Published online May 28, 2016. doi: 10.4329/wjr.v8.i5.530
Peer-review started: September 7, 2015
First decision: December 7, 2015
Revised: January 25, 2016
Accepted: February 16, 2016
Article in press: February 17, 2016
Published online: May 28, 2016
Processing time: 263 Days and 9.7 Hours
AIM: To validate the feasibility of digital tomosynthesis of the abdomen (DTA) combined with contrast enhanced ultrasound (CEUS) in assessing complications after endovascular aortic aneurysm repair (EVAR) by using computed tomography angiography (CTA) as the gold standard.
METHODS: For this prospective study we enrolled 163 patients (123 men; mean age, 65.7 years) referred for CTA for EVAR follow-up. CTA, DTA and CEUS were performed at 1 and 12 mo in all patients, with a maximum time interval of 2 d.
RESULTS: Among 163 patients 33 presented complications at CTA. DTA and CTA correlated for the presence of complications in 32/33 (96.96%) patients and for the absence of complications in 127/130 (97.69%) patients; the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of DTA were 97%, 98%, 91%, 99%, and 98%, respectively. CEUS and CTA correlated for the presence of complications in 19/33 (57.57%) patients and for the absence of complications in 129/130 (99.23%) patients; the sensitivity, specificity, PPV, NPV and accuracy of CEUS were 58%, 99%, 95%, 90%, and 91%, respectively. Sensitivity, specificity and accuracy of combining DTA and CEUS together in detecting EVAR complications were 77%, 98% and 95%, respectively.
CONCLUSION: Combining DTA and CEUS in EVAR follow-up has the potential to limit the use of CTA only in doubtful cases.
Core tip: Follow-up of endovascular aortic aneurysm repair: A preliminary study to validate the feasibility of digital tomosynthesis of the abdomen combined with contrast enhanced ultrasound.
- Citation: Mazzei MA, Guerrini S, Mazzei FG, Cioffi Squitieri N, Notaro D, de Donato G, Galzerano G, Sacco P, Setacci F, Volterrani L, Setacci C. Follow-up of endovascular aortic aneurysm repair: Preliminary validation of digital tomosynthesis and contrast enhanced ultrasound in detection of medium- to long-term complications. World J Radiol 2016; 8(5): 530-536
- URL: https://www.wjgnet.com/1949-8470/full/v8/i5/530.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i5.530
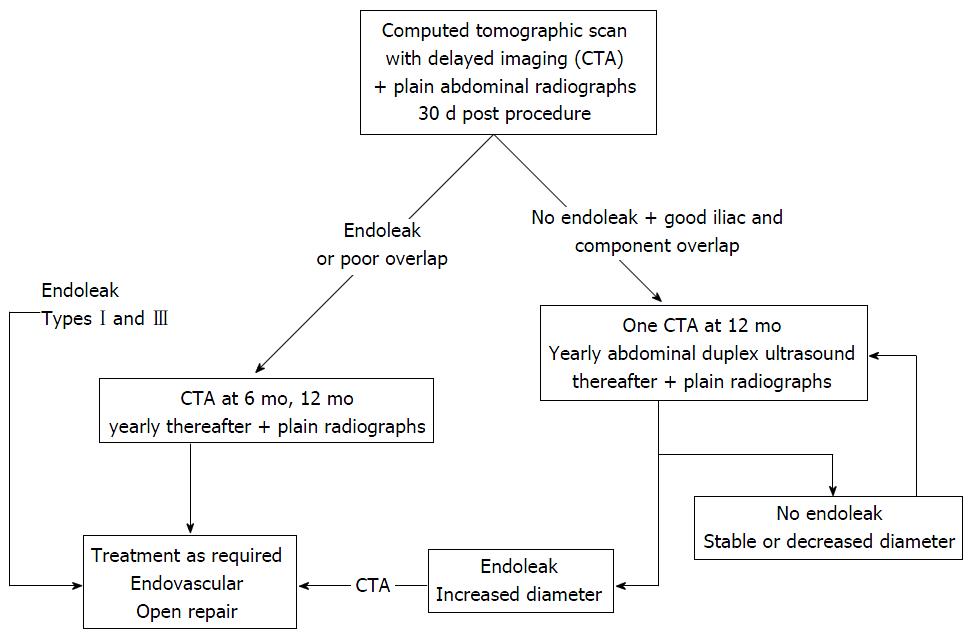
Endovascular aortic aneurysm repair (EVAR) is a safe technique first described by Blankensteijn et al[1]. It is associated with a significant reduction in perioperative mortality and morbidity compared to open repair[2]. The technical success of stent-graft implantation is well known and relatively safe but new data on the long-term efficacy of EVAR are emerging. In fact, after aortic-endovascular replacement, several medium- and long-term complications, such as graft failure (stenosis, angulation, kinking, device migration, stent fractures and modular disconnections), which may be associated with endoleak (extra-luminal leakage of the aneurysmal sac), the most common complication of EVAR, have been observed in cases of lifelong surveillance (Table 1)[2-4]. Follow-up of EVAR is therefore essential to diagnose and treat graft complications[5]. Current EVAR follow-up guidelines suggest computed tomography angiography (CTA) as the best method for detecting graft complications and endoleaks. CTA is recommended one month after EVAR and again at 6 and 12 mo in the case of endoleak at the first follow-up examination (Figure 1).
| Endoleak (type) | Source of perigraft flow |
| I | Attachment site |
| A | Proximal end of the stentgraft |
| B | Distal end of the stentgraft |
| C | Iliac occluder |
| II | Branch leaks without attachment site leaks |
| A | Simple: One patent branch |
| B | Complex: Two or more patent branches |
| III | Stentgraft defect |
| A | Junctional leak or modular disconnect |
| B | Fabric holes |
| IV | Stentgraft fabric porosity < 30 d after placement |
| Endoleak (time of detection) | Primary, present from time of EVAR Secondary, appearing after prior negative CTA |
| Endotension | AAA enlargement with increased intrasac pressure after EVAR without visualised endoleak on delayed contrast CTA |
Although CTA is the best method for complete non-invasive post-procedural assessment of aortic stent-grafting, current data on CTA in patients with impaired renal function or known allergic reaction to iodinated contrast medium encourage the use of alternative techniques of detecting complications, such as magnetic resonance angiography (MRA), Doppler ultrasound (DU) and plain radiography[5]. Other major concerns about the frequent use of CTA in EVAR follow-up are the cost and the cumulative amount of exposure to ionizing radiation with potential lifetime cancer risk, though the latter seems questionable from a radiobiological point of view since EVAR patients are usually over 65 years of age[6,7].
Plain radiography, using a standardised protocol with antero-posterior and lateral projections is suitable for follow-up assessment of angulation, kinking, device migration, stent fractures and modular disconnections, including material fatigue. This technique obviously has limits for the evaluation of aneurysm diameter and endoleaks, whereas digital tomosynthesis (DT), a new radiographic technique that can produce an arbitrary number of section images of a patient from a single pass of the X-ray tube, offers the potential to improve plain radiography, overcoming its limitations. DU is also recommended with plain radiography at 12-mo follow-up in the absence of endoleaks at the first CTA follow-up[4]. DU is a fast, easy, economical imaging technique that detects and images endoleak flow direction. Moreover, the lack of radiation exposure or nephrotoxicity makes it attractive, especially combined with intravenous contrast-enhancement [contrast-enhanced ultrasound (CEUS)], which has proven as accurate as CTA for detecting endoleaks and measuring abdominal aortic aneurysm diameter during EVAR follow-up. However, substantial interobserver variability, in measuring the diameter of aneurysm sacs and in detecting endoleaks, and lack of information about stent-graft integrity and migration, mean that DU has to be sustained by other diagnostic examinations for EVAR follow-up[6,8,9]. MRA with a blood pool contrast agent has been proposed as an accurate diagnostic tool for endoleak detection and in particular for type II endoleaks, but has limitations, such as patient cooperation, clinical contraindications, scanner availability and cost[10,11].
Considering the previous statements, the aim of the present study was to validate the feasibility of digital tomosynthesis of the abdomen (DTA) combined with CEUS, against CTA, for assessing medium- and long-term complications after EVAR, in a preliminary cohort of patients.
Institutional review board approval, as well as informed consent from all subjects, was obtained for this prospective study.
Between June 2013 and December 2014, 216 patients were referred to our centre for CTA of the abdomen for follow-up of EVAR. Fifty-three patients were excluded for the following reasons: 40 patients because of thoracic endografting and 13 because of renal failure. The remaining 163 patients (123 men, 40 women; mean age, 65.7 years; range, 53-96 years) were enrolled. CTA, DTA and CEUS were performed at 1 and 12 mo in all patients and also at 6 mo after EVAR in 41 out of 163 patients, due to a non-surgical complication detected at the first follow-up. The time interval between DTA/CEUS and CTA was no longer than 2 d.
CTA: All patients underwent 64-detector row computed tomography (CT) scans (Discovery HD 750, General Electric Healthcare, Milwaukee, United States). Abdominal aortic examination (from pelvic brim to thoracic outlet) was performed by a spiral technique in a caudo-cranial direction with the patient in a supine position. Patients were instructed to hold the breath during helical imaging to avoid motion artifacts. Pre-contrast scans were not performed. After a scout-view scan, an intravenous injection of 1.5 mL/kg non-ionic contrast material (Iomeprol 400 mg iodine/mL; Iomeron 400, Bracco Diagnostics, Milan, Italy), followed by 40 mL saline solution was administered with an 18-gauge needle via the antecubital vein, using a dual-barrel injector (4 mL/s flow rate, CT Motion, Ulrich Medical, Ulm, Germany). Arterial phase images were obtained 4 s after bolus detection in the suprarenal aorta. The following technical parameters were used: Effective slice thickness 1.25 mm, collimation 40 mm, beam pitch 0.969, reconstruction interval 0.8 mm, tube voltage 140 kVp and reference mAs 250/700. Automatic tube current modulation was used to minimize radiation exposure. A standard reconstruction algorithm was used.
DTA: In our study, we used a multipurpose digital tomosynthesis system (VolumeRAD option of Definium 8000, General Electric Healthcare, United States). The X-ray tube provided an uninterrupted vertical or horizontal movement for 10 s, achieving 60 low-dose projection images within ± 15° in the peripheral radiographic view. A continuous tube charge was used for each exposure which was determined by a scout image collected prior to the digital tomosynthesis acquisition. A user adjustable dose factor was used to multiply the automatic exposure control determined tube charge used for the scout image. This was then distributed over the low dose projections and the consequential exposure of each projection was then fixed to the closest tube current time setting. The low-dose projections were then merged to reconstruct section images of the aorta. Twenty-five acquisitions in the antero-posterior view were made at 85 kVp, 630 mA, 32.88 mAs, and 52.4 milliseconds, while the patient was in a supine position. A total of 35 slices were taken (reconstruction slice spacing was taken between 1 and 50 mm with a step of 1 mm[12]).
CEUS: All CEUS examinations were performed by two radiologists, with 2 and 5 years of expertise, respectively, blind to CTA findings. The scans were performed with an Esaote MyLab 70 XVG (Esaote, Florence, Italy), equipped with a convex 3.5-MHz probe. A typical US examination started with standard B-mode investigation to measure aneurysm sac diameter (outer wall to outer wall, dimensions recorded as the mean of three measurements). Blood flow from the main body of the endograft to the femoral arteries was then analyzed in a pulse wave mode. CEUS was performed after injection of 2.5 mL of SonoVue (Bracco Diagnostics, Milan, Italy), the only second-generation contrast agent approved in Italy, flushed with 5 mL of isotonic saline solution through an intravenous cannula. Endoleak detection was performed at a low mechanical index (0.2e0.3) with the focus positioned behind the aorta to delay bubble destruction. We classified endoleaks according to “Reporting standards for endovascular aortic aneurysm repair” (2002)[7].
All images were analyzed independently by two readers with 7 and 3 years of experience in vascular radiology, respectively. Differences were resolved by consensus. The two readers were blind to all clinical and pathological data. The DTA and CEUS images were read in random order. The readers recorded any findings considered a possible sign of EVAR complications and rated them on a 2-point scale according to the level of confidence in the presence or not of EVAR complication. The EVAR complications identified by CTA were regarded as the gold standard for comparing the DTA and CEUS results rated by the readers.
CTA scans were analyzed in the arterial phase, by an expert radiologist, on a reconstruction and image interpretation console (Advantage Workstation 4.4, General Electric Healthcare, Milwaukee, Wis, United States), adjusting the image level, window and enlargement values each time, and routinely using a 2D multiplanar reconstruction technique (coronal, sagittal and oblique planes) in order to better evaluate the possibily of complications after EVAR.
The complications detected by both diagnostic techniques were collected, and the results expressed as mean ± SD. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were calculated both separately and in combination between DTA and CEUS.
The statistical review of the study was performed by a biomedical statistician. A McNemar’s χ2 test was used to identify differential complications detected by two imaging techniques.
EVAR complications were detected by CTA in 33/163 (22.08%) patients with endoleak in 23/33 (69.69%) patients. Among the 33 patients with complications, 29 had a single complication (5 graft fractures, 9 graft dislocations, 6 graft migrations and 9 graft stenoses) whereas 4 patients had two complications (one patient had graft fracture and migration, two patients had graft fracture plus dislocations and one patient had a graft migration plus stenosis), resulting in a total of 37 complications in 33 patiens. The other 130 (78.78%) out of 163 patients had no complications detected by CTA. Considering patient-level analysis DTA and CTA concurred on presence of complications (true positive) in 32/33 (96.96%) patients and absence of complications (true negative) in 127/130 (97.69%) patients. In the remaining 4 patients DTA and CTA were not concordant (DTA detected 1 false negative case and 3 false positives), thus showing a sensitivity of 97%, a specificity of 98%, PPV of 91%, NPV of 99%, and accuracy of 98%. Considering patient-level analsysis, CEUS and CTA concurred on presence of complications (true positive) in 19/33 (57.57%) patients and absence of complications (true negative) in 129/130 (99.23%) patients. In the remaining 15 patients CEUS and CTA were not concordant (CEUS detected 1 false positive case and 14 false negatives), thus showing a sensitivity, specificity, PPV, NPV, and accuracy of 58%, 99%, 95%, 90%, and 91%, respectively. For the 37 complications in 33 patients, DTA and CTA concurred on presence/absence of complications in 97% (36/37 complications); the only complication missed by DTA was a graft dislocation associated with a small endoleak detected by CTA. By contrast, CEUS and CTA concurred on presence/absence of complications in 62% (23/37). The 14 complications missed by CEUS were: 7 cases of graft stenosis, 3 graft dislocations and 4 graft migrations. Eight of them were not associated with endoleak. The data are summarized in Tables 2 and 3.
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| DTA | 97% | 98% | 91% | 99% | 98% |
| CEUS | 58% | 99% | 95% | 90% | 91% |
| Complication | Total (37) | DTA (36) | CEUS (23) |
| Graft fracture | 5 | 5 | 5 |
| Graft dislocation | 9 | 8 | 6 |
| Graft migration | 6 | 6 | 2 |
| Graft stenosis | 9 | 9 | 2 |
| Graft fracture and migration | 2 | 2 | 2 |
| Graft fracture and dislocation | 4 | 4 | 4 |
| Graft migration and stenosis | 2 | 2 | 2 |
Complications were detected by both imaging modalities (DTA and CEUS) in 18 out of 33 patients (54.54%, 22/37 complications). McNemar’s χ2 test confirmed that the two techniques were equivalent (P > 0.001). Sensitivity, specificity and accuracy in detecting EVAR complications obtained by combining DTA and CEUS were 77%, 98% and 95%, respectively.
CTA is considered the gold standard technique for follow-up after EVAR, although it raises several major concerns for the lifelong surveillance that these patients require[4]. One is the cumulative dose of radiation to which patients are exposed, which has prompted attempts to reconsider the necessity of CTA in the follow-up of EVAR and to explore the possibility of reducing the effective dose associated with CTA[13-15]. From a radiobiological point of view, however, the cumulative dose of radiation is a marginal problem for lifetime cancer risk, because the mean age of patients undergoing EVAR and follow-up is usually over 65 years[16-19]. More important concers regarding CTA in EVAR follow-up are undoubtedly represented by the costs, both for patients and national health systems, and contrast-induced nephropathy (CIN) that causes a progressive decline in renal function during long-term follow-up. The latter side-effect is associated with increased morbidity, mortality, and financial burden on the healthcare system[20-22]. A safer and more cost-effective alternative to CTA in follow-up of EVAR is therefore necessary. DT is a new radiological technique with better accuracy than plain radiography. It exploits a series of very-low-dose projection images acquired with a digital detector during a single sweep of the X-ray tube over a limited angle. The data are reconstructed with a filtered back-projection algorithm to generate a set of images with slice interval and start and end heights defined by the user. An advantage is that DT removes overlying structures and provides more information about the structures of interest. Its limitations are that only slices parallel to the detector plane can be obtained and the loss of overview with blurring of the tissues outside the region of interest. The technique has mostly been used for breast and chest imaging, and recently also for the urinary tract[12,23,24].
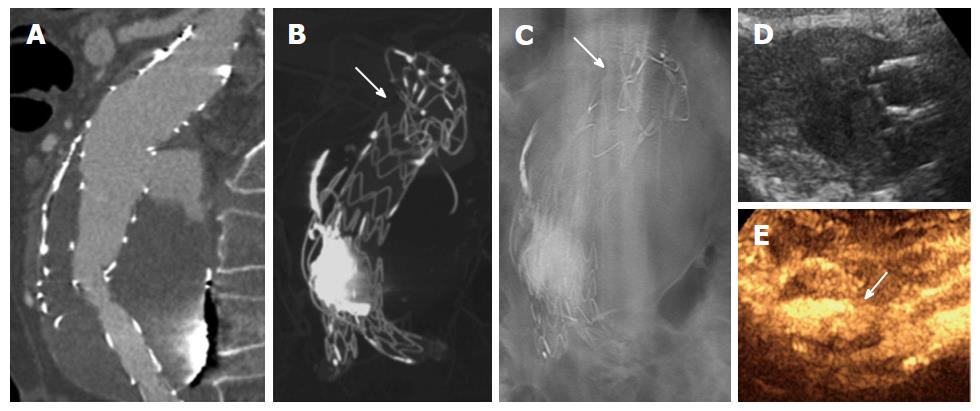
Our preliminary results show that DTA combined with CEUS is effective for diagnosis of complications after EVAR. In particular, DTA showed good accuracy and NPV (98% and 99% respectively), correctly identifying all graft fractures and migrations, but sometimes underestimating small endoleaks, which on the contrary were easily recognized by CEUS, a technique that should be performed by radiologists with experience in vascular pathologies and EVAR complications. In our population, the only false positive from DTA (a graft dislocation with a small endoleak) was correctly diagnosed by CEUS. On the other hand, CEUS (accuracy of 91% and NPV of 90%), especially if performed by an unskilled radiologist, could miss graft stenosis (7 cases in our population) and some graft migrations or dislocations (4 and 3, respectively, in our population), especially when there are no leaks or the associated endoleaks are very small. However, both DTA and CEUS correctly recognized the complications that required urgent treatment (9 patients) (Figure 2). Advantages of CEUS are the lack of ionizing radiations and contrast medium for renal function. However, this technique could be affected by body morphology (obesity, ascites, bowel gas) and echo reflection from the stent graft, even if the latter is minimized by the use of contrast medium that increases the ultrasound signal[6]. Advantages of DTA are the possibility of imaging the graft in different planes and a radiation dose only slightly higher than of plain digital radiography but substantially lower than the dose required for high- and even low-dose CTA protocols. A disadvantage is that aneurysmal sac enlargement cannot be correctly detected, with the exception for cases with a previous DTA examination, hence there is a need to combine it with a second imaging modality, such as CEUS. Some limitations of our study are that the population and follow-up period were both too small for validation worthy of EVAR follow-up guidelines. Nevertheless, CTA remains the standard reference for detecting complications after EVAR, with almost perfect sensitivity of direct graft complication imaging and visualization of secondary CT signs of graft complications (such as endoleaks and aneurysmal sac enlargement). However, EVAR follow-up imaging has changed since the introduction of minimally invasive aortic repair, with a significant shift towards less invasive surveillance protocols. Our results confirm the possibility of using DTA combined with CEUS as a cost-effective diagnostic protocol alternative to CTA in EVAR follow-up. This diagnostic protocol has the potential to limit the use of CTA in doubtful cases and cases requiring reintervention or with an unfavorable anatomy, significantly reducing costs and risk of CIN as well as overall radiation dose received by patients[25-31].
Endovascular aortic aneurysm repair (EVAR) is a safe technique associated with a significant reduction in perioperative mortality and morbidity compared to open repair. The technical success of stent-graft implantation is well known but after aortic-endovascular replacement, several medium- and long-term complications have been observed in cases of lifelong surveillance. Follow-up of EVAR is therefore essential to promptly diagnose and treat graft complications.
Current EVAR follow-up guidelines suggest computed tomography angiography (CTA) as the best method for detecting graft complications and endoleaks. The primary aim of this project is to find a new way to perform the EVAR follow-up, tailoring the imaging protocol per single patient, with a significant reduction of dose-exposure and risk of contrast-induced nephropathy (CIN).
To be known, this is the first study using digital tomosynthesis of the abdomen (DTA), combined with contrast enhanced ultrasound (CEUS) in assessing complications after EVAR by using CTA as the gold standard.
The authors’ results confirm the possibility of using DTA combined with CEUS as a cost-effective diagnostic protocol alternative to CTA in EVAR follow-up, with the potential to limit the use of CTA in doubtful cases and in cases requiring reintervention or with an unfavorable anatomy, significantly reducing costs and risk of CIN as well as overall radiation dose received by patients.
DTA; CEUS; EVAR; CTA; magnetic resonance angiography; Doppler ultrasound.
The study was well designed and performed, the results well supported their conclusion, and the manuscript is well organized as well.
P- Reviewer: Liu GJ, Yang H S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Li D
| 1. | Blankensteijn JD, de Jong SE, Prinssen M, van der Ham AC, Buth J, van Sterkenburg SM, Verhagen HJ, Buskens E, Grobbee DE. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352:2398-2405. [PubMed] |
| 2. | White GH, Yu W, May J, Chaufour X, Stephen MS. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis, and management. J Endovasc Surg. 1997;4:152-168. [PubMed] |
| 3. | Bastos RM, Razuk Filho A, Blasbalg R, Caffaro RA, Karakhanian WK, Rocha AJ. A multidetector tomography protocol for follow-up of endovascular aortic aneurysm repair. Clinics (Sao Paulo). 2011;66:2025-2029. [PubMed] |
| 4. | Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, van Herwaarden JA, Holt PJ, van Keulen JW, Rantner B. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 Suppl 1:S1-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1008] [Cited by in RCA: 1038] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 5. | Thurnher S, Cejna M. Imaging of aortic stent-grafts and endoleaks. Radiol Clin North Am. 2002;40:799-833. [PubMed] |
| 6. | Perini P, Sediri I, Midulla M, Delsart P, Mouton S, Gautier C, Pruvo JP, Haulon S. Single-centre prospective comparison between contrast-enhanced ultrasound and computed tomography angiography after EVAR. Eur J Vasc Endovasc Surg. 2011;42:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, Matsumura JS, May J, Veith FJ, Fillinger MF. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048-1060. [PubMed] |
| 8. | Mazzei MA, Guerrini S, Cioffi Squitieri N, Cagini L, Macarini L, Coppolino F, Giganti M, Volterrani L. The role of US examination in the management of acute abdomen. Crit Ultrasound J. 2013;5 Suppl 1:S6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Mazzei MA, Cioffi Squitieri N, Guerrini S, Stabile Ianora AA, Cagini L, Macarini L, Giganti M, Volterrani L. Sigmoid diverticulitis: US findings. Crit Ultrasound J. 2013;5 Suppl 1:S5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Habets J, Zandvoort HJ, Reitsma JB, Bartels LW, Moll FL, Leiner T, van Herwaarden JA. Magnetic resonance imaging is more sensitive than computed tomography angiography for the detection of endoleaks after endovascular abdominal aortic aneurysm repair: a systematic review. Eur J Vasc Endovasc Surg. 2013;45:340-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Setacci F, Sirignano P, De Donato G, Chisci E, Galzerano G, Cappelli A, Palasciano G, Setacci C. Endovascular approach for ruptured abdominal aortic aneursyms. J Cardiovasc Surg (Torino). 2010;51:313-317. [PubMed] |
| 12. | Mermuys K, De Geeter F, Bacher K, Van De Moortele K, Coenegrachts K, Steyaert L, Casselman JW. Digital tomosynthesis in the detection of urolithiasis: Diagnostic performance and dosimetry compared with digital radiography with MDCT as the reference standard. AJR Am J Roentgenol. 2010;195:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | White HA, Macdonald S. Estimating risk associated with radiation exposure during follow-up after endovascular aortic repair (EVAR). J Cardiovasc Surg (Torino). 2010;51:95-104. [PubMed] |
| 14. | Buffa V, Solazzo A, D’Auria V, Del Prete A, Vallone A, Luzietti M, Madau M, Grassi R, Miele V. Dual-source dual-energy CT: dose reduction after endovascular abdominal aortic aneurysm repair. Radiol Med. 2014;119:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Goetti R, Winklehner A, Gordic S, Baumueller S, Karlo CA, Frauenfelder T, Alkadhi H. Automated attenuation-based kilovoltage selection: preliminary observations in patients after endovascular aneurysm repair of the abdominal aorta. AJR Am J Roentgenol. 2012;199:W380-W385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Brambilla M, Cerini P, Lizio D, Vigna L, Carriero A, Fossaceca R. Cumulative radiation dose and radiation risk from medical imaging in patients subjected to endovascular aortic aneurysm repair. Radiol Med. 2015;120:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284. [PubMed] |
| 18. | Verhoeven EL, Oikonomou K, Ventin FC, Lerut P, Fernandes E Fernandes R, Mendes Pedro L. Is it time to eliminate CT after EVAR as routine follow-up? J Cardiovasc Surg (Torino). 2011;52:193-198. [PubMed] |
| 19. | Ten Bosch JA, Rouwet EV, Peters CT, Jansen L, Verhagen HJ, Prins MH, Teijink JA. Contrast-enhanced ultrasound versus Computed tomography angiography for surveillance of endovascular abdominal aortic aneurysm repair. J Vasc Interv Radiol. 2010;21:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | de Donato G, Setacci C, Chisci E, Setacci F, Giubbolini M, Sirignano P, Galzerano G, Cappelli A, Pieraccini M, Palasciano G. Abdominal aortic aneurysm repair in octogenarians: myth or reality? J Cardiovasc Surg (Torino). 2007;48:697-703. [PubMed] |
| 21. | Nicola R, Shaqdan KW, Aran K, Mansouri M, Singh A, Abujudeh HH. Contrast-Induced Nephropathy: Identifying the Risks, Choosing the Right Agent, and Reviewing Effective Prevention and Management Methods. Curr Probl Diagn Radiol. 2015;44:501-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Mazzei MA, Guerrini S, Cioffi Squitieri N, Imbriaco G, Mazzei FG, Volterrani L. Non-obstructive mesenteric ischemia after cardiovascular surgery: not so uncommon. Ann Thorac Cardiovasc Surg. 2014;20:253-255. [PubMed] |
| 23. | McAdams HP, Samei E, Dobbins J, Tourassi GD, Ravin CE. Recent advances in chest radiography. Radiology. 2006;241:663-683. [PubMed] |
| 24. | Park JM, Franken EA, Garg M, Fajardo LL, Niklason LT. Breast tomosynthesis: present considerations and future applications. Radiographics. 2007;27 Suppl 1:S231-S240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Törnqvist P, Dias NV, Resch T. Optimizing imaging for aortic repair. J Cardiovasc Surg (Torino). 2015;56:189-195. [PubMed] |
| 26. | Scaglione M, Salvolini L, Casciani E, Giovagnoni A, Mazzei MA, Volterrani L. The many faces of aortic dissections: Beware of unusual presentations. Eur J Radiol. 2008;65:359-364. [PubMed] |
| 27. | Mazzei MA, Volterrani L. Errors in multidetector row computed tomography. Radiol Med. 2015;120:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Setacci C, De Donato G, Setacci F, Chisci E, Perulli A, Galzerano G, Sirignano P. Management of abdominal endograft infection. J Cardiovasc Surg (Torino). 2010;51:33-41. [PubMed] |
| 29. | Setacci F, Sirignano P, de Donato G, Chisci E, Iacoponi F, Galzerano G, Palasciano G, Cappelli A, Setacci C. AAA with a challenging neck: early outcomes using the Endurant stent-graft system. Eur J Vasc Endovasc Surg. 2012;44:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Setacci F, Sirignano P, de Donato G, Galzerano G, Messina G, Guerrini S, Mazzei MA, Setacci C. Two-year-results of Endurant stent-graft in challenging aortic neck morphologies versus standard anatomies. J Cardiovasc Surg (Torino). 2014;55:85-92. [PubMed] |
| 31. | Speziale F, Sirignano P, Setacci F, Menna D, Capoccia L, Mansour W, Galzerano G, Setacci C. Immediate and two-year outcomes after EVAR in “on-label” and “off-label” neck anatomies using different commercially available devices. analysis of the experience of two Italian vascular centers. Ann Vasc Surg. 2014;28:1892-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |