue stating that the arterial flow might decrease in the late stage HCC[19,20]. The role of wash-in and wash-out enhancement patterns in MR imaging is by now well established. Ninety-one point seven percent of all lesions in our series presented wash-in enhancement pattern whereas only 69.4% presented typical wash-out pattern. In particular wash-out was found more frequent with progressive dedifferentiation of the tumor (P < 0.005). Interestingly, we found no correlation between tumor size and signal pattern in MRI, and also no correlation to the degree of tumor vascularization or even arterialisation. So, the tumor size is no guarantor for the presence of a more dedifferentiated HCC. The growth pattern (encapsulated vs infiltrative) proved to be independent of the perfusion parameters. Finally, we found also no match between the magnitude of perfusion parameters measured by VPCT and MR-signal characteristics that classified them into “typical” and/or “atypical”. In general perfusion parameters show a high variation which is not dependant on tumor grading and size.
There are limitations to our study. First, this was a retrospective analysis of MRI findings in HCC-patients undergoing VPCT for TACE monitoring. Second, MRI studies were performed on different MR-scanners using conventional imaging sequences without the use of liver-specific contrast agent. Third, histologic distribution of G1 to G3 tumors throughout this subgroup was uneven. Fourth, the number of evaluated patients is low which affects also the subgroup distribution and finally the power of statistical evaluation.
In summary, our preliminary study shows that there was no correlation between HCC perfusion parameters and any of the possible MR-signal characteristics as well as tumor size. However HPI measured with help of VPCT was very high in all biopsy proven HCCs. For this purpose, we advocate the additional use of perfusion-based liver tumor identification by VPCT for accurate HCC detection and characterisation in case of unclear MR signal characteristics. This preliminary data should be empowered by further research on this field to assess the potential additional benefit of VPCT for HCC diagnosis.
Magnetic resonance (MR) imaging to detect and characterize liver lesions is considered the gold standard to detect hepatocellular carcinomas (HCC). However HCC lesions do not always present typical signal constellations and contrast dynamics. Volume perfusion computed tomography (VPCT) is a method which has been recently introduced to evaluate perfusion based functional parameters of liver lesions.
It has been shown that VPCT can adequately detect the degree of arterialization of liver lesions. However it was not clear whether different MR signal constellations present with different perfusion parameters as measured by VPCT.
This is the first study to correlate different MR signal characteristics of HCC lesions with functional parameters as assessed by VPCT. It was demonstrated that all HCC lesions showed a high degree of arterialization independent of tumor size or MR signal constellation.
In case of lesions with unclear MR signal characteristics the additional use of VPCT can be an additional hint to diagnose HCCs.
VPCT measures tissue density after intravenous contrast agent application. For this purpose, the total liver volume is scanned at multiple different points in time. The density in two different region of interests, in an afferent artery and the desired tissue, is compared in the subsequent mathematical analysis to calculate perfusion based parameters.
This manuscript has perfectly determined if functional perfusion parameters by means of VPCT differ in HCC lesions with typical and atypical MR pattern including enhancement patterns and the correlation with histology. They found hepatic perfusion index measured with help of VPCT was very high in all HCC lesions and the arterial liver perfusion decreased with increasing tumor dedifferentiation. The contents would give significant information.
Manuscript Source: Invited manuscript
Specialty Type: Radiology, Nuclear Medicine and Medical Imaging
Country of Origin: Germany
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gao GW, Tomizawa M, Zhang ZM, Zhu X S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4265] [Article Influence: 236.9] [Reference Citation Analysis (2)] |
| 2. | Duran R, Chapiro J, Schernthaner RE, Geschwind JF. Systematic review of catheter-based intra-arterial therapies in hepatocellular carcinoma: state of the art and future directions. Br J Radiol. 2015;88:20140564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 4. | Cruite I, Tang A, Sirlin CB. Imaging-based diagnostic systems for hepatocellular carcinoma. AJR Am J Roentgenol. 2013;201:41-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Zheng SG, Xu HX, Liu LN. Management of hepatocellular carcinoma: The role of contrast-enhanced ultrasound. World J Radiol. 2014;6:7-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Schraml C, Kaufmann S, Rempp H, Syha R, Ketelsen D, Notohamiprodjo M, Nikolaou K. Imaging of HCC-Current State of the Art. Diagnostics (Basel). 2015;5:513-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | van den Bos IC, Hussain SM, Dwarkasing RS, Hop WC, Zondervan PE, de Man RA, IJzermans JN, Walker CW, Krestin GP. MR imaging of hepatocellular carcinoma: relationship between lesion size and imaging findings, including signal intensity and dynamic enhancement patterns. J Magn Reson Imaging. 2007;26:1548-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Ichikawa T, Saito K, Yoshioka N, Tanimoto A, Gokan T, Takehara Y, Kamura T, Gabata T, Murakami T, Ito K. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol. 2010;45:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Mita K, Kim SR, Kudo M, Imoto S, Nakajima T, Ando K, Fukuda K, Matsuoka T, Maekawa Y, Hayashi Y. Diagnostic sensitivity of imaging modalities for hepatocellular carcinoma smaller than 2 cm. World J Gastroenterol. 2010;16:4187-4192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Kadoya M, Matsui O, Takashima T, Nonomura A. Hepatocellular carcinoma: correlation of MR imaging and histopathologic findings. Radiology. 1992;183:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 195] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Wanless IR. Nodular regenerative hyperplasia, dysplasia, and hepatocellular carcinoma. Am J Gastroenterol. 1996;91:836-837. [PubMed] |
| 12. | Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Kim CK, Lim JH, Park CK, Choi D, Lim HK, Lee WJ. Neoangiogenesis and sinusoidal capillarization in hepatocellular carcinoma: correlation between dynamic CT and density of tumor microvessels. Radiology. 2005;237:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Shinmura R, Matsui O, Kobayashi S, Terayama N, Sanada J, Ueda K, Gabata T, Kadoya M, Miyayama S. Cirrhotic nodules: association between MR imaging signal intensity and intranodular blood supply. Radiology. 2005;237:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Efremidis SC, Hytiroglou P, Matsui O. Enhancement patterns and signal-intensity characteristics of small hepatocellular carcinoma in cirrhosis: pathologic basis and diagnostic challenges. Eur Radiol. 2007;17:2969-2982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Hecht EM, Holland AE, Israel GM, Hahn WY, Kim DC, West AB, Babb JS, Taouli B, Lee VS, Krinsky GA. Hepatocellular carcinoma in the cirrhotic liver: gadolinium-enhanced 3D T1-weighted MR imaging as a stand-alone sequence for diagnosis. Radiology. 2006;239:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Chung YE, Park MS, Park YN, Lee HJ, Seok JY, Yu JS, Kim MJ. Hepatocellular carcinoma variants: radiologic-pathologic correlation. AJR Am J Roentgenol. 2009;193:W7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Ueda K, Terada T, Nakanuma Y, Matsui O. Vascular supply in adenomatous hyperplasia of the liver and hepatocellular carcinoma: a morphometric study. Hum Pathol. 1992;23:619-626. [PubMed] |
| 19. | Kaufmann S, Horger T, Oelker A, Kloth C, Nikolaou K, Schulze M, Horger M. Characterization of hepatocellular carcinoma (HCC) lesions using a novel CT-based volume perfusion (VPCT) technique. Eur J Radiol. 2015;84:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Tajima T, Honda H, Taguchi K, Asayama Y, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, Shimada M, Masuda K. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol. 2002;178:885-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |