Published online Jul 28, 2016. doi: 10.4329/wjr.v8.i7.668
Peer-review started: October 6, 2015
First decision: January 15, 2016
Revised: March 24, 2016
Accepted: April 7, 2016
Article in press: April 11, 2016
Published online: July 28, 2016
Processing time: 301 Days and 17.5 Hours
Crohn’s disease affects more than 500000 individuals in the United States, and about 25% of cases are diagnosed during the pediatric period. Imaging of the bowel has undergone dramatic changes in the past two decades. The endoscopy with biopsy is generally considered the diagnostic reference standard, this combination can evaluates only the mucosa, not inflammation or fibrosis in the mucosa. Actually, the only modalities that can visualize submucosal tissues throughout the small bowel are the computed tomography (CT) enterography (CTE) with the magnetic resonance enterography (MRE). CT generally is highly utilized, but there is growing concern over ionizing radiation and cancer risk; it is a very important aspect to keep in consideration in pediatric patients. In contrast to CTE, MRE does not subject patients to ionizing radiation and can be used to detect detailed morphologic information and functional data of bowel disease, to monitor the effects of medical therapy more accurately, to detect residual active disease even in patients showing apparent clinical resolution and to guide treatment more accurately.
Core tip: Magnetic resonance enterography is an effective imaging modality to diagnosis, evaluating and follow-up of Crohn’s disease (CD) in pediatric patient and novel magnetic resonance imaging application, such as motility studies, spectroscopy, diffusion weighted imaging, molecular and hybrid imaging are extremely interesting and might contribute to diagnosis and managment of CD.
- Citation: Masselli G, Mastroiacovo I, De Marco E, Francione G, Casciani E, Polettini E, Gualdi G. Current tecniques and new perpectives research of magnetic resonance enterography in pediatric Crohn’s disease. World J Radiol 2016; 8(7): 668-682
- URL: https://www.wjgnet.com/1949-8470/full/v8/i7/668.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i7.668
A recent study shows the increase of the incidence of Crohn’s disease (CD) during childhood and adolescence[1,2]. In childhood, rates of CD increase from the first year of life, with highest rates in teenage years. The highest rate of CD occurs during adolescence. Between 11 to 30 years old, is registered the highest number of diagnosis.
The economic effects of pediatric and adult CD are staggering and are estimated to be between $10.9 billion and $15.5 billion per year in the United Sates alone[3].
Diagnosis and follow-up of bowel CD in children and adults are typically based on a combination of patient history and physical examination, such as disease activity surveys [e.g., the Pediatric Crohn’s Disease Activity Index (CDAI)], laboratory assessment, endoscopy with biopsy, and imaging[4].
Clinical subjective CD activity measurements, including the Physician Global Assessment, the Harvey-Bradshaw Index, CDAI and the Pediatric Crohn’s Disease Activity Index (PCDAI), are predominantly subjective measurements[3-6].
While several studies[7-10] have demonstrated no correlation between MRE (Magnetic Resonance Enterography) findings and the CDAI, other studies[11-13,14-18] have shown a correlation.
In particular, only two pediatric studies have compared MRE to PCDAI; Laghi et al[14] demonstrated a statistically significant correlation between disease on MRE and PCDAI, instead Alexopoulou et al[15] demonstrated no correlation between magnetic resonance (MR) percentage of CE (%CE) with pediatric CDAI.
These studies[14,15,19,20] showing discordance between inflammation on endoscopy and subjective activity index measurements and suggests that subjective clinical activity measurements do not essentially reflect mucosal findings with an intrinsic unreliability of subjective techniques for assessment of bowel wall and extra-enteric soft tissue pathology.
Various imaging modalities can be used to diagnose and follow up pediatric patients with CD, including radiography, fluoroscopic studies, ultrasonography, CT, and MR imaging. Traditionally, pediatric CD has been diagnosed and monitored over time by using endoscopy and fluoroscopic endoluminal contrast material-enhanced studies (e.g., upper gastrointestinal series, small bowel follow-through, and contrast enema).
Endoscopy with biopsy is considered as gold standard in diagnosis CD in particular in lesions localized in terminal segment of the ileum and for capability of obtaining samples for histopathologic assessment[3].
However endoscopic techniques have some limitations including poor accessibility to remaining part of the small bowel (except for difficult and time-consuming enteroscopy), risk of bowel perforation and limited ability to evaluate extraluminal structures.
Therefore, these optical techniques alone may under-represent the extent of disease, particularly when considering that the mucosa has a high capacity for repair. Capsule endoscopy, although attractive, cannot be performed in very young children, does not allow for tissue sampling and is not reimbursed by national health service.
In the last ten years, the methods used to visualize the bowel, including computed tomography (CT) enterography and MR enterography in pediatric patients with CD, are increased. CT and MR enterography are similar imaging tests, both capable to identify CD in a sensitive and specific manner. The ECCO guidelines established the highest diagnostic accuracy of MR and CT enterography or enteroclysis imaging modalities. CT enterography, compared to MRE[21,22], has an high diagnostic value and offers a lot of advantages as: Excellent spatial resolution, more willingness of CT scanner, low costs and shorter examination times.
However CT enterography, compared with MRE, have the major disadvantage of exposure to ionizing radiation, that’s why it should not be routinely used in children. Even though the mean CT dose index has decreased in recent years with advances such as iterative reconstruction, it is still higher than ionizing radiation-free of MR enterography[23,24].
In pediatric patients has been detected the similitude between the MR enterography and CT enterography, by the Appropriateness Criteria of the American College of Radiology. One recent study[25], explained that the second technique was extremely accurate in detective active inflammation and in the absence of active inflammation, MR enterography showed high precision to detect the mural fibrosis[26].
The progress in MR techniques and the development of new sequences with short exposition time, which are less sensitive to motion artifacts, has enabled acquiring good quality images of abdominal organs in children and currently it is considered the method of choice for the evaluation of CD severity and activity as well as for monitoring treatment effectiveness in children[3].
The recent development of faster pulse sequences, in fact, gives an opportunity to provide a movie cine images[27]. Cine imaging allows the observation of bowel movements in a short period and in real time. It provides high temporal, spatial and contrast resolution for monitoring bowel peristalsis[28]. MR enterography also allows for imaging that high-lights multiple determinants of image contrast (e.g., T1 and T2 relaxivity, DWI, pre- and postcontrast imaging, etc.)[4]. New techniques as PET-MRI, molecular imaging and MR spectroscopy are trying out for improving diagnosis, management and treatment of CD. Aim of this review is to describe current techniques of pediatric MR enterography interpret bowel disease findings and propose new MR techniques regarding CD.
An important role is a proper patient preparation to guaranteed high-quality MR enterography images. The patients were instructed to eat light meals in the day before the examination and to be fasting in the day of the examination. To an adeguate small bowel distension, are necessary large amounts of oral fluid intake and the oral contrast agents to provide the luminal distension. Contrast agents could give same collateral effects such as diarrhea, abdominal pain and nausea. The oral contrast agents can be divided into 3 groups[29,30]: Biphasic [e.g., water, polyethylene glycol (PEG) and others], negative (e.g., contrast media with iron particles), or positive (e.g., gadolinium) contrast agents[29].
PEG is the most commonly used in MR enterography, appear as low signal intensity on T1 and high signal intensity on T2 images and these properties could supplies convenience in the detection of active inflammation (in particular on post-contrast T1W images)[30]. Ajaj et al[31] developed a mixed solution with 4 different oral contrast agents (water, lactulose, low-dose barium sulfate with sorbitol and methylcellulose) with which the diagnostic quality of the MRE images was further superior respect to the MRE images obtained using lactulose solution in the adequate lumen distension of small bowels, is more tolerable and the adverse effects are less respect to the oral contras agents routinely used[32-34].
A study of Masselli et al[35] has showed that intravenous glucagone, when administrated to children and adolescent undergoing MR enterography, improves visualization of the small and large bowel on post contrast T1 weighted 3D gradient Echo (GRE) images, including the terminal ileum. Small bowel distention in pediatric and young population was achieved by oral administration of 600-1000 mL (in general 900 mL) of PEG or water (when patients cannot tolerate the taste of consistency of VoLumen) during a 45-min period before the examination[29,30].
Antiperistaltic agent such as hyoscine butylbromide (Buscopan; Boehringer Ingelheim, Germany) or glucagon (Glucagen, Novo Nordisk, Bagsvaerd, Denmark) are used intravenously in patients to improve visualization of the bowel at MR imaging.
There are few published data[36-40] about the use of these spasmolytic medications in pediatric patients undergoing MR enterography. 1-2 mL/s. of intravenous gadolinium chelates are administrated through a peripheral intravenous catheter by using a power injection; the amount of contrast material injected is based on patient weight (0.1 mmol/kg of body weight). Imaging procedure was performed with the patient lying prone or supine.
An information sheet is delivered to all patients, who may find the description of the procedure and any risks related to ingestion of PEG[41-43].
MR imaging examination are performed with a 1.5 T MR imaging system and two six-channel phased - array abdominal coils. During the exam are used MR pulse sequences with insignificant sensibilities to motion artifacts (e.g., FISP, FFE, SSFP, FIESTA).
First of all, are acquired coronal and axial images (FISP) to evaluate adequate bowel distension with consecutive T2-weighted coronal and axial images (with half Fourier RARE sequences that minimize the artifacts due to small-bowel peristalsis), following by intravenously administration of Buscopan and after T1-weighted spoiled gradient echo and coronal T1-weighted fat-saturated sequences. Axial and coronal T1 GRE sequences, in arteria and portal phase, are obtained after giving gadolinium chelate (0.2 mg/kg with an injection rate of 3 mL/s) and the protocol is completed by diffusion-weighted study.
Common indications for pediatric MR enterography involve evaluating suspected, following up known CD and appraising its complications. The first indication is identified CD and differentiate it from other small-bowel disease. Subsequently must determine the number, length and location of the segment involved; if a stenosis is present, must classify it as inflammatory (distinguishing between mild, moderate and severe disease) or fibrous subtypes.
Differentiation between the subtypes is clinically important because active inflammation is usually treated medically unless there are extramural complication, while fibrostenotic disease characterized by obstructive symptoms, often requires surgery[19,44-46]. Finally, it’s important to assess the presence of mesenteric complication such as abscesses and fistulas and monitor response to medical therapy. There are many findings of CD at MR enterography that can be separated into intestinal and extraintestinal findings. Intestinal findings include bowel wall thickening, mucosal hyperenhancement, mural stratification, skip lesions, luminal narrowing, strictures and fistulas.
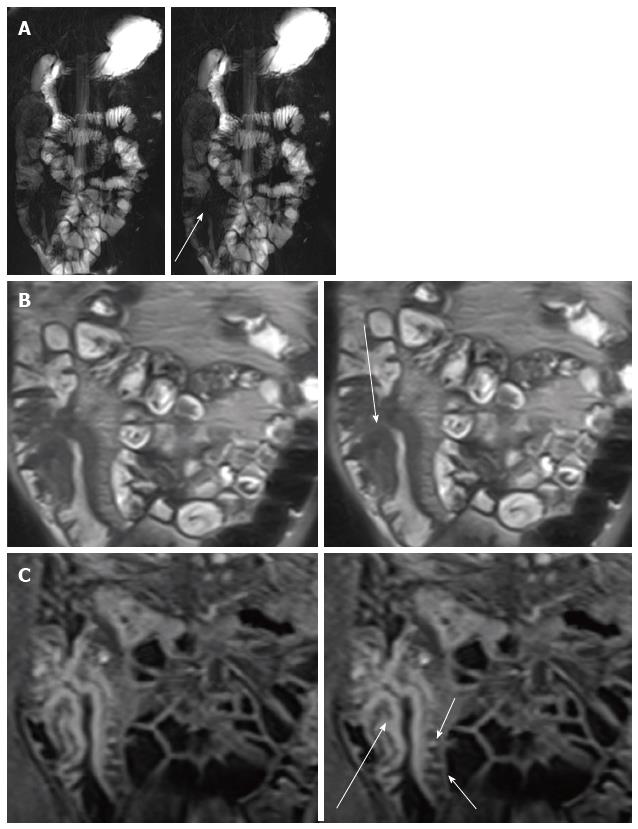
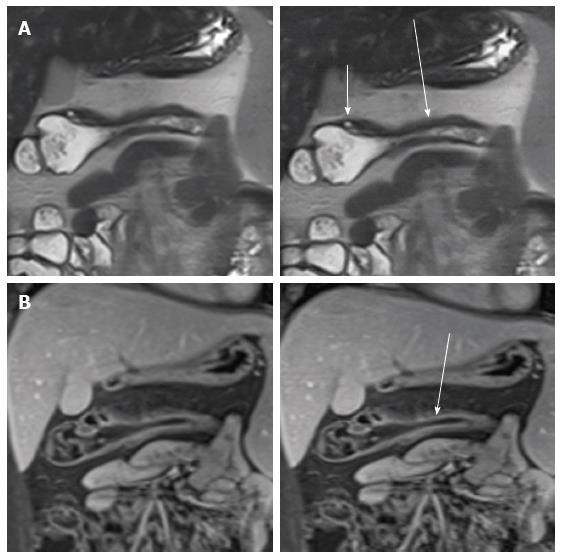
Masselli et al[47] suggested an imaging-based classification of small bowel CD subtypes correlate with clinical classifications. They radiologically classified CD into four groups: Active inflammatory, fibrostenotic, fistulizing/perforating, and reparative or regenerative subtype that could be differentiate by MR imaging due to its accuracy for the detection of morphologic and functional abnormalities, early CDs changes and help the clinicial plan appropriate therapy. The visualization of early changes due to CD, such as ulcerations and subtle wall thickening, can depict by high-resolution (thin-section) true FISP and half-Fourier RARE images (Figure 1)[48,49].
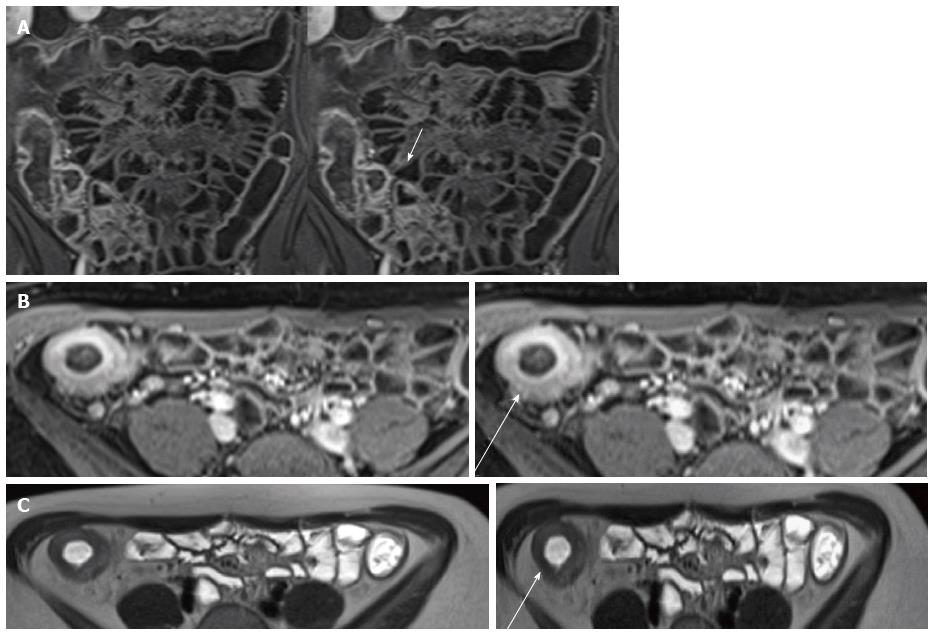
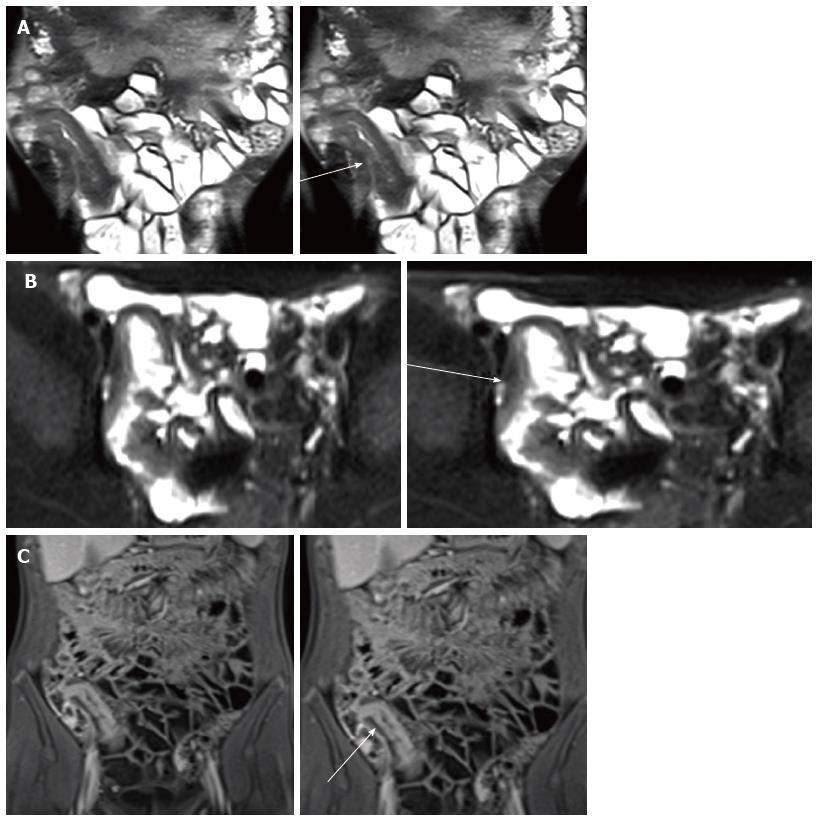
Bowel wall thickening is the common finding of CD with sensitivity of 83%-91% and a specificity of 86%-100% at MR enterography[50]. It is defined as a bowel wall thickness measuring more than 3 mm in a distended loop and may be eccentric or concentric and smooth or nodular and several study[5,43]. Masselli et al[42] confirmed that the terminal ileum is the most common localiazation of bowel wall thickness (Figure 2).
Stratified contrast enhancement with avid enhancement of the mucosa relative to the submucosa and muscular layers helps to confirm active CD[51] (Figure 1C). High signal intensity in T2-weighted images indicates wall edema and it is an acute inflammation’s sign[52] (Figure 3).
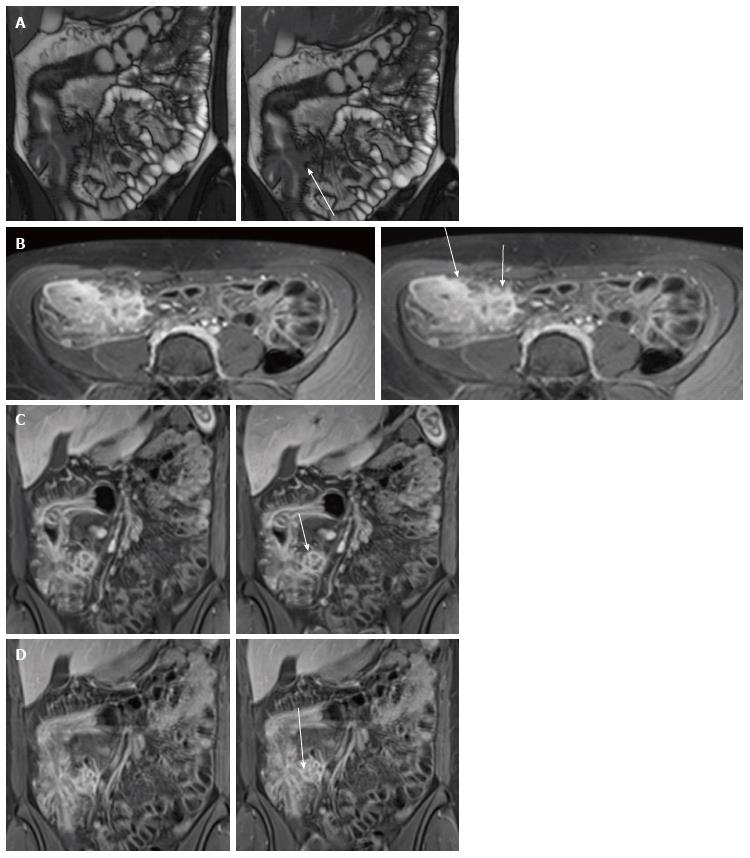
Mucosal increased enhancement with submucosal edema is so-called “stratified type of bowel enhancement” and has been especially related to acute disease[53] (Figure 4).
Mural stratification with engorged vasa recta that penetrate the bowel wall perpendicular to the bowel lumen (“comb sign”) which suggest that the disease is clinically active, advanced and extensive (Figure 1C)[49,54,55] and mesenteric lymphadenopathy, another finding in active disease[56]. On high-resolution SSFP image with fat suppression, it is possible to appreciate aphthous ulcers and transmural ulcers in the bowel wall[57] (Figure 5).
In the fibrostenotic disease subtype, instead, small bowel obstruction is the principal clinical manifestation and it is characterized as a fixed narrowing of the bowel, wall thickening and marked prestenotic dilatation, the latter less likely responsive to medical therapy[58].
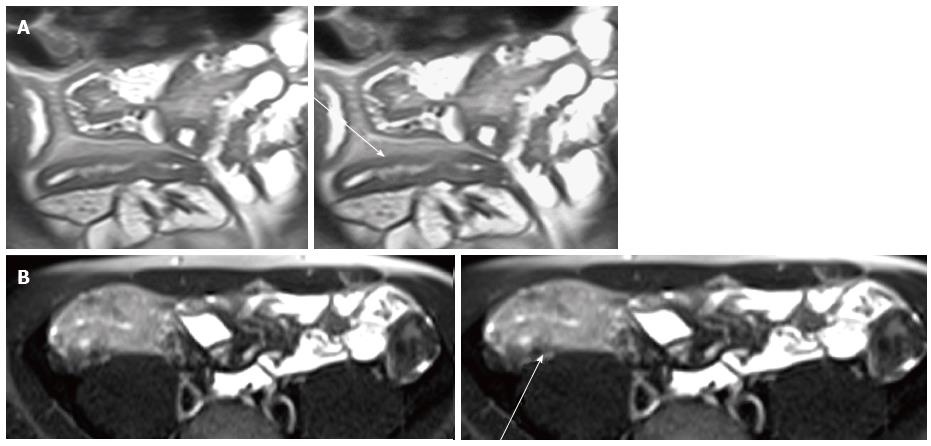
Chronic fibrotic strictures have low intensity signal on T1 and T2 sequences with inhomogeneous contrast enhancement (lack of mural inflammation) edema and surrounding mesenteric hyperemia[59,60] and in many patients are likely due to a combination of active and chronic inflammation and fibrosis[61] (Figure 6) and on MR cine imaging, it appears as a peristaltic bowel segments with mural thickening and luminal narrowing[48,62].
As pediatric CD, uncommonly manifests initially as a strictures[63], the cumulative incidence of strictures increases with time, from 5.5% at 1-year following diagnosis to 20.5% at 10 years[64].
In the fistulizing-penetrating subtype, involvement of bowel wall can range from superficial erosion and aphthous ulcers to fistula or perforation[61,65-67].
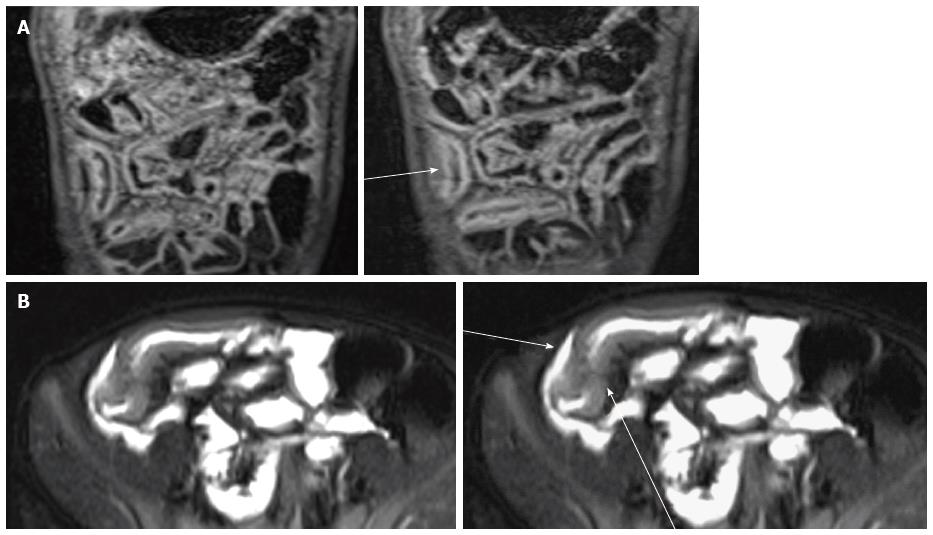
These tracts develop in 8.2% of pediatric CD patients at 1 year after diagnosis and 24.5% at 10 years[64] and they are commonly associated with a stricture. By definition, sinus tracts are blind and may extend into adjacent structures (e.g., mesentery, retroperitoneal musculature or abdominal wall), whereas fistula tracts communicate with a second epithelialized surface, as bowel, skin and genitourinary tract[67-69] (Figure 7).
These tracts are commonly recognized on single-shot FSE, balanced SSFE and fat-saturated T2-weighted FSE pulse images (with a sensitivity ranges from 83.3% to 84.4% and specificity of 100%[33,56,66,70]) as linear or stellate hypointense or hyperintense (if fluid-filled) abnormalities and also commonly enhance on post-contrast T1-weighted images[3]. Diagnostic advanced in CD: DWI, Perfusion, Motility Imaging, Magnetic Resonance Spectroscopy (MRS), and PET-MRI. Inflammatory process of the bowel wall in CD may be evaluated by most of these techniques that, as yet, have been applied to neurologic or oncologic disease.
However, over recent years, this imaging techniques showed great interest also in the evaluation and characterization of the bowel wall abnormalities of the CD and further developments, nowadays purely experimental (molecular imaging and PET-MRI), will provide relevant information on the status of inflammatory cells[71].
Diffusion-weighted magnetic resonance imaging used the diffusion of water molecules in biological tissues (intracellular, extracellular and vascular space) to produce images by random translation motion, known as Brownian motion, that in cells is more restricted than in extracellular or intravascular spaces[72,73].
The apparent diffusion coefficient (ADCs) is quantitative expressions of the diffusion characteristics of tissue. Its values decrease with increased tissue cellularity or cell density and may help in the quantitative analysis of disease activity[74,75]. In inflammatory disease of the bowel[75,76] the increased cellularity lead to rescrict diffusion (low ADC values) and high signal of DVI showed high sensitivity (86%-94%) and specificity (81.4%-84.8%) in inflammatory disease of the bowel with 94% of sensitivity and 88% of specificity by using an ADC threshold of 2.4 × 10-3 mm2/s[75,77-81].
An observational prospective study[79] with 130 CD patients reported that, at certain apparent diffusion coefficient, sensitivity and specificity of discriminating active from non-active CD were 96.6% and 98.1% respectively, for the colon/rectum, and 85.9% and 81.6%, respectively for ileum. They also reported high interobserver agreement.
A recent study[80] involved 31 CD patients with ileal involvement, compared DWI with conventional MRE in estimating inflammation in small bowel CD; DWI hyperintensity was highly correlated with disease activity evaluated using conventional MRE.
Koh et al[76] reported that a segmental magnetic resonance score (MR-score-S) based on DWI values and on other MRI parameters, detected endoscopic inflammation with a sensitivity and specificity of 58.33% and 84.48% in CD. In another study, Hordonneau et al[78] compared the ADC values of inactive intestinal segments (from jejunum to rectum) with ADC values of active intestinal segments on the basis of signal intensity in DWI sequences (as expressed in the values of b equal to 800 s/mm2) and they found a restriction of diffusion at the level of active segments as compared to non-active ones, without use of oral contrast.
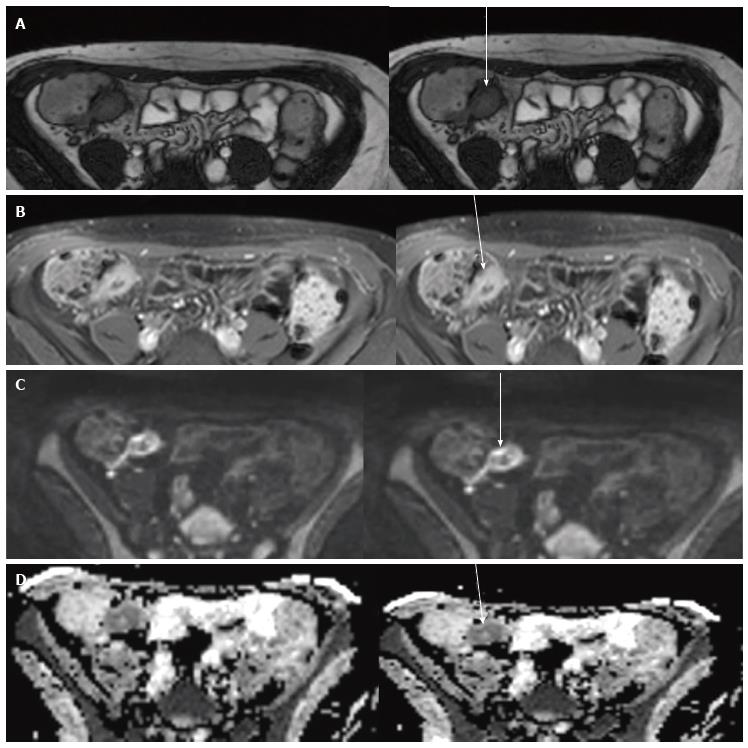
Preliminary studies[71,82] suggest that active wall inflammation in CD determines a restricted diffusion and it may be helpful in clinical practice to identify the sites of active CD, particularly when using biphasic intestinal contrast agents (Figure 8).
Although other studies are needed to define the practical clinical value of DWI; it is unclear if restricted diffusion is applied to acute wall inflammation (edema) only or wall fibrosis only or inflammation associated with fibrosis.
Dynamic contrast-enhanced MRI (DCE-MRI), gives information about physiological tissue characteristics and it is enable to provide quantitative and semiquantitative measurements of perfusion, in this case, of bowel wall, on the bases of the kinetics of contrast media uptake and wash-out[83,84].
In CD, acute inflammation, is characterized by an increase of vascular perfusion correlated with the activation of angiogenesis due to continuous epithelial damage and vascular remodeling of the intestinal mucosa.
This abnormal distribution of arteries increases the accuracy of DCE-MRI in the determination of disease activity through a quantitative or a semiquantitative approach. The first approach is based on the evaluation of two parameters (volume transfer coefficient Ktrans and extracellular volume fraction Ve) directly related to the uptake and wash-out of contrast, applying at intravascular vs extravascular - extracellular space. The second approach, instead, assessed parameters directly derived by the time-enhancement curve, area under the curve, enhancement slope, time to pick enhancement and enhancement ratio, which are easier and faster to be calculated but are not directly related to pathophysiology[71].
However, few study, performed on small series, have been published on the accuracy of DCE-MRI in CD diverging results mainly related to technical limitation (motion artifacts) resulting in measurements misregistration[81,85,86].
Many pathologies affecting the small bowel such as diabetes, dyspepsia, irritable bowel disease and visceral neuropathies, can alter its motility. CD also affects motility of inflamed small bowel segments. The use of MRI could have an impact on the research of small bowel physiology and pathologies. MR motility imaging used to evaluated CD related affected bowel segments showed an increase in the member of lesions in each patient and significant increase in the overall number of patients with CD lesions[87-90].
The images must be acquired before the application of a spasmolitic drug such as glucagon or n-hyoscine[71]. The sequences to acquire small bowel motility is a fast cine sequences using T2-W SSFP or echo planar imaging sequences with a maximum repetition time of 1 s and slice thickness of 10 mm.
A retrospective study of Patak et al[91] correlated MR-detectable motility alterations of the terminal ileum with biopsy documented active and chronic changes in CD. It analyzed 43 patients and the evaluation was done between motility (classified as normal, hypomotility and complete arrest) and local biopsy. Histopathology correlated with grading of motility alterations in both active and chronic signs. It seems that the motility changes are more a grading for severity of the disease than a predictor of activity. Another study of Maccioni et al[71] showed an inverse correlation between the contraction frequency via MRI and both the blood levels of C-reactive protein and focal levels of calprotectin.
In conclusion, motility can be quantified by MR imaging, but further studies are necessary to classify motility disorder assessed by MR.
MR molecular imaging and MRS are still two experimental techniques, have both the capability to study molecular composition of inflammatory bowel wall, identifying the metabolites involved in physiological and pathological process[71].
MRS is already routinely used in many malignant conditions such as brain, breast and prostate cancer and it provides to determine the distribution of metabolites associated with the relevant pathology producing predictive pattern of resonant frequencies corresponding to molecular arrangement of some atomic nuclei susceptible to perturbation, typically protons. The structural, or chemical information regarding the reaction of the nuclei can be obtained and after the examination is performed the data in a one-dimensional Nuclear MR (NMR) frequency spectrum[89,90]. Even thought spectroscopy can by perform on different nuclei; the most common nuclei used are those that not require exogenous label such as 31P, 1H and 23Na which generate spectra from endogenous metabolites. Spectroscopy of hydrogenous nucleus (1HNMR) is the most widely studies in MRS and the feasibility of metabonomics in clinical studies was suggested by the analysis of 1HNMR on plasma and urine samples obtained from healthy studies.
The 1HNMR spectra obtained were analyzed using principal component analysis to generate metabonomic data. This approach has been suggested as a quantitative measurement of metabolic response in CD. Biochemical analysis of fecal extracts has been studies to reflects biochemical changes of bowel disease in patient with CD and by employing 1HNMR to spectroscopy multivariate pattern recognition techniques was reported to differentiate two IBD[91].
Lately there was a rise of experimental studies regarding the research of small metabolites (as TCA) cycle intermediates for the screening of metabolic biomarkers in serum urine, fecal extracts and colon tissue in patients with IBD.
A study using in vitro1HNMR reported that patients with IBD showed similar metabolic profile in macroscopically involved and uninvolved colonic mucosa compared with that of control[92,93].
In the mucosa of active phase of UC and CD, have been observed lower concentration of amino-acids, membrane components, lactate and succinate and an increase of alpha-glucose compared with normal mucosa of controls. Instead, during chronic inflammation there was a decreasing of levels of proteins and carbohydrate due to deterioration of mucosa integrity. An analysis of the fecal extract of both CD and UC patients, showed reducted levels of butyrate, acetate, methylamine and trimethalamine and a high amount of aminoacids, implying malabsorbition. Metabolic difference in fecal profiles were more marked in the CD group because of the extent of the disease and the analysis reported that glycerol resonances were a feature of patients with CD[91].
In a study based on urinary metabolomic, individual with IBD can be distinguished from healthy ones by difference on the levels of TCA cycles intermediates, aminoacid and gut microflora metabolites[94].
NMR has also shown possibilities to differentiate between UC and CD, which is not always easy on clinical practice and 1HNMR in particular could be used as part of metabonomics to diagnosis with other disease with similar signs and symptoms[19].
A recent study, in fact, focused on findings of metabolic biomarkers and the correlation with serum zinc in CD patients, suggested two amino acid valina and isoleucina - as differentiating metabolites for CD diagnosi[95]. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT depend on the accumulation of FDG in metabolically active cells and is used for detect active disease sites of CD trought a standardized uptake values (SUV) that reveal areas of inflammation and it can be used also in the analysis of response to treatment[96,97].
However 18FDG PET has some limits to detect detailed morphologic information therefore benefits of combined use of CT and MR enterography. 18 FDG-PET/CT enterography (CTE) increases the sensitivity and specificity in the detection and monitoring of disease because provides morphological, physiological and metabolic information and may also allow distinction between active or fibrotic strictures[98].
Patients fast for 4-6 h before the scan and the blood glucose level is checked to detect hyperglycemia. A weight-based dose of 3.3 MBq/kg (0.09 mCi/kg) was sufficient for diagnostic PET imaging in patients with CD. The patient should drink approximately 1350 mL of a refrigerated neutral oral contrast agent, such as VoLumen (Bracco Diagnostics, Inc., Princeton, NJ), for 45-60 min during the 18F-FDG uptake period. For CTE protocols, 80-150 mL of non-ionic contrast material is injected at 3-5 mL/s and with a 70 s scan delay. PET acquisitions are usually obtained, beginning at the bottom of the pelvis and finishing at the top of the diaphragm.
Malham et al[97] compared the measurement of disease activity with 18F-FDG PET/CT with endoscopic biopsies and reported a sensitivity of 82% and specificity of 97%, using 18F-FDG PET/CT and other studies have reported sensitivity of 85%-98% and specificity of 50%-89%[99-101].
Combination of PET with FDG and MRI, have been shown to be useful for diagnostic evaluation of a variety of inflammatory processes and CD could be a candidate target of this novel technique[102], with several advantages respect to PET/CT or SPECT/CT or MRI alone due to MRI excellent soft tissue contrast, diffusion weighted imaging, dynamic contrast enhanced imaging, fMRI and MR spectroscopy, improving the sensitivity and specificity of diagnosis and follow-up treatment monitoring the possibility of earlier response evaluation[64,103].
PET/MRI system can be either simultaneous or sequential.
Simultaneous imaging systems have major advantages as compared to conventional PET/TC systems, due to an identical position of the patient during image acquisition, whit a substantial reduction in motion artifacts due to heart beating. An important advantage of MRI compared to CT is its superior functional soft tissue analysis, for example for inflammation, dynamic perfusion, and identification of different tissue types, such as edema and fibrosis.
While MRI mainly provides exquisite morphological details in human tissue, PET investigates the human body at the molecular level enabling the acquisition of exquisite functional data in particular when PET is combined also with functional MRI like DWI, spectroscopy, and combined new paramagnetic nano-cell-contrast agent with radiolabelled probes for histological characterization of tissue.
However the major advantage compared with the clinical PET/CT systems of today is the absence of radiation burden, an important features in pediatric patient. Molecular MRI allows an hybrid between PET and MRI with cells like lymphocyte/macrophage marked with radionuclides or fluorescent or MRI contrast agent.
Tracking of lymphocyte/macrophage migration could give important information about pathological processes and helping to monitoring response to treatment. The use of nuclear medicine techniques and MRI, nowadays purely experimental, is essential for detection of active inflammatory cells and cytokines in IBD. The primary target of PET-MRI in IBD must be to evaluate if connected PET-MRI with targeted molecular imaging and various MRI techniques is able to find IBD with precision.
MR entrygraphy is an effective imaging modality to diagnosis, evaluating and follow-up of CD in pediatric patient while sparing children and adolescent from the potentially harmful effects of ionizing radiation exposure. Novel MRI applications such as motility studies, spectroscopy, DWI, molecular and hybrid imaging (PET-MRI), might contribute to diagnosis and management of CD but further studies are necessary to assess the diagnosis value of these newer MRI application which are not fully predictable but extremely interesting in the evaluation of CD.
Specialty Type: Radiology, Nuclear Medicine and Medical Imaging
Country of Origin: Italy
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Algin O, Chiba T S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Diefenbach KA, Breuer CK. Pediatric inflammatory bowel disease. World J Gastroenterol. 2006;12:3204-3212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 2. | Malaty HM, Fan X, Opekun AR, Thibodeaux C, Ferry GD. Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatr Gastroenterol Nutr. 2010;50:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Mollard BJ, Smith EA, Dillman JR. Pediatric MR enterography: technique and approach to interpretation-how we do it. Radiology. 2015;274:29-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Podgórska J, Pacho R, Albrecht P. MR enterography imaging of Crohn’s disease in pediatric patients. Pol J Radiol. 2014;79:79-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Day AS, Ledder O, Leach ST, Lemberg DA. Crohn’s and colitis in children and adolescents. World J Gastroenterol. 2012;18:5862-5869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Griffiths AM, Hugot JP, Crohn disease. Ontario, Canada: BC Decker 2004; . |
| 7. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 792] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 8. | Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 9. | Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1527] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 10. | Sidhu R, Sanders DS, Morris AJ, McAlindon ME. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut. 2008;57:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Hall B, Holleran G, Costigan D, McNamara D. Capsule endoscopy: High negative predictive value in the long term despite a low diagnostic yield in patients with suspected Crohn’s disease. United European Gastroenterol J. 2013;1:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Wold PB, Fletcher JG, Johnson CD, Sandborn WJ. Assessment of small bowel Crohn disease: noninvasive peroral CT enterography compared with other imaging methods and endoscopy--feasibility study. Radiology. 2003;229:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 231] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Laghi A, Borrelli O, Paolantonio P, Dito L, Buena de Mesquita M, Falconieri P, Passariello R, Cucchiara S. Contrast enhanced magnetic resonance imaging of the terminal ileum in children with Crohn’s disease. Gut. 2003;52:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Alexopoulou E, Roma E, Loggitsi D, Economopoulos N, Papakonstantinou O, Panagiotou I, Pahoula I, Kelekis NL. Magnetic resonance imaging of the small bowel in children with idiopathic inflammatory bowel disease: evaluation of disease activity. Pediatr Radiol. 2009;39:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | D’Incà R, Caccaro R. Measuring disease activity in Crohn’s disease: what is currently available to the clinician. Clin Exp Gastroenterol. 2014;7:151-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3526] [Article Influence: 271.2] [Reference Citation Analysis (5)] |
| 18. | Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 755] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 19. | Yoon K, Change KT, Lee HJ. MRI for Crohn’s disease: Present and Future BioMed Research International Article, 2015. |
| 20. | Sinha R, Rajiah P, Ramachandran I, Sanders S, Murphy PD. Diffusion-weighted MR imaging of the gastrointestinal tract: technique, indications, and imaging findings. Radiographics. 2013;33:655-676; discussion 676-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Dillman JR, Adler J, Zimmermann EM, Strouse PJ. CT enterography of pediatric Crohn disease. Pediatr Radiol. 2010;40:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Towbin AJ, Sullivan J, Denson LA, Wallihan DB, Podberesky DJ. CT and MR enterography in children and adolescents with inflammatory bowel disease. Radiographics. 2013;33:1843-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Kambadakone AR, Prakash P, Hahn PF, Sahani DV. Low-dose CT examinations in Crohn’s disease: Impact on image quality, diagnostic performance, and radiation dose. AJR Am J Roentgenol. 2010;195:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Huprich JE, Rosen MP, Fidler JL, Gay SB, Grant TH, Greene FL, Lalani T, Miller FH, Rockey DC, Sudakoff GS. ACR Appropriateness Criteria on Crohn’s disease. J Am Coll Radiol. 2010;7:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Gee MS, Nimkin K, Hsu M, Israel EJ, Biller JA, Katz AJ, Mino-Kenudson M, Harisinghani MG. Prospective evaluation of MR enterography as the primary imaging modality for pediatric Crohn disease assessment. AJR Am J Roentgenol. 2011;197:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Quencer KB, Nimkin K, Mino-Kenudson M, Gee MS. Detecting active inflammation and fibrosis in pediatric Crohn’s disease: prospective evaluation of MR-E and CT-E. Abdom Imaging. 2013;38:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Courtier J, Ohliger M, Rhee SJ, Terreblanche O, Heyman MB, MacKenzie JD. Shooting a moving target: use of real-time cine magnetic resonance imaging in assessment of the small bowel. J Pediatr Gastroenterol Nutr. 2013;57:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Wakamiya M, Furukawa A, Kanasaki S, Murata K. Assessment of small bowel motility function with cine-MRI using balanced steady-state free precession sequence. J Magn Reson Imaging. 2011;33:1235-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Lauenstein TC, Schneemann H, Vogt FM, Herborn CU, Ruhm SG, Debatin JF. Optimization of oral contrast agents for MR imaging of the small bowel. Radiology. 2003;228:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Lomas DJ, Graves MJ. Small bowel MRI using water as a contrast medium. Br J Radiol. 1999;72:994-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Ajaj W, Goyen M, Schneemann H, Kuehle C, Nuefer M, Ruehm SG, Goehde SC, Lauenstein TC. Oral contrast agents for small bowel distension in MRI: influence of the osmolarity for small bowel distention. Eur Radiol. 2005;15:1400-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Kuehle CA, Ajaj W, Ladd SC, Massing S, Barkhausen J, Lauenstein TC. Hydro-MRI of the small bowel: effect of contrast volume, timing of contrast administration, and data acquisition on bowel distention. AJR Am J Roentgenol. 2006;187:W375-W385. [PubMed] |
| 33. | Masselli G, Casciani E, Polettini E, Lanciotti S, Bertini L, Gualdi G. Assessment of Crohn’s disease in the small bowel: Prospective comparison of magnetic resonance enteroclysis with conventional enteroclysis. Eur Radiol. 2006;16:2817-2827. [PubMed] |
| 34. | Umschaden HW, Szolar D, Gasser J, Umschaden M, Haselbach H. Small-bowel disease: comparison of MR enteroclysis images with conventional enteroclysis and surgical findings. Radiology. 2000;215:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Masselli G. Small Bowel Imaging: clinical application of the different imaging modalities- A comprehensive Review. ISRN Pathol. 2013;2013:13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Masselli G, Casciani E, Polettini E, Gualdi G. Comparison of MR enteroclysis with MR enterography and conventional enteroclysis in patients with Crohn’s disease. Eur Radiol. 2008;18:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 37. | Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, Prassopoulos P. MR enteroclysis: technical considerations and clinical applications. Eur Radiol. 2002;12:2651-2658. [PubMed] |
| 38. | Lee SS, Kim AY, Yang SK, Chung JW, Kim SY, Park SH, Ha HK. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 39. | Gupta A, Postgate AJ, Burling D, Ilangovan R, Marshall M, Phillips RK, Clark SK, Fraser CH. A prospective study of MR enterography versus capsule endoscopy for the surveillance of adult patients with Peutz-Jeghers syndrome. AJR Am J Roentgenol. 2010;195:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Cronin CG, Lohan DG, Mhuircheartaigh JN, McKenna D, Alhajeri N, Roche C, Murphy JM. MRI small-bowel follow-through: prone versus supine patient positioning for best small-bowel distention and lesion detection. AJR Am J Roentgenol. 2008;191:502-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Maccioni F, Al Ansari N, Mazzamurro F, Civitelli F, Viola F, Cucchiara S, Catalano C. Detection of Crohn disease lesions of the small and large bowel in pediatric patients: diagnostic value of MR enterography versus reference examinations. AJR Am J Roentgenol. 2014;203:W533-W542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Masselli G, Picarelli A, Di Tola M, Libanori V, Donato G, Polettini E, Piermattei A, Palumbo P, Pittalis A, Saponara A. Celiac disease: evaluation with dynamic contrast-enhanced MR imaging. Radiology. 2010;256:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Torkzad MR, Ullberg U, Nyström N, Blomqvist L, Hellström P, Fagerberg UL. Manifestations of small bowel disease in pediatric Crohn’s disease on magnetic resonance enterography. Inflamm Bowel Dis. 2012;18:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Oto A, Kayhan A, Williams JT, Fan X, Yun L, Arkani S, Rubin DT. Active Crohn’s disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging. 2011;33:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 45. | Griffin N, Grant LA, Anderson S, Irving P, Sanderson J. Small bowel MR enterography: problem solving in Crohn’s disease. Insights Imaging. 2012;3:251-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Maglinte DD, Gourtsoyiannis N, Rex D, Howard TJ, Kelvin FM. Classification of small bowel Crohn’s subtypes based on multimodality imaging. Radiol Clin North Am. 2003;41:285-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Masselli G. The gastrointestinal string sign. Radiology. 2007;242:632-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Masselli G, Gualdi G. MR imaging of the small bowel. Radiology. 2012;264:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 49. | Sinha R, Verma R, Verma S, Rajesh A. MR enterography of Crohn disease: part 2, imaging and pathologic findings. AJR Am J Roentgenol. 2011;197:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Masselli G, Casciani E, Polettini E, Laghi F, Gualdi G. Magnetic resonance imaging of small bowel neoplasms. Cancer Imaging. 2013;13:92-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 51. | Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E, Bloom S, Cohen R, Windsor A, Obichere A, Hansmann A. Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology. 2009;252:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 52. | Rodriguez P, Mendez R, Matute F, Hernandez P, Mendoza JL. Imaging Crohn disease: MR enterography. J Comput Assist Tomogr. 2014;38:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Prassopoulos P, Papanikolaou N, Grammatikakis J, Rousomoustakaki M, Maris T, Gourtsoyiannis N. MR enteroclysis imaging of Crohn disease. Radiographics. 2001;21 Spec No:S161-S172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Madureira AJ. The comb sign. Radiology. 2004;230:783-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Gourtsoyiannis NC, Grammatikakis J, Papamastorakis G, Koutroumbakis J, Prassopoulos P, Rousomoustakaki M, Papanikolaou N. Imaging of small intestinal Crohn’s disease: comparison between MR enteroclysis and conventional enteroclysis. Eur Radiol. 2006;16:1915-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 56. | Leyendecker JR, Bloomfeld RS, DiSantis DJ, Waters GS, Mott R, Bechtold RE. MR enterography in the management of patients with Crohn disease. Radiographics. 2009;29:1827-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Masselli G, Polettini E, Casciani E, Bertini L, Vecchioli A, Gualdi G. Small-bowel neoplasms: prospective evaluation of MR enteroclysis. Radiology. 2009;251:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 58. | Al-Hawary MM, Zimmermann EM, Hussain HK. MR imaging of the small bowel in Crohn disease. Magn Reson Imaging Clin N Am. 2014;22:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Sinha R, Rajiah P, Murphy P, Hawker P, Sanders S. Utility of high-resolution MR imaging in demonstrating transmural pathologic changes in Crohn disease. Radiographics. 2009;29:1847-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Gore R, Masselli G, Caroline D. Crohn’s disease of thesmall bowel in Textbook of Gastrointestinal Radiology. Philadelphia: Saunders Elsevier 2008; 781-806. [DOI] [Full Text] |
| 61. | Froehlich JM, Waldherr C, Stoupis C, Erturk SM, Patak MA. MR motility imaging in Crohn’s disease improves lesion detection compared with standard MR imaging. Eur Radiol. 2010;20:1945-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 62. | Masselli G, Gualdi G. CT and MR enterography in evaluating small bowel diseases: when to use which modality? Abdom Imaging. 2013;38:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 63. | Gupta N, Bostrom AG, Kirschner BS, Ferry GD, Gold BD, Cohen SA, Winter HS, Baldassano RN, Abramson O, Smith T. Incidence of stricturing and penetrating complications of Crohn’s disease diagnosed in pediatric patients. Inflamm Bowel Dis. 2010;16:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Casciani E, Masselli G, Di Nardo G, Polettini E, Bertini L, Oliva S, Floriani I, Cucchiara S, Gualdi G. MR enterography versus capsule endoscopy in paediatric patients with suspected Crohn’s disease. Eur Radiol. 2011;21:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 65. | Schmidt S, Chevallier P, Bessoud B, Meuwly JY, Felley C, Meuli R, Schnyder P, Denys A. Diagnostic performance of MRI for detection of intestinal fistulas in patients with complicated inflammatory bowel conditions. Eur Radiol. 2007;17:2957-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Toma P, Granata C, Magnano G, Barabino A. CT and MRI of paediatric Crohn disease. Pediatr Radiol. 2007;37:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Tolan DJ, Greenhalgh R, Zealley IA, Halligan S, Taylor SA. MR enterographic manifestations of small bowel Crohn disease. Radiographics. 2010;30:367-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 68. | Booya F, Akram S, Fletcher JG, Huprich JE, Johnson CD, Fidler JL, Barlow JM, Solem CA, Sandborn WJ, Loftus EV. CT enterography and fistulizing Crohn’s disease: clinical benefit and radiographic findings. Abdom Imaging. 2009;34:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | Herrmann KA, Michaely HJ, Zech CJ, Seiderer J, Reiser MF, Schoenberg SO. Internal fistulas in Crohn disease: magnetic resonance enteroclysis. Abdom Imaging. 2006;31:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Masselli G, Brizi MG, Menchini L, Minordi L, Vecchioli Scaldazza A. Magnetic Resonance Enteroclysis imaging of Crohn’s. Radiol Med. 2005;110:221-233. [PubMed] |
| 71. | Stejskal EO, Tanner JE. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys. 1965;42:288-292. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6259] [Cited by in RCA: 5055] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 72. | Chavhan GB, Alsabban Z, Babyn PS. Diffusion-weighted imaging in pediatric body MR imaging: principles, technique, and emerging applications. Radiographics. 2014;34:E73-E88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1418] [Cited by in RCA: 1462] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 73. | Zhu J, Xu JR, Gong HX, Zhou Y. Updating magnetic resonance imaging of small bowel: imaging protocols and clinical indications. World J Gastroenterol. 2008;14:3403-3409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol. 2009;16:597-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 75. | Oussalah A, Laurent V, Bruot O, Bressenot A, Bigard MA, Régent D, Peyrin-Biroulet L. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut. 2010;59:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 76. | Koh DM, Miao Y, Chinn RJ, Amin Z, Zeegen R, Westaby D, Healy JC. MR imaging evaluation of the activity of Crohn’s disease. AJR Am J Roentgenol. 2001;177:1325-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 249] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 77. | Kiryu S, Dodanuki K, Takao H, Watanabe M, Inoue Y, Takazoe M, Sahara R, Unuma K, Ohtomo K. Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J Magn Reson Imaging. 2009;29:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 78. | Hordonneau C, Buisson A, Scanzi J, Goutorbe F, Pereira B, Borderon C, Da Ines D, Montoriol PF, Garcier JM, Boyer L. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol. 2014;109:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 79. | Buisson A, Joubert A, Montoriol PF, Da Ines D, Hordonneau C, Pereira B, Garcier JM, Bommelaer G, Petitcolin V. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment Pharmacol Ther. 2013;37:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 80. | Schmid-Tannwald C, Agrawal G, Dahi F, Sethi I, Oto A. Diffusion-weighted MRI: role in detecting abdominopelvic internal fistulas and sinus tracts. J Magn Reson Imaging. 2012;35:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 81. | Gowland P, Mansfield P, Bullock P, Stehling M, Worthington B, Firth J. Dynamic studies of gadolinium uptake in brain tumors using inversion-recovery echo-planar imaging. Magn Reson Med. 1992;26:241-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Florie J, Wasser MN, Arts-Cieslik K, Akkerman EM, Siersema PD, Stoker J. Dynamic contrast-enhanced MRI of the bowel wall for assessment of disease activity in Crohn’s disease. AJR Am J Roentgenol. 2006;186:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 83. | Oto A, Fan X, Mustafi D, Jansen SA, Karczmar GS, Rubin DT, Kayhan A. Quantitative analysis of dynamic contrast enhanced MRI for assessment of bowel inflammation in Crohn’s disease pilot study. Acad Radiol. 2009;16:1223-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Taylor SA, Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E, Forbes A, Cohen R, Windsor A, Obichere A. Mural Crohn disease: correlation of dynamic contrast-enhanced MR imaging findings with angiogenesis and inflammation at histologic examination--pilot study. Radiology. 2009;251:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 85. | Shyn PB. 18F-FDG positron emission tomography: potential utility in the assessment of Crohn’s disease. Abdom Imaging. 2012;37:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Patak MA, Cullmann J, Szeucs-Farkas Z. MR motility measurement for the evaluation of Crohn’s disease activity compared to biopsy, In: Book of abstract of ESGAR (European Society of Gastrointestinal and Abdominal Radiology) Annual Congress, Venice: 2011. |
| 87. | Mullins ME. MR spectroscopy: truly molecular imaging; past, present and future. Neuroimaging Clin N Am. 2006;16:605-618, viii. [PubMed] |
| 88. | Glaudemans AW, Maccioni F, Mansi L, Dierckx RA, Signore A. Imaging of cell trafficking in Crohn’s disease. J Cell Physiol. 2010;223:562-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 89. | Marchesi JR, Holmes E, Khan F, Kochhar S, Scanlan P, Shanahan F, Wilson ID, Wang Y. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J Proteome Res. 2007;6:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 475] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 90. | Sharma U, Singh RR, Ahuja V, Makharia GK, Jagannathan NR. Similarity in the metabolic profile in macroscopically involved and un-involved colonic mucosa in patients with inflammatory bowel disease: an in vitro proton ((1)H) MR spectroscopy study. Magn Reson Imaging. 2010;28:1022-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 91. | Storr M, Vogel HJ, Schicho R. Metabolomics: is it useful for inflammatory bowel diseases? Curr Opin Gastroenterol. 2013;29:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 92. | Stephens NS, Siffledeen J, Su X, Murdoch TB, Fedorak RN, Slupsky CM. Urinary NMR metabolomic profiles discriminate inflammatory bowel disease from healthy. J Crohns Colitis. 2013;7:e42-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 93. | Fathi F, Majari-Kasmaee L, Mani-Varnosfaderani A, Kyani A, Rostami-Nejad M, Sohrabzadeh K, Naderi N, Zali MR, Rezaei-Tavirani M, Tafazzoli M. 1H NMR based metabolic profiling in Crohn’s disease by random forest methodology. Magn Reson Chem. 2014;52:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 94. | Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJ. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med. 2010;51:1937-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 95. | Louis E, Ancion G, Colard A, Spote V, Belaiche J, Hustinx R. Noninvasive assessment of Crohn’s disease intestinal lesions with (18)F-FDG PET/CT. J Nucl Med. 2007;48:1053-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 96. | Däbritz J, Jasper N, Loeffler M, Weckesser M, Foell D. Noninvasive assessment of pediatric inflammatory bowel disease with ¹⁸F-fluorodeoxyglucose-positron emission tomography and computed tomography. Eur J Gastroenterol Hepatol. 2011;23:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Malham M, Hess S, Nielsen RG, Husby S, Høilund-Carlsen PF. PET/CT in the diagnosis of inflammatory bowel disease in pediatric patients: a review. Am J Nucl Med Mol Imaging. 2014;4:225-230. [PubMed] |
| 98. | Lemberg DA, Issenman RM, Cawdron R, Green T, Mernagh J, Skehan SJ, Nahmias C, Jacobson K. Positron emission tomography in the investigation of pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Löffler M, Weckesser M, Franzius C, Schober O, Zimmer KP. High diagnostic value of 18F-FDG-PET in pediatric patients with chronic inflammatory bowel disease. Ann N Y Acad Sci. 2006;1072:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Meisner RS, Spier BJ, Einarsson S, Roberson EN, Perlman SB, Bianco JA, Taylor AJ, Einstein M, Jaskowiak CJ, Massoth KM. Pilot study using PET/CT as a novel, noninvasive assessment of disease activity in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 101. | Jadvar H, Colletti PM. Competitive advantage of PET/MRI. Eur J Radiol. 2014;83:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 102. | Glaudemans AW, Quintero AM, Signore A. PET/MRI in infectious and inflammatory diseases: will it be a useful improvement? Eur J Nucl Med Mol Imaging. 2012;39:745-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 103. | Vandenberghe S, Marsden PK. PET-MRI: a review of challenges and solutions in the development of integrated multimodality imaging. Phys Med Biol. 2015;60:R115-R154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |